Abstract
To investigate the endoplasmic reticulum (ER) Ca2+ stores in plant cells, we generated tobacco (Nicotiana tabacum; NT1) suspension cells and Arabidopsis plants with altered levels of calreticulin (CRT), an ER-localized Ca2+-binding protein. NT1 cells and Arabidopsis plants were transformed with a maize (Zea mays) CRT gene in both sense and antisense orientations under the control of an Arabidopsis heat shock promoter. ER-enriched membrane fractions from NT1 cells were used to examine how altered expression of CRT affects Ca2+ uptake and release. We found that a 2.5-fold increase in CRT led to a 2-fold increase in ATP-dependent 45Ca2+ accumulation in the ER-enriched fraction compared with heat-shocked wild-type controls. Furthermore, after treatment with the Ca2+ ionophore ionomycin, ER microsomes from NT1 cells overproducing CRT showed a 2-fold increase in the amount of 45Ca2+ released, and a 2- to 3-fold increase in the amount of 45Ca2+ retained compared with wild type. These data indicate that altering the production of CRT affects the ER Ca2+ pool. In addition, CRT transgenic Arabidopsis plants were used to determine if altered CRT levels had any physiological effects. We found that the level of CRT in heat shock-induced CRT transgenic plants correlated positively with the retention of chlorophyll when the plants were transferred from Ca2+-containing medium to Ca2+-depleted medium. Together these data are consistent with the hypothesis that increasing CRT in the ER increases the ER Ca2+ stores and thereby enhances the survival of plants grown in low Ca2+ medium.
Calcium is an essential second messenger that controls a variety of cellular functions (Bush, 1993, 1995; Sanders et al., 1999). The efficacy of calcium as a signaling molecule is dependent on tightly regulated transport and storage. Ca2+ is stored in organelles, e.g. endoplasmic reticulum (ER), vacuole, mitochondria and chloroplasts, and the cell wall. Although the vacuole is the main Ca2+ sequestration site in plant cells, the ER has also been suggested to play an important role in regulating Ca2+ homeostasis (Klusener et al., 1995). Calcium is also a required micronutrient and lack of calcium can be detrimental to plant growth and development (Marshner, 1986). Plants grown on calcium-deficient media are more susceptible to plant pathogens, and show reduced growth of the apical meristem, chlorotic leaves, softening of tissues, and cell wall breakdown (Simon, 1978). The cell's sensitivity and response to various stresses, such as salinity, cold, and Ca2+ deficiency, is dependent on its ability to sequester and use Ca2+ from internal Ca2+-signaling stores (Hirschi, 1999, 2001; Miseta et al., 1999; Cessna and Low, 2001). The ability to modulate intracellular Ca2+ pools therefore could provide a means for plants to gain resistance to various external stresses.
The ER contains a variety of Ca2+-binding proteins such as the molecular chaperone binding protein (BiP), calnexin, and calreticulin (CRT). Of these, CRT is responsible for the main Ca2+-retaining pool in plants (Hassan et al., 1995). CRT is an evolutionarily conserved protein containing an HDEL retention sequence for ER lumen localization in plants. It has a globular N domain and two Ca2+-binding regions; a high-affinity, low-capacity P domain, and a low-affinity and high-capacity Ca2+-binding C domain. The C domain of the mammalian CRT can sequester at least 25 mol Ca2+ per mole protein (for review, see Krause and Michalak, 1997; Michalak et al., 1999; Corbett and Michalak, 2000). Because of its high Ca2+-binding capacity, CRT has been suggested to be involved in Ca2+ signaling (Camacho and Lechleiter, 1995; Mery et al., 1996; John et al., 1998). Furthermore, CRT has been proposed to be involved in chaperone activity (Denecke et al., 1995; Nauseef et al., 1995; Hebert et al., 1996; Otteken and Moss, 1996; Crofts et al., 1999; Saito et al., 1999), cell adhesion (Coppolino et al., 1997), gene expression (Burns et al., 1994; Dedhar et al., 1994), apoptosis (Nakamura et al., 2000; Taguchi et al., 2000), and in store-operated Ca2+ fluxes through the plasma membrane (Mery et al., 1996; Fasolato et al., 1998; Llewelyn et al., 1998; Xu et al., 2000).
The Ca2+-binding properties of CRT are similar in both mammalian and plant homologs (Chen et al., 1994; Mery et al., 1996). Although no reports have characterized CRT's Ca2+-storing ability or its potential role in Ca2+ signaling in plants, several reports in mammalian systems have shown that increased production of CRT increases cellular Ca2+ levels and affects the response of cytosolic Ca2+ to external stimuli (Bastianutto et al., 1995; Mery et al., 1996; Opas et al., 1996). Using mouse L fibroblast cells, Mery et al. (1996) showed that an increase in CRT leads to an increase in the ER Ca2+-signaling pool. Addition of either extracellular ATP, an activator of the P2y purinergic receptors, or ionomycin plus thapsigargin resulted in a 1.5-fold increase in cytosolic Ca2+ in CRT overexpressing lines compared with wild-type lines (Mery et al., 1996). In addition, it was shown in CRT overexpressing HeLa cells that stimulation by two agonists, ATP and histamine, added in succession resulted in increased cytosolic Ca2+ after the second agonist (Bastianutto et al., 1995). The increase in cytosolic Ca2+ was observed in both fura-2 loaded CRT-overexpressing cells, and in CRT-overexpressing cells cotransfected with aequorin. The magnitude of the response varied with the stimulus used and the physiological status of the cell, consistent with the hypothesis that CRT can function not only as a Ca2+-buffering device but also as a regulator of agonist-triggered Ca2+ release.
In view of the complexity of the role of CRT in the regulation of cellular Ca2+ homeostasis and of the yet unexplored properties of CRT in plants, we decided to investigate whether perturbation of CRT levels could affect ER Ca2+ in tobacco (Nicotiana tabacum; NT1) suspension cells and Arabidopsis plants. Here, we show that CRT levels can be selectively modulated in plant cells using a heat shock-inducible promoter and a maize (Zea mays) CRT cDNA. 45Ca2+ measurements were carried out in ER-enriched membrane vesicles generated from NT1 cells. Heat shock-induced production of CRT caused a significant increase in ER-accumulated Ca2+ in vitro