Abstract
Mavacamten is the first and only cardiac myosin inhibitor approved in 5 continents for the treatment of adults with symptomatic New York Heart Association class II and III obstructive hypertrophic cardiomyopathy. An evidence‐based rationale was used to develop individualized mavacamten dosing, guided by commonly used clinical parameters. Echocardiography is recommended as part of routine clinical assessment of patients with hypertrophic cardiomyopathy, and left ventricular (LV) outflow tract gradient and LV ejection fraction are parameters that can be readily assessed and monitored by echocardiography. Therefore, an echocardiography‐based, clinically guided dose‐titration strategy was developed to optimize patient benefit from mavacamten for the treatment of symptomatic obstructive hypertrophic cardiomyopathy while minimizing the risk of LV ejection fraction reduction. Results from clinical trials paired with extensive modeling and simulation analyses support a dose‐titration and monitoring strategy based on serial echocardiographic measures of Valsalva LV outflow tract gradient and LV ejection fraction. This dosing approach allows for the identification of the lowest individualized mavacamten dose and exposure required to provide improvements in LV outflow tract obstruction, functional capacity, and symptoms. Mavacamten is primarily metabolized by CYP2C19 (cytochrome P450 2C19), and CYP2C19 metabolizer phenotype has an effect on mavacamten exposure. Therefore, this approach has also been demonstrated to provide a favorable safety profile irrespective of patients' CYP2C19 metabolizer status. The dose‐titration strategy includes additional considerations for the potential onset of systolic dysfunction in the context of intercurrent illness, and for the potential of drug–drug interactions with inhibitors and substrates of cytochrome P450 enzymes. This posology is reflected in the mavacamten US prescribing information.
Keywords: echocardiogram, individualized dosing, left ventricular ejection fraction, left ventricular outflow tract gradient, mavacamten, obstructive hypertrophic cardiomyopathy
Subject Categories: Cardiomyopathy, Treatment
Nonstandard Abbreviations and Acronyms
- C max
maximum serum concentration
- CYP
cytochrome P450
- FDA
US Food and Drug Administration
- HCM
hypertrophic cardiomyopathy
- LTE
long‐term extension
- USPI
US prescribing information
Mavacamten is the first and only cardiac myosin inhibitor approved by the US Food and Drug Administration (FDA), the European Medicines Agency, and other regulatory bodies in 5 continents for the treatment of adults with symptomatic New York Heart Association functional class II and III obstructive hypertrophic cardiomyopathy (HCM). 1 , 2 Mavacamten reduces the number of myosin heads that can bind with actin, thus normalizing the probability of force‐producing (systolic) and residual (diastolic) cross‐bridge formation. 1 , 3 , 4 Mavacamten reduces excess contractility and dynamic left ventricular (LV) outflow tract (LVOT) obstruction and improves myocardial energetics and cardiac filling pressures in patients with HCM. 1 , 3 , 5 Mavacamten is rapidly absorbed and exhibits pharmacokinetics characterized by a biphasic elimination profile, and is primarily metabolized through hepatic pathways involving CYP (cytochrome P450) enzymes, including CYP2C19, CYP3A4, and CYP2C9. 5 , 6 Genetic polymorphisms in CYP2C19 are known to affect the CYP2C19 metabolic pathway and can result in differences in drug exposure by metabolic phenotype. 7 Mavacamten has a half‐life of 6 to 9 days in CYP2C19 normal metabolizers and 23 days in CYP2C19 poor metabolizers, and area under the concentration–time curve was increased by 241% in poor metabolizers compared with normal metabolizers following a single dose of mavacamten 15 mg. 1 Furthermore, the time to reach steady‐state concentrations of mavacamten was found to be considerably longer in CYP2C19 poor metabolizers (approximately 28 weeks) than in normal metabolizers (approximately 6 weeks). 8 Based on its mechanism of action, reductions in LVOT gradient and LV ejection fraction (LVEF) are expected pharmacological effects of mavacamten. Both LVOT gradient and LVEF are parameters readily measured and assessed by echocardiography.
Treatment guidelines from the European Society of Cardiology recommend consideration of mavacamten in addition to a beta‐blocker (or, if this is not possible, with verapamil or diltiazem) to improve symptoms in adult patients with resting or provoked LVOT obstruction (Class IIa, level of evidence A). The guidelines also recommend consideration of mavacamten as monotherapy in symptomatic patients with LVOT gradients who are intolerant or have contraindications to beta blockers, verapamil or diltiazem, or disopyramide (Class IIa, level of evidence B). 9 However, in the absence of evidence from clinical studies that directly compare mavacamten versus existing therapies, guidelines from the European Society of Cardiology currently recommend the use of beta‐blockers and nondihydropyridine calcium channel blockers as first‐line therapies for symptom management, and the addition of disopyramide in refractory cases (Class I, level of evidence B). 9 The 2024 American Heart Association/American College of Cardiology guidelines provide similar recommendations for the use of cardiac myosin inhibitors (such as mavacamten) as a treatment in adult patients with obstructive HCM who have persistent symptoms despite therapy with beta‐blockers or nondihydropyridine calcium channel blockers. 10 Limited placebo‐controlled data support the off‐label use of beta‐blocker and calcium channel blocker agents for treatment of HCM, which were developed for other conditions and not specifically for HCM, provide variable symptomatic relief, and do not target the underlying pathophysiology of the disease. 11 , 12 , 13 Mavacamten is the first pharmacological therapy with the potential to modify the underlying pathophysiology of HCM, as demonstrated in preclinical models. 4 , 14
In the pivotal phase 3, randomized, double‐blind, placebo‐controlled EXPLORER‐HCM trial (Clinical Study to Evaluate Mavacamten in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy; NCT03470545), mavacamten dose was titrated to 1 of 4 strengths (2.5, 5, 10, and 15 mg) based on each patient's clinical status over 30 weeks of treatment, with administration of a 5 mg oral capsule once daily (QD) being the uniform starting dosage. 15 , 16 Mavacamten plasma concentration ≥1000 ng/mL or LVEF <50% were used as temporary treatment interruption criteria. Patients could resume treatment at the next lowest available dose if mavacamten plasma concentration was <1000 ng/mL and LVEF was ≥50% at the follow‐up visit. 15 The goal of the dosing regimen used in EXPLORER‐HCM was to achieve Valsalva LVOT gradient <30 mm Hg and maintain LVEF >50% while adjusting mavacamten dose when the drug exposure was outside the range of 350 to 700 ng/mL (with the expectation that the regimen would maintain exposures between 350 ng/mL and 1000 ng/mL). 15 Mavacamten was shown to be superior to placebo in achieving the composite functional primary end point and all secondary end points, including changes from baseline in peak oxygen consumption, New York Heart Association class, and Kansas City Cardiomyopathy Questionnaire‐Clinical Summary Score after 30 weeks of treatment. 16 These changes were concomitant with a sustained improvement in resting, Valsalva, and postexercise LVOT gradient. Consistent with its mechanism of action, mavacamten treatment resulted in a mean LVEF decrease of 3.9% compared with 0.01% with placebo, from a hyperdynamic mean baseline of 74%. Despite this, mean LVEF remained within normal range (>50%) throughout the study period with few individual instances of left ventricular systolic dysfunction (7 patients in the mavacamten group with transient reductions in LVEF <50% compared with 2 patients in the placebo group). Safety and tolerability with treatment were similar to placebo, and adverse events were mostly mild. 16 In the EXPLORER‐HCM cardiac magnetic resonance substudy, patients in the mavacamten group experienced significant reductions from baseline to week 30 in LV mass index, LV mass, maximum LV wall thickness, left atrial volume index, and absolute intracellular myocardial mass index compared with placebo. 17 The change from baseline to week 30 in late gadolinium enhancement (6 SDs), a marker of myocardial fibrosis, was similar between treatment groups. 17
The ongoing, long‐term extension (LTE) study MAVA‐LTE (A Long‐Term Safety Extension Study of Mavacamten in Adults Who Have Completed MAVERICK‐HCM or EXPLORER‐HCM; NCT03723655) has enrolled patients who completed EXPLORER‐HCM (referred to as the EXPLORER‐LTE cohort) following a posttreatment washout period in the parent study. 18 The dosing algorithm in the EXPLORER‐LTE cohort of MAVA‐LTE used a mavacamten starting dosage of 5 mg/day for all patients. Interim results from the EXPLORER‐LTE cohort with median follow‐up of 101 weeks (interim data cutoff: May 31, 2022) similarly showed rapid and sustained beneficial effects of mavacamten treatment on LVOT gradients, New York Heart Association classification, and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) concentrations as in the parent study. 19
Here, we describe the evidence‐based rationale for the FDA‐approved, echocardiography‐based dosing scheme in the US prescribing information (USPI) for mavacamten in patients with symptomatic obstructive HCM. 1 The goal of this review is to help clinicians and potential prescribers to understand how clinical trial data, pharmacokinetics/pharmacodynamics, and exposure–response modeling support individualized dosing of mavacamten in clinical practice based on echocardiographic parameters. At the time of writing, 1866 patients have been treated with mavacamten using this posology in a US real‐world setting, with results consistent to those observed in the clinical trials. 20
Rationale for Individualized Dose‐Titration Strategy
Results of sequential clinical studies paired with extensive model‐based analyses were used to establish a starting dose and dose‐titration strategy that could achieve effective and well‐tolerated exposure of mavacamten within a target therapeutic range in individual patients with symptomatic obstructive HCM. To achieve mavacamten exposure consistent with the therapeutic range that leads to improvements in symptoms and LVOT obstruction, while maintaining normal LVEF, individual patients may require higher or lower oral daily mavacamten doses. Furthermore, patients with HCM exhibit heterogeneity in cardiac structure and function. 21 An echocardiogram‐guided, individualized dose‐titration approach therefore enables the identification of the lowest effective dose for each patient based on clinical assessment of their response over a series of visits. This approach accounts for various patient baseline characteristics (eg, body weight, genetic differences in drug‐metabolizing enzymes, disease‐specific characteristics, concomitant medications, Valsalva LVOT gradient, LVEF) and, if required, also allows for dose adjustments to be made for medical circumstances that can affect exposure or LVEF (eg, presence of intercurrent illness, concomitant and interacting medications). 1
In the phase 2 PIONEER‐HCM study (A Phase 2 Open‐Label Pilot Study Evaluating MYK‐461 in Subjects With Symptomatic Hypertrophic Cardiomyopathy and Left Ventricular Outflow Tract Obstruction; NCT02842242), a wide exposure–response range was investigated to identify the target therapeutic range in which mavacamten reduced LVOT obstruction while maintaining LVEF within normal limits. The target therapeutic range was determined to be 350 to 695 ng/mL, whereas plasma concentrations above 1000 ng/mL were initially hypothesized to excessively reduce LVEF beyond what was necessary to achieve a clinically meaningful response. 22 These findings subsequently informed the dose‐titration strategy implemented in EXPLORER‐HCM. This dosing algorithm used a combination of central laboratory‐read, blinded echocardiographic measures and mavacamten plasma concentration levels <350 ng/mL to uptitrate, and plasma concentrations >700 ng/mL to downtitrate, while a concentration >1000 ng/mL was implemented as a dose‐interruption criterion. 15 Modeling analysis of mavacamten concentration along with Valsalva LVOT gradient and LVEF observations from EXPLORER‐HCM, PIONEER‐HCM, MAVA‐LTE, and the open‐label extension study PIONEER‐OLE (PIONEER‐Open‐Label Extension; NCT03496168) demonstrated that monitoring of Valsalva LVOT gradient and LVEF by echocardiography was adequate to assess the expected effect of mavacamten and that reduced LVEF was not always associated with concomitant elevated mavacamten plasma concentration. 23 The use of real‐time, clinical site‐read echocardiography parameters to guide dose adjustments in the EXPLORER‐LTE cohort of MAVA‐LTE provided further evidence that avoidance of excess reduction in LVEF could be monitored through periodic echocardiographic imaging without reliance on the mavacamten plasma concentration to inform titration. Nevertheless, in the absence of data, the initial study design of MAVA‐LTE included mavacamten plasma concentration of >1000 ng/mL as a prespecified criterion for temporary treatment interruption, as in EXPLORER‐HCM. Initial data from MAVA‐LTE demonstrating the lack of correlation between high mavacamten plasma concentrations and clinically relevant adverse events informed the removal of this criterion from the study protocol on February 2, 2022. 18 , 24 Based on these findings, as well as extensive pharmacokinetic modeling and simulations, a dose‐titration strategy guided by periodic echocardiography was developed for clinical use.
Echocardiography as a Primary Tool for Dose Selection and Monitoring
Echocardiographic assessments of Valsalva LVOT gradient and LVEF guiding mavacamten dosing were implemented and clinically evaluated in the MAVA‐LTE and VALOR‐HCM (A Study to Evaluate Mavacamten in Adults With Symptomatic Obstructive HCM Who Are Eligible for Septal Reduction Therapy; NCT04349072) studies. 18 , 25 , 26 The Valsalva maneuver is a commonly used breathing method performed during an echocardiogram to estimate LVOT gradient with provocation, and is more cost‐effective, less time consuming, and more feasible to record serially in symptomatic patients than postexercise LVOT gradient (Table 1). 27 , 28 , 29 , 30 Guidelines from the American Heart Association/American College of Cardiology and the American Society of Echocardiography recommend the use of echocardiography for diagnosis and monitoring of treatment efficacy and consideration for invasive therapy (eg, myectomy or alcohol septal ablation) in patients with obstructive HCM. 28 , 31 Known challenges associated with accurate assessment of cardiac function by echocardiography include signal contamination of LVOT gradients by mitral regurgitation, resting echocardiography underestimating the presence or severity of LVOT obstruction, and the quantification of LVEF in hearts that have heterogenous regions of hypertrophy. 32 , 33 , 34 LVOT gradients can also be highly variable on a day‐to‐day basis and are known to be influenced by factors such as exercise, medication, posture during gradient assessment, and the individual's understanding of how to correctly perform the Valsalva maneuver. 29 , 30 , 35 , 36 Therefore, it is important for cardiologists and sonographers to be familiar with the caveats associated with echocardiographic evaluation of obstruction and systolic function, to perform echocardiography measurements in a standardized environment (ideally by the same group of trained professionals), and to adhere to guideline‐approved best practices. For patients with HCM and a resting LVOT gradient <50 mm Hg, use of provocative maneuvers during echocardiography is specifically recommended. 28 Mavacamten posology uses the measurement of Valsalva LVOT gradient at each visit to inform dosing, regardless of resting LVOT gradient. 1 Although regular echocardiographic assessments can be a time and economic burden for both patients and providers, direct observation of an individual patient's Valsalva LVOT gradient and LVEF response mitigates the need to perform therapeutic drug monitoring, which determines benefit–risk in a less timely manner. In the EXPLORER‐LTE cohort of the MAVA‐LTE study, site‐read measures of Valsalva LVOT gradient and LVEF were the only echocardiographic parameters used to guide dosing decisions in patients receiving mavacamten. 18 This approach to dosing decisions without consideration of mavacamten concentration levels was also used effectively in the phase 3 VALOR‐HCM study, which evaluated the ability of mavacamten to reduce the need for septal reduction therapy in patients with symptomatic obstructive HCM. 25 , 26 , 37 , 38 Overall, despite the aforementioned potential caveats associated with echocardiographic assessment of Valsalva LVOT gradient and LVEF, these measures have been shown to be suitable and effective for titration of mavacamten dose in a clinical setting. Subsequently, through the extensive use of modeling and simulation, an algorithm was developed that resulted in further optimization of the dose‐titration regimen of mavacamten for clinical use; this is reflected in the current USPI. 1 , 23 , 39
Table 1.
The Valsalva Maneuver During Echocardiography*
| Question | Answer |
|---|---|
| Why is the Valsalva maneuver used to assess LVOT gradient during the management of obstructive HCM? |
|
| How do I perform an adequate Valsalva maneuver? |
|
| How do I know if the Valsalva maneuver is performed adequately? |
|
Treatment Initiation
Per FDA‐approved USPI, the recommended starting dosage of mavacamten is a 5 mg capsule taken orally QD without regard to food, with four available dose strengths of 2.5, 5, 10, and 15 mg. Stepwise downtitrations to 2.5 mg QD can occur at weeks 4 and 8 in the presence of early pharmacodynamic response (eg, Valsalva LVOT gradient reduction <20 mm Hg). LVEF is expected to remain in the normal range during this initiation phase when patients are receiving 2.5 mg or 5 mg per day. Subsequent stepwise uptitrations can occur at week 12 (to a maximum dose of 10 mg) and every 12 weeks thereafter (to a maximum dose of 15 mg). To minimize risk, week 24 is the first visit following treatment initiation in which the total range of approved dose strengths could be prescribed. 1 The recommendations for mavacamten treatment initiation are outlined in Figure 1. A 5 mg starting dose of mavacamten was used in multiple phase 2 and 3 clinical studies, including EXPLORER‐HCM, 16 the ongoing EXPLORER‐LTE cohort of MAVA‐LTE, 18 , 19 the MAVERICK‐HCM (A Phase 2 Study of Mavacamten in Adults With Symptomatic Non‐Obstructive Hypertrophic Cardiomyopathy; NCT03442764) trial in patients with nonobstructive HCM, 40 and the VALOR‐HCM trial. 25 , 37 , 38 Across the mavacamten arms of EXPLORER‐HCM, EXPLORER‐LTE, and VALOR‐HCM, no patient experienced LVEF <50% before the first visit (week 4; ie, the first opportunity for dose downtitration). 16 , 18 , 25 Therefore, these findings emphasize that a starting dose of mavacamten 5 mg is generally well tolerated in patients with obstructive HCM.
Figure 1. Initiation phase study schema.
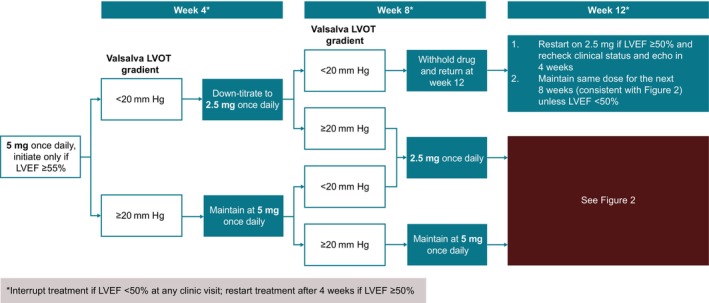
Echo indicates echocardiogram; LVEF, left ventricular ejection fraction; and LVOT, left ventricular outflow tract.
In the phase 3 EXPLORER‐CN study (A Study to Evaluate the Efficacy and Safety of Mavacamten in Chinese Adults With Symptomatic Obstructive HCM; NCT05174416) in Chinese patients with symptomatic obstructive HCM, the starting mavacamten dosage was 2.5 mg QD. 41 This reduced starting dosage was chosen because Chinese patients have a lower average body weight and a higher prevalence of CYP2C19 poor metabolizer phenotypes than patients assessed in the EXPLORER‐HCM study. 42 , 43 Despite this, 48 of 54 patients in the mavacamten arm (88.9%) were receiving a 5 mg or 10 mg dose at the end of the double‐blind, placebo‐controlled treatment period. 41 Improvements in LVOT gradients with mavacamten treatment were observed from week 4, and a significant improvement in Valsalva LVOT gradient with mavacamten versus placebo was observed over 30 weeks of treatment. 41 A consistent benefit on Valsalva LVOT gradient was observed across CYP2C19 metabolizer phenotypes. Improvements in resting LVOT gradient, New York Heart Association class, Kansas City Cardiomyopathy Questionnaire‐Clinical Summary Score, and NT‐proBNP levels were also observed in the mavacamten group compared with the placebo group following 30 weeks of treatment, while safety and tolerability were similar between treatment groups. Overall, the results of the EXPLORER‐CN study demonstrated that a mavacamten 2.5 mg starting dose followed by titration to clinical parameters provided an acceptable efficacy and safety profile in Chinese patients regardless of CYP2C19 phenotype. 41
Initial Follow‐Up Assessment
In clinical trials of mavacamten, follow‐up assessment 4 weeks after mavacamten initiation was used to identify patients with an early pharmacodynamic response (eg, rapidly reduced Valsalva LVOT gradient). 18 , 25 , 26 Patients who exhibited such a response during the first 4 weeks could include poor metabolizers who may require a dose reduction to avoid escalating effective drug levels. In MAVA‐LTE and VALOR‐HCM, Valsalva LVOT gradient <30 mm Hg at week 4 was used as a criterion for downtitration to 2.5 mg QD. 18 , 25 , 26 Overall, this approach demonstrated that the first follow‐up visit after initiation of mavacamten treatment can reasonably occur after 4 weeks. The threshold of <30 mm Hg at week 4 used in MAVA‐LTE and VALOR‐HCM for down‐titration necessitated subsequent uptitration in a large number of patients due to a rebound of the Valsalva LVOT gradient ≥30 mm Hg at subsequent visits. Therefore, the threshold for downtitration used in the approved dosing regimen is Valsalva LVOT gradient <20 mm Hg. Qualification of the use of a <20 mm Hg threshold in in silico model‐based simulations versus a < 30 mm Hg threshold demonstrated that a <20 mm Hg threshold would result in fewer patients being downtitrated at week 4. Therefore, these results suggested that a <20 mm Hg threshold would allow for early identification of patients who are more likely to require down‐titration (eg, CYP2C19 poor metabolizers) while minimizing the proportion of patients who would require subsequent uptitration back to a 5 mg dose. 39
Titration Phase
In the EXPLORER‐HCM trial, dose uptitration opportunities occurred at weeks 8 and 14 based on central reading of assessments (Valsalva LVOT gradient, LVEF, plasma concentration) from weeks 6 and 12, respectively. The 6‐week intervals were designed to allow the majority of patients to approach steady‐state drug concentrations before the next uptitration opportunity. Dose adjustments were designed to achieve a Valsalva LVOT gradient <30 mm Hg and a mavacamten plasma concentration <700 ng/mL while maintaining an LVEF >50%. 16 In the EXPLORER‐LTE cohort of MAVA‐LTE, dose adjustment opportunities occurred at week 4 (downtitration) and weeks 8 and 12 (uptitration) based on local site‐read echocardiogram results for Valsalva LVOT gradient and resting LVEF, and at week 24 based on site‐read, postexercise LVOT gradient. 18 In the FDA‐approved dosing regimen for clinical use, the starting dosage is 5 mg QD and 2 downtitration opportunities are offered after initiating mavacamten, at weeks 4 and 8 based on early efficacy (e.g., if a patient's Valsalva LVOT gradient is <20 mm Hg in the first weeks of treatment, this may indicate a patient who requires only 2.5 mg QD). The first uptitration opportunity occurs more gradually in the approved regimen than in the clinical trials, at week 12. 1 These revisions represent conservative safeguards that accommodate the extremes of the patient population, most notably poor metabolizers who may benefit from an early opportunity to down‐titrate. This approach additionally ensures that most patients, including poor metabolizers, have an opportunity to approach steady‐state concentrations of mavacamten before up‐titration.
In EXPLORER‐HCM, the EXPLORER‐LTE cohort of MAVA‐LTE, and VALOR‐HCM, dose increases were considered for patients with Valsalva LVOT gradient ≥30 mm Hg and LVEF ≥50%. 15 , 18 , 26 However, an LVEF threshold of ≥55% is used for uptitration in the approved dosing regimen to allow a buffer for potential further effect on LVEF. A follow‐up echocardiogram 4 weeks after any dose increase is recommended to monitor LVEF response. 1 Overall, the downtitration and uptitration criteria in the approved dosing regimen have been established with the aim of optimizing the benefit for each individual patient while ensuring a favorable safety profile and limiting the number of dose adjustments required. The key differences between the mavacamten posology used in clinical trials and the posology used in the USPI are detailed in Table 2. 1 , 6 , 16 , 25 , 39
Table 2.
Comparison of Pivotal Trial and Approved Mavacamten Posology*
| Studied posology in mavacamten clinical trials | USPI approved posology | Rationale |
|---|---|---|
| 5 mg QD starting dosage for all patients | Same as EXPLORER‐HCM | Consistent starting dose allows simpler titration strategy for all patients |
| Pharmacokinetics therapeutic drug monitoring and echocardiogram‐guided dose adjustment | Echocardiogram‐guided dose adjustment | Echocardiogram data serve as the most directly relevant clinical marker |
| Down‐titration at week 4 if Valsalva LVOT gradient <30 mm Hg | Down‐titration at weeks 4 and 8 if Valsalva LVOT gradient <20 mm Hg |
Gradient threshold changed to limit unnecessary down‐titration at week 4 and as an added safety precaution at week 8 Additional potential dose reduction at week 8 reduces the risk of LVEF <50% in patients with cytochrome P450 2C19 poor metabolizer phenotype |
| Uptitration opportunities at weeks 8 and 14 | Uptitration opportunity at week 12 (and every 12 weeks thereafter) | More gradual (conservative) uptitration interval when moving from clinical studies to the real world; ultimately achieves similar results |
| Uptitration if LVEF ≥50% | Uptitration if Valsalva LVOT gradient ≥30 mm Hg and LVEF ≥55% | Provides a larger safety margin to minimize occurrences of LVEF <50% during uptitration because some effect on LVEF is expected |
| Permanent discontinuation if LVEF <30% | Permanent discontinuation upon the second occurrence of LVEF <50% at mavacamten 2.5 mg QD |
Allows for consideration of real‐world situations and decision‐making. The patient can resume treatment following temporary interruption for LVEF <50% if LVEF recovers upon reassessment However, the patient is unlikely to tolerate treatment if LVEF reduces <50% more than twice on lowest available dose |
Maintenance
Longer‐term interim efficacy and safety results from the EXPLORER‐LTE cohort of MAVA‐LTE, using echocardiogram‐guided dosing, support a monitoring strategy guided by site‐measured clinical parameters. 18 After the week 24 visit, patients were evaluated every 12 weeks in the EXPLORER‐LTE cohort. At the latest interim data cutoff, the median mavacamten dose was 5 mg QD at all study visits through week 120. Furthermore, 82.5% and 85.1% of patients were receiving mavacamten ≤10 mg QD at week 96 and week 120, respectively. This may suggest that most patients underwent only 1 dose adjustment at most during long‐term treatment. Improvements observed in LVOT gradients, symptoms, and NT‐proBNP concentrations were sustained, durable, and consistent with those seen in the EXPLORER‐HCM parent study. Furthermore, the long‐term safety profile of mavacamten in the EXPLORER‐LTE cohort was also consistent with EXPLORER‐HCM. 19 Over a median follow‐up of 62 weeks and 317 patient‐years of exposure, 12 patients (5.2%) had transient reductions in LVEF <50%, of whom 8 experienced the event (while on treatment) following the week 24 visit. Of these 8 patients, 5 had intercurrent illness at the time of LVEF reduction (including 4 events of atrial fibrillation/flutter) and 6 resumed treatment following LVEF recovery to ≥50% (including 1 patient who discontinued treatment and later reenrolled into the study). 18 Assessments of cardiac function (including LVEF) by echocardiography were consistent between site‐read and central laboratory‐read values, thus confirming the feasibility of using these measures in the clinic. 18 , 19 Patients who completed the EXPLORER‐HCM cardiac magnetic resonance substudy and enrolled in MAVA‐LTE had the option to participate in the MAVA‐LTE cardiac magnetic resonance substudy. Cardiac magnetic resonance data in these patients demonstrated sustained reductions in LV mass index, maximum LV wall thickness, absolute myocardial mass index, and left atrial volume index from week 24 to week 96 of MAVA‐LTE. 24 The efficacy and safety results from the EXPLORER‐LTE cohort of MAVA‐LTE, therefore, demonstrate that long‐term echocardiography‐guided dosing can be reliably used in routine clinical practice to manage patients receiving mavacamten.
In the approved dosing regimen, once patients achieve a stable mavacamten dose that results in LVEF >55% and Valsalva LVOT gradient <30 mm Hg, or in LVEF 50% to 55% regardless of Valsalva LVOT gradient, patients should be monitored every 12 weeks. 1 The recommendations for treatment maintenance are outlined in Figure 2. Follow‐up visits should include both clinical and echocardiographic assessments to monitor LVEF and to check for heart failure symptoms. Reductions in LVEF can occur at any time throughout the course of treatment, although those that occur later in treatment tend to occur owing to intercurrent events (eg, paroxysms of atrial fibrillation or infection) rather than mavacamten dose variation. 16
Figure 2. Titration and maintenance phase.
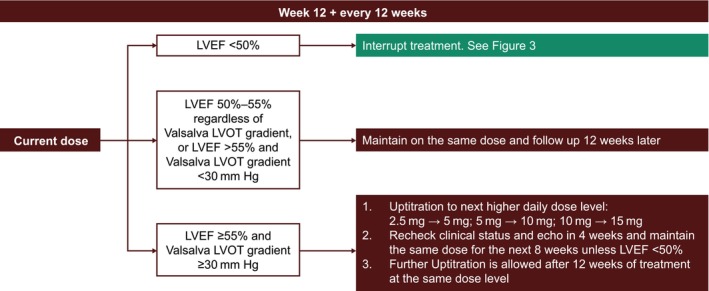
Echo indicates echocardiogram; LVEF, left ventricular ejection fraction; and LVOT, left ventricular outflow tract.
The following vignette provides an example of a hypothetical scenario that may be encountered in a clinical setting.
A 55‐year‐old man receiving metoprolol 25 mg QD initiated treatment with mavacamten 5 mg QD. At baseline, his Valsalva LVOT gradient was 60 mm Hg and LVEF 70%. The initial follow‐up echocardiogram after 4 weeks of treatment showed the patient had a Valsalva LVOT gradient of 35 mm Hg and an LVEF of 65%. The patient's dosage was therefore maintained at 5 mg QD for the next 4 weeks. At the week 8 visit, the patient's echocardiogram showed a Valsalva LVOT gradient of 36 mm Hg and an LVEF of 63%; the patient's dosage was therefore maintained at 5 mg QD for an additional 4 weeks. At the week 12 visit, the patient's echocardiogram showed a Valsalva LVOT gradient of 38 mm Hg and an LVEF of 65%, which resulted in an uptitration of dosage to 10 mg QD. Per protocol, the patient underwent a repeat echocardiogram 4 weeks after the dose increase, which showed Valsalva LVOT gradient and LVEF measurements were consistent with the week 12 visit. The follow‐up visit 12 weeks after dose increase showed the patient's Valsalva LVOT gradient had dropped to 27 mm Hg while LVEF remained at 65%. Thus, the patient was maintained at 10 mg QD dosage. Subsequent follow‐up visits every 12 weeks showed the patient remained within the designated Valsalva LVOT gradient threshold of <30 mm Hg and LVEF ≥55%. Therefore, he continued to receive mavacamten 10 mg QD indefinitely while being monitored every 12 weeks for heart failure symptoms and Valsalva LVOT gradient and LVEF by echocardiographic assessment.
Treatment Interruption and Discontinuation
Temporary interruption of mavacamten in patients experiencing LVEF <50% was prespecified in the protocols of EXPLORER‐HCM and MAVA‐LTE and is also recommended in the approved posology, as outlined in Figure 3. 16 , 18 Cases occurring in the clinical studies have previously been described. 16 , 18 , 19 , 25 , 37 , 38 Most patients initiating mavacamten treatment will have elevated LVEF, and a mild decrease over time is expected. However, it is very important to monitor LVEF at every visit; if LVEF reduces to <50%, an interruption is recommended followed by a reassessment in 4 weeks. In patients with a transient reduction in LVEF to <50%, dosing may resume at the next lower dose level once the patient's condition has stabilized and LVEF has recovered to ≥50% at least 4 weeks after treatment interruption. Initiation or uptitration of concomitant medications that affect mavacamten metabolism or medical conditions should also be considered as a potential cause for reduction in LVEF, and close medical supervision is recommended. 1
Figure 3. Treatment interruption at any clinic visit if LVEF <50%.
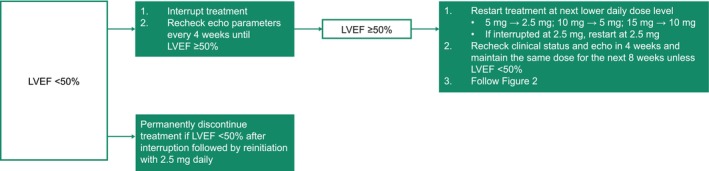
Echo indicates echocardiogram; LVEF, left ventricular ejection fraction.
In VALOR‐HCM, patients could elect to discontinue mavacamten at any time and proceed to septal reduction therapy, preferably performed at a comprehensive HCM treatment center, after a recommended washout period of 6 weeks or greater. Patients who discontinued mavacamten to undergo septal reduction therapy were assessed within 14 days of discontinuation and were followed every 24 weeks from the date of the procedure to week 128 of the study. 26 By week 56, 3 of 56 patients (5.4%) from the original mavacamten group and 3 of 52 patients (5.8%) from the placebo group (who had crossed over to mavacamten treatment at week 16) elected to undergo septal reduction therapy. 38
Based on limited human data, systolic dysfunction is the most likely result of mavacamten overdose. Management of mavacamten overdose consists of cessation of treatment in addition to medically supportive measures to maintain hemodynamic stability, such as close monitoring of LVEF, vital signs, and clinical status. 1 Early administration of activated charcoal may be considered to reduce absorption of mavacamten in the event of acute overdose 44 ; data supporting the beneficial effect of this measure are forthcoming.
The following vignette provides an example of a hypothetical scenario in which a patient with an intercurrent serious infection experiences LVEF <50% in a clinical setting.
A 68‐year‐old woman who had been responding optimally to mavacamten 5 mg QD complained of a dry cough and congestion, which had been ongoing for a few weeks. The patient also reported a fever (maximum temperature of 102 °F), shortness of breath, pain in her back and ribs (due to coughing), body aches, and night sweats. The patient sought treatment at an urgent care center and was prescribed albuterol. Three days later, the patient was hospitalized, and a chest radiograph revealed right lower‐lobe pneumonia. Laboratory testing confirmed bacterial pneumonia, and the patient was treated with albuterol, azithromycin, ceftriaxone, ipratropium, methylprednisolone, and sodium chloride. An ECG confirmed normal sinus rhythm. A transthoracic echocardiogram showed an LVEF of 48%; mavacamten treatment was therefore temporarily discontinued. The patient's symptoms improved over the course of the hospitalization. After 4 days, the event was considered to be resolved and the patient was discharged and prescribed albuterol, doxycycline, and steroids. A follow‐up echocardiogram 4 weeks later showed Valsalva LVOT gradient of 69 mm Hg and LVEF of 74%. Given that the patient's condition had stabilized and reduction in LVEF was most likely due to pneumonia, which had since resolved, dosing was resumed at 5 mg QD. The patient's echocardiogram should be rechecked in 4 weeks, and the same dose could be maintained for the next 8 weeks provided that LVEF remains ≥50%. Subsequently, the patient would continue to be dosed and maintained or further titrated based on ongoing results of monitoring.
Owing to the potential for systolic dysfunction development in patients with HCM with a serious intercurrent illness, such as acute onset atrial fibrillation or serious infection, reassessment of LVEF should be considered, regardless of timing from last dose adjustment. Atrial fibrillation is common in HCM, occurring in approximately 20% of patients and can be associated with systolic dysfunction, particularly in the setting of rapid ventricular response. 45 , 46 , 47 In EXPLORER‐HCM, the number of patients who experienced a serious adverse event of atrial fibrillation was similar between those randomized to mavacamten or placebo (2 versus 4 patients, respectively). 16 Of these patients, 1 assigned to the mavacamten group with a history of ongoing atrial fibrillation experienced a transient reduction in LVEF <50% following atrial fibrillation ablation during the protocol‐defined 8‐week washout period. 16 In the EXPLORER cohort of MAVA‐LTE, 21 patients experienced an adverse event of atrial fibrillation over 315 patient‐years of exposure, 11 of whom had new onset atrial fibrillation. Of the 12 patients who experienced a transient reduction in LVEF <50%, 5 had an intercurrent illness of atrial fibrillation/flutter at the time of reduced LVEF. Overall, the proportion of patients in the EXPLORER cohort of MAVA‐LTE who experienced an adverse event or serious adverse event of atrial fibrillation (9.1% and 3%, respectively) was similar to that reported in the placebo group of EXPLORER‐HCM (7.0% and 3%, respectively). 18 Treatment may also need to be interrupted for worsening symptoms of heart failure. Patients who interrupt mavacamten treatment while receiving the 2.5 mg QD dosage may restart at 2.5 mg, per clinician's decision. If patients experience a second LVEF reduction <50% while receiving 2.5 mg, the recommendation is to permanently discontinue dosing.
If an intercurrent illness was a contributing factor to decrease in LVEF, it is recommended that the condition be treated or improved before rechecking LVEF and restarting mavacamten. Once restarted, the echocardiogram should be rechecked 4 weeks later, and the same dose maintained for the subsequent 8 weeks (if LVEF ≥50%). If Valsalva LVOT gradient ≥30 mm Hg and LVEF ≥55% persist, the patient may be uptitrated to the next available higher dose (Figure 2). 1
The following vignette provides an example of a hypothetical scenario in which a patient with intercurrent atrial fibrillation experiences LVEF <50% in a clinical setting.
A 46‐year‐old man experienced new onset atrial fibrillation with rapid ventricular response confirmed with an ECG while taking mavacamten 10 mg QD. Transthoracic echocardiogram showed LVEF of 45% while in atrial fibrillation. Mavacamten was discontinued and normal sinus rhythm was restored with cardioversion. The patient was prescribed rivaroxaban. Three weeks later, the patient experienced several episodes of palpitations and was hospitalized for recurrent paroxysmal atrial fibrillation. He reported no chest pain or syncope. Spontaneous reversion to sinus rhythm occurred during monitoring. At a follow‐up visit, an echocardiogram showed Valsalva LVOT gradient of 80 mm Hg and LVEF of 70%. With atrial fibrillation believed to be the cause of the LVEF < 50% event, the patient was restarted with mavacamten 5 mg and was reexamined 4 weeks later; the echocardiogram showed Valsalva LVOT gradient of 69 mm Hg and LVEF of 70%. The patient can be maintained on the same 5 mg dose for the next 8 weeks unless LVEF <50%. After 8 weeks, the site‐read echocardiogram showed Valsalva LVOT gradient of 60 mm Hg and LVEF of 70%; therefore, the dose can be uptitrated to 10 mg after 12 weeks of treatment with 5 mg.
Drug Interactions With CYP2C19 and CYP3A4 Inhibitors and Inducers
Mavacamten is primarily metabolized hepatically by CYP2C19 and to a lesser extent by CYP3A4 and CYP2C9 enzymes. 1 Potential drug interactions with certain CYP inhibitors and inducers must, therefore, be considered, and some are contraindicated (Table 3). 1 , 48 , 49 , 50 , 51 , 52 , 53 An additive negative inotropic effect of mavacamten with other drugs that reduce cardiac contractility can be expected. Of note, in EXPLORER‐HCM, 92% of patients were receiving beta blockers or calcium channel blockers at baseline, and almost all maintained their background HCM therapy throughout the study or required only minor adjustments. 16 The USPI recommends avoiding concomitant use of mavacamten with disopyramide in combination with calcium channel blockers because such use has been associated with LV systolic dysfunction and heart failure symptoms in 1 patient. 1 , 38 Nevertheless, 14 patients (25.0%) in the original mavacamten group of the VALOR‐HCM study received the study drug concomitantly with disopyramide, including 3 patients (5.4%) who received mavacamten, disopyramide and calcium channel blockers (with or without beta blockers), and no new safety concerns were reported in these patients over the 56‐week treatment period. 25 , 38 Following the initial 16‐week treatment period, a further 3 patients (5.8%) who crossed over from placebo to mavacamten treatment received mavacamten concomitantly with disopyramide and calcium channel blockers (with or without beta blockers). 38 Further studies need to be performed assess the safety and efficacy of combining mavacamten therapy with disopyramide and calcium channel blockers. If concomitant therapy with a negative inotrope is initiated, or if the dose of a negative inotrope is increased, LVEF should be closely monitored until a stable dose and clinical response have been achieved.
Table 3.
Established and Potentially Significant Pharmacokinetic Drug Interactions with Mavacamten*
| Category | Drug type | Clinical impact | Prevention or management per USPI | Drug interaction study results |
|---|---|---|---|---|
| Impact of other drugs on mavacamten |
Moderate‐to‐strong CYP2C19 inhibitors (eg, omeprazole 40 mg; ticlopidine) or Strong CYP3A4 inhibitors (eg, ketoconazole; ritonavir copackaged with nirmatrelvir) |
|
|
|
| Moderate‐to‐strong CYP2C19 or CYP3A4 inducers (eg, rifampin; carbamazepine) |
|
|
|
|
| Weak CYP2C19 inhibitors (eg, omeprazole 20 mg) or moderate CYP3A4 inhibitors (eg, verapamil; diltiazem) |
|
|
|
|
| Impact of mavacamten on other drugs | CYP3A4 (eg, midazolam), CYP2C9 (eg, tolbutamide), and CYP2C19 (eg, omeprazole) substrates |
|
|
|
| Hormonal contraceptives (eg, progestin; ethinyl estradiol) |
|
|
|
A phase 1 drug–drug interaction study of a 31‐day course of omeprazole 20 mg (a weak CYP2C19 inhibitor) concomitantly administered with a single dose of mavacamten 15 mg in healthy volunteers resulted in a moderate increase in mavacamten overall exposure, with no significant effect on mavacamten absorption. 48 A study of healthy volunteers receiving a 28‐day course of verapamil 240 mg (a moderate CYP3A4 inhibitor) with a single dose of mavacamten 25 mg resulted in a minimal increase in mavacamten area under the concentration–time curve and a 52% increase in peak mavacamten exposure. 48 The results of the 2 studies suggested that initiation of mavacamten treatment on a background of weak CYP2C19 or moderate CYP3A4 inhibitors was well tolerated and could be accommodated in the dosing posology without modification. 48 Concomitant use of mavacamten with strong inhibitors of CYP3A4 is contraindicated. Therefore, mavacamten should be discontinued if treatment with a strong CYP3A4 inhibitor, such as the antiviral medication ritonavir 100 mg copackaged with nirmatrelvir 150 mg, is required. 49
Mavacamten itself is an inducer of CYP3A4, CYP2C9, and CYP2C19, and concomitant use of mavacamten with CYP enzyme substrates, such as hormonal oral contraceptives, may reduce the plasma concentration of these drugs. To assess if repeated administration of mavacamten 15 mg affected the exposure of oral contraceptives, a prospective, 2‐period crossover study was performed in 13 healthy female volunteers receiving treatment with ethinyl estradiol 35 μg and norethindrone 1 mg. Modest increases in area under the concentration–time curves were observed for ethinyl estradiol and norethindrone following administration with mavacamten compared with before mavacamten administration (geometric mean ratio: ≤1.22), while maximum observed concentration and biological half‐life of ethinyl estradiol and norethindrone were unaffected by coadministration. 50 Nevertheless, close monitoring is recommended when mavacamten is used in combination with CYP enzyme substrates and is of particular importance in women of childbearing potential who may be taking hormonal contraceptives (Table 3). The absence of pregnancy should be confirmed in women of childbearing potential before initiation of mavacamten. Patients should also be advised to use effective contraceptives during mavacamten treatment and for 4 months after the last dose; if a patient becomes pregnant during this period, health care providers should contact the drug manufacturer. 1 A pregnancy safety study for mavacamten is ongoing.
In the United States, mavacamten is currently available only through a restricted Risk Evaluation and Mitigation Strategy program. 54 This program helps to monitor the known or potential risks associated with a drug product and is required by the FDA to ensure the continued positive benefit–risk profile of a drug. For mavacamten, the Risk Evaluation and Mitigation Strategy program is applied because of the risk of heart failure due to systolic dysfunction. To prescribe mavacamten, patients, health care providers, and pharmacies must enroll and comply with the ongoing monitoring requirements of the Risk Evaluation and Mitigation Strategy program. Only certified pharmacies can dispense mavacamten to patients who are authorized to receive the drug.
Conclusions
Results from clinical trials support the rationale for a clinically guided dose‐titration and monitoring strategy for mavacamten that considers interindividual variability, with some patients requiring higher or lower drug doses to achieve a target therapeutic range and beneficial clinical response. Echocardiography‐guided management of mavacamten dosing can be used to identify the lowest individualized dose needed to achieve therapeutic benefit while maintaining LVEF in the normal range (Figure 4).
Figure 4. Clinically guided dose‐titration strategy for mavacamten in patients with obstructive HCM.
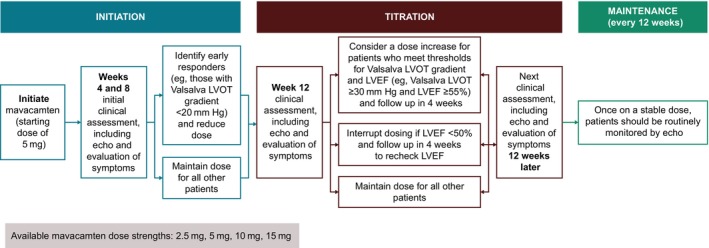
Results from clinical trials support a clinically guided dose‐titration and monitoring strategy for mavacamten in clinical practice that uses echocardiography in conjunction with clinical status to identify the lowest individualized dose needed to achieve therapeutic benefit while maintaining LVEF in the normal range. In the dosing algorithm shown here, patients are initiated with a 5 mg starting dose, with initial follow‐up assessments after 4 and 8 weeks to identify early responders who may benefit from a dose reduction. The first assessment for possible dose increase is recommended 12 weeks after treatment initiation. Similar to the clinical studies, if LVEF is <50% at any time, mavacamten dosing should be interrupted and LVEF rechecked in 4 weeks. Echo indicates echocardiogram; HCM, hypertrophic cardiomyopathy; LVEF, left ventricular ejection fraction; and LVOT, left ventricular outflow tract.
Sources of Funding
This work was supported by Bristol Myers Squibb, Princeton, NJ.
Disclosures
Dr Owens has received payments as a consultant to Cytokinetics and MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb. Dr Desai serves as a consultant for MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb, and Medtronic, LLC. Dr Wheeler has received research support and consulting fees from MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb, and research support from Cytokinetics. Ms. Rodonski has received personal fees for serving on an advisory board for MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb. Dr Merali and Dr Sehnert are employees of Bristol Myers Squibb and receive stock or stock options from Bristol Myers Squibb. Dr Saberi has received payments as a consultant to MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb and Cytokinetics.
Acknowledgments
Writing and editorial assistance were provided by Thomas Crighton, PhD, of Oxford PharmaGenesis, Oxford, UK, and Kim Fuller, PhD, of Lumanity Scientific Inc., Yardley, PA, USA, and funded by Bristol Myers Squibb.
Author Contributions: All authors contributed to the development of the article, critically revised the article for important intellectual content, and approved the final version.
This article was sent to Sakima A. Smith, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 13.
REFERENCES
- 1. CAMZYOS (mavacamten) . Prescribing Information. Princeton, NJ: Bristol‐Myers Squibb Company; 2024. Accessed June 14, 2024. https://packageinserts.bms.com/pi/pi_camzyos.pdf. [Google Scholar]
- 2. CAMZYOS (mavacamten) . Summary of Product Characteristics. Dublin, Ireland: Bristol‐Myers Squibb Pharma EEIG; 2023. Accessed April 22, 2024. https://www.ema.europa.eu/en/documents/product‐information/camzyos‐epar‐product‐information_en.pdf. [Google Scholar]
- 3. Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H, Rogers CS, Gorham JM, Wong FL, Morck MM, et al. Deciphering the super relaxed state of human beta‐cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A. 2018;115:E8143–E8152. doi: 10.1073/pnas.1809540115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kawas RF, Anderson RL, Ingle SRB, Song Y, Sran AS, Rodriguez HM. A small‐molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J Biol Chem. 2017;292:16571–16577. doi: 10.1074/jbc.M117.776815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grillo MP, Erve JCL, Dick R, Driscoll JP, Haste N, Markova S, Brun P, Carlson TJ, Evanchik M. In vitro and in vivo pharmacokinetic characterization of mavacamten, a first‐in‐class small molecule allosteric modulator of beta cardiac myosin. Xenobiotica. 2019;49:718–733. doi: 10.1080/00498254.2018.1495856 [DOI] [PubMed] [Google Scholar]
- 6. Perera V, Gretler DD, Seroogy JD, Chiang M, Palmisano M, Florea V. Pharmacokinetic drug‐drug interaction study of mavacamten with verapamil in healthy subjects. Poster presented at: Annual College of Clinical Pharmacology (ACCP) Annual Meeting. North Bethesda, MD, USA. September 25–272022.
- 7. Sienkiewicz‐Oleszkiewicz B, Wiela‐Hojeńska A. CYP2C19 polymorphism in relation to the pharmacotherapy optimization of commonly used drugs. Pharmazie. 2018;73:619–624. doi: 10.1691/ph.2018.8689 [DOI] [PubMed] [Google Scholar]
- 8. Centre for Drug Evaluation and Research . Clinical pharmacology review. Mavacamten. 2022. Accessed December 7, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/214998Orig1s000ClinPharmR.pdf.
- 9. Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales‐Villa R, Basso C, Bezzina CR, Biagini E, Blom NA, de Boer RA, et al. 2023 ESC guidelines for the management of cardiomyopathies: developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur Heart J. 2023;44:3503–3626. doi: 10.1093/eurheartj/ehad194 [DOI] [PubMed] [Google Scholar]
- 10. Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, Day SM, Dearani JA, Epps KC, Evanovich L, Ferrari VA, et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy. Circulation. 2024;149:e1239–e1311. doi: 10.1161/CIR.0000000000001250 [DOI] [PubMed] [Google Scholar]
- 11. Ammirati E, Contri R, Coppini R, Cecchi F, Frigerio M, Olivotto I. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Fail. 2016;18:1106–1118. doi: 10.1002/ejhf.541 [DOI] [PubMed] [Google Scholar]
- 12. Papadakis M, Basu J, Sharma S. Mavacamten: treatment aspirations in hypertrophic cardiomyopathy. Lancet. 2020;396:736–737. doi: 10.1016/S0140-6736(20)31793-1 [DOI] [PubMed] [Google Scholar]
- 13. Sherrid MV. Drug therapy for hypertrophic cardiomypathy: physiology and practice. Curr Cardiol Rev. 2016;12:52–65. doi: 10.2174/1573403X1201160126125403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, Henze M, Kawas R, Oslob JD, Rodriguez HM, et al. A small‐molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science. 2016;351:617–621. doi: 10.1126/science.aad3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ho CY, Olivotto I, Jacoby D, Lester SJ, Roe M, Wang A, Waldman CB, Zhang D, Sehnert AJ, Heitner SB. Study design and rationale of EXPLORER‐HCM: evaluation of mavacamten in adults with symptomatic obstructive hypertrophic cardiomyopathy. Circ Heart Fail. 2020;13:e006853. doi: 10.1161/CIRCHEARTFAILURE.120.006853 [DOI] [PubMed] [Google Scholar]
- 16. Olivotto I, Oreziak A, Barriales‐Villa R, Abraham TP, Masri A, Garcia‐Pavia P, Saberi S, Lakdawala NK, Wheeler MT, Owens A, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER‐HCM): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2020;396:759–769. doi: 10.1016/S0140-6736(20)31792-X [DOI] [PubMed] [Google Scholar]
- 17. Saberi S, Cardim N, Yamani M, Schulz‐Menger J, Li W, Florea V, Sehnert AJ, Kwong RY, Jerosch‐Herold M, Masri A, et al. Mavacamten favorably impacts cardiac structure in obstructive hypertrophic cardiomyopathy: EXPLORER‐HCM cardiac magnetic resonance substudy analysis. Circulation. 2021;143:606–608. doi: 10.1161/CIRCULATIONAHA.120.052359 [DOI] [PubMed] [Google Scholar]
- 18. Rader F, Oręziak A, Choudhury L, Saberi S, Fermin D, Wheeler MT, Abraham TP, Garcia‐Pavia P, Zwas DR, Masri A, et al. Mavacamten treatment for symptomatic obstructive hypertrophic cardiomyopathy: interim results from the MAVA‐LTE study, EXPLORER‐LTE cohort. JACC Heart Fail. 2024;12:164–177. doi: 10.1016/j.jchf.2023.09.028 [DOI] [PubMed] [Google Scholar]
- 19. Garcia‐Pavia P, Oreziak A, Masri A, Barriales‐Villa R, Abraham TP, Owens AT, Lakdawala NK, Saberi S, Wang A, Wheeler MT, et al. Long‐term effects of mavacamten treatment in obstructive hypertrophic cardiomyopathy (HCM): updated cumulative analysis of the EXPLORER cohort of MAVA‐long‐term extension (LTE) study up to 120 weeks. Oral presentation at: European Society of Cardiology Congress. August 25–28, 2023.
- 20. Martinez MW, Ferri L, Patel N, Minton N, Lockman J, Cheung M, Coiro M, Afsari S, Seto D. The CAMZYOS (mavacamten) risk evaluation and mitigation strategy program: results from 10 months post‐launch. J Am Coll Cardiol. 2024;83:354. doi: 10.1016/S0735-1097(24)02344-1 [DOI] [Google Scholar]
- 21. Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121:749–770. doi: 10.1161/CIRCRESAHA.117.311059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heitner SB, Jacoby D, Lester SJ, Owens A. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: a clinical trial. Ann Intern Med. 2019;170:741–748. doi: 10.7326/M18-3016 [DOI] [PubMed] [Google Scholar]
- 23. Merali S, Salinger DH, Perera V, Thaneer N, Back H, Seroogy JD, Gretler DD, Sehnert AJ, Palmisano M, Roy A. Exposure‐response modeling of mavacamten in adults with hypertrophic cardiomyopathy (HCM). Poster presented at: 13th American Conference of Pharmacometrics (ACoP13). Aurora, CO, USA. October 30–November 2, 2022.
- 24. Saberi S, Kramer CM, Oreziak A, Masri A, Barriales‐Villa R, Abraham TP, Lakdawala NK, Wang A, Choudhury L, Rader F, et al. 96‐week cardiac magnetic resonance (CMR) results of treatment with mavacamten from the EXPLORER cohort of the MAVA‐Long‐Term Extension (LTE) study in patients (pts) with obstructive hypertrophic cardiomyopathy (HCM). Poster presented at: American College of Cardiology (ACC)/World Congress of Cardiology (WCC) 2023. New Orleans, LA, USA. March 4–6; 2023.
- 25. Desai MY, Owens A, Geske JB, Wolski K, Naidu SS, Smedira NG, Cremer PC, Schaff H, McErlean E, Sewell C, et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy. J Am Coll Cardiol. 2022;80:95–108. doi: 10.1016/j.jacc.2022.04.048 [DOI] [PubMed] [Google Scholar]
- 26. Desai MY, Wolski K, Owens A, Naidu SS, Geske JB, Smedira NG, Schaff H, Lampl K, McErlean E, Sewell C, et al. Study design and rationale of VALOR‐HCM: evaluation of mavacamten in adults with symptomatic obstructive hypertrophic cardiomyopathy who are eligible for septal reduction therapy. Am Heart J. 2021;239:80–89. doi: 10.1016/j.ahj.2021.05.007 [DOI] [PubMed] [Google Scholar]
- 27. Ghazal SN. Valsalva maneuver in echocardiography. J Echocardiogr. 2017;15:1–5. doi: 10.1007/s12574-016-0310-8 [DOI] [PubMed] [Google Scholar]
- 28. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2020;142:e533–e557. doi: 10.1161/CIR.0000000000000938 [DOI] [PubMed] [Google Scholar]
- 29. Jensen MK, Havndrup O, Pecini R, Dalsgaard M, Hassager C, Helqvist S, Kelbæk H, Jørgensen E, Køber L, Bundgaard H. Comparison of Valsalva manoeuvre and exercise in echocardiographic evaluation of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Eur J Echocardiogr. 2010;11:763–769. doi: 10.1093/ejechocard/jeq063 [DOI] [PubMed] [Google Scholar]
- 30. Kumar S, Van Ness G, Bender A, Yadava M, Minnier J, Ravi S, McGrath L, Song HK, Heitner SB. Standardized goal‐directed Valsalva maneuver for assessment of inducible left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2018;31:791–798. doi: 10.1016/j.echo.2018.01.022 [DOI] [PubMed] [Google Scholar]
- 31. Nagueh SF, Phelan D, Abraham T, Armour A, Desai MY, Dragulescu A, Gilliland Y, Lester SJ, Maldonado Y, Mohiddin S, et al. Recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: an update from the American Society of Echocardiography, in collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2022;35:533–569. doi: 10.1016/j.echo.2022.03.012 [DOI] [PubMed] [Google Scholar]
- 32. Cardim N, Galderisi M, Edvardsen T, Plein S, Popescu BA, D'Andrea A, Bruder O, Cosyns B, Davin L, Donal E, et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging. 2015;16:280. doi: 10.1093/ehjci/jeu291 [DOI] [PubMed] [Google Scholar]
- 33. Shah JS, Esteban MT, Thaman R, Sharma R, Mist B, Pantazis A, Ward D, Kohli SK, Page SP, Demetrescu C, et al. Prevalence of exercise‐induced left ventricular outflow tract obstruction in symptomatic patients with non‐obstructive hypertrophic cardiomyopathy. Heart. 2008;94:1288–1294. doi: 10.1136/hrt.2007.126003 [DOI] [PubMed] [Google Scholar]
- 34. Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11:260–274. doi: 10.1016/j.jcmg.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 35. Geske JB, Sorajja P, Ommen SR, Nishimura RA. Left ventricular outflow tract gradient variability in hypertrophic cardiomyopathy. Clin Cardiol. 2009;32:397–402. doi: 10.1002/clc.20594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kizilbash AM, Heinle SK, Grayburn PA. Spontaneous variability of left ventricular outflow tract gradient in hypertrophic obstructive cardiomyopathy. Circulation. 1998;97:461–466. doi: 10.1161/01.CIR.97.5.461 [DOI] [PubMed] [Google Scholar]
- 37. Desai MY, Owens AT, Geske JB, Wolski K, Saberi S, Wang A, Sherrid MV, Cremer PC, Naidu SS, Smedira N, et al. Dose‐blinded myosin inhibition in patients with obstructive HCM referred for septal reduction therapy: outcomes through 32‐weeks. Circulation. 2023;147:850–863. doi: 10.1161/CIRCULATIONAHA.122.062534 [DOI] [PubMed] [Google Scholar]
- 38. Desai MY, Owens A, Wolski K, Geske JB, Saberi S, Wang A, Sherrid M, Cremer PC, Lakdawala NK, Tower‐Rader A, et al. Mavacamten in patients with hypertrophic cardiomyopathy referred for septal reduction: week 56 results from the VALOR‐HCM randomized clinical trial. JAMA Cardiol. 2023;8:969–977. doi: 10.1001/jamacardio.2023.3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Merali S, Sehnert AJS, David H, Roy A, Palmisano M, Perera V. Modeling and simulation to investigate dose titration regimen for mavacamten in adults with obstructive hypertrophic cardiomyopathy (HCM). Poster presented at: 13th American Conference of Pharmacometrics (ACoP13). Aurora, CO, USA. October 30–November 2. 2022.
- 40. Ho CY, Mealiffe ME, Bach RG, Bhattacharya M, Choudhury L, Edelberg JM, Hegde SM, Jacoby D, Lakdawala NK, Lester SJ, et al. Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2020;75:2649–2660. doi: 10.1016/j.jacc.2020.03.064 [DOI] [PubMed] [Google Scholar]
- 41. Tian Z, Li L, Li X, Ja W, Zhang Q, Li Z, Peng D, Yang P, Ma W, Wang F, et al. Effect of mavacamten on Chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER‐CN randomized clinical trial. JAMA Cardiol. 2023;8:957–965. doi: 10.1001/jamacardio.2023.3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen L, Qin S, Xie J, Tang J, Yang L, Shen W, Zhao X, Du J, He G, Feng G, et al. Genetic polymorphism analysis of CYP2C19 in Chinese Han populations from different geographic areas of mainland China. Pharmacogenomics. 2008;9:691–702. doi: 10.2217/14622416.9.6.691 [DOI] [PubMed] [Google Scholar]
- 43. Koopmans AB, Braakman MH, Vinkers DJ, Hoek HW, van Harten PN. Meta‐analysis of probability estimates of worldwide variation of CYP2D6 and CYP2C19. Transl Psychiatry. 2021;11:141. doi: 10.1038/s41398-020-01129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. CAMZYOS (mavacamten) . Canadian Product Monograph. Montreal, Canada: Bristol‐Myers Squibb Canada; 2022. Accessed April 22, 2024. https://www.bms.com/assets/bms/ca/documents/productmonograph/CAMZYOS_EN_PM.pdf [Google Scholar]
- 45. Rowin EJ, Hausvater A, Link MS, Abt P, Gionfriddo W, Wang W, Rastegar H, Estes NAM, Maron MS, Maron BJ. Clinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation. 2017;136:2420–2436. doi: 10.1161/CIRCULATIONAHA.117.029267 [DOI] [PubMed] [Google Scholar]
- 46. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure. Circulation. 2009;119:2516–2525. doi: 10.1161/CIRCULATIONAHA.108.821306 [DOI] [PubMed] [Google Scholar]
- 47. Marcusohn E, Kobo O, Postnikov M, Epstein D, Agmon Y, Gepstein L, Hellman Y, Zukermann R. Left ventricular systolic dysfunction due to atrial fibrillation: clinical and echocardiographic predictors. Card Fail Rev. 2021;7:e16. doi: 10.15420/cfr.2021.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perera V, Gretler DD, Seroogy JD, Chiang M, Palmisano M, Florea V. Effects of omeprazole and verapamil on the pharmacokinetics, safety, and tolerability of mavacamten: two drug‐drug interaction studies in healthy participants. Clin Pharmacol Drug Dev. 2023;12:1241–1251. doi: 10.1002/cpdd.1332 [DOI] [PubMed] [Google Scholar]
- 49. CAMZYOS (mavacamten) . REMS patient brochure. Princeton, NJ: Bristol Myers Squibb; 2022. Accessed December 7, 2023. https://www.camzyosrems.com/assets/commercial/us/camzyosrems/en/pdf/Camzyos‐REMS‐Patient‐Brochure.pdf [Google Scholar]
- 50. Chiang M, Sychterz C, Gaohua L, Perera V, Gretler DD, Florea V, Merali S. Drug‐drug interaction potential of mavacamten with oral contraceptives: results from a clinical pharmacokinetic study and a physiologically based pharmacokinetic model. J Clin Pharmacol. 2023;63:1275–1282. doi: 10.1002/jcph.2298 [DOI] [PubMed] [Google Scholar]
- 51. Chiang M, Sychterz C, Perera V, Merali S, Palmisano M, Templeton IE, Gaohua L. Physiologically based pharmacokinetic modeling and simulation of mavacamten exposure with drug‐drug interactions from CYP inducers and inhibitors by CYP2C19 phenotype. Clin Pharmacol Ther. 2023;114:922–932. doi: 10.1002/cpt.3005 [DOI] [PubMed] [Google Scholar]
- 52. CAMZYOS (mavacamten) dosing guide. Princeton, NJ: Bristol Myers Squibb; 2022. Accessed December 7, 2023. https://www.camzyoshcp.com/dosing‐guide. [Google Scholar]
- 53. PAXLOVID™ (nirmatrelvir tablets; ritonavir tablets) emergency use authorization fact sheet for healthcare providers. Pfizer Inc., New York, NY. Accessed December 7, 2023. https://www.fda.gov/media/155050/download.
- 54. CAMZYOS (mavacamten) . REMS program overview. Princeton, NJ: Bristol Myers Squibb; 2022. Accessed December 7, 2023. https://www.camzyosrems.com/assets/commercial/us/camzyosrems/en/pdf/Camzyos‐REMS‐Program‐Overview.pdf. [Google Scholar]


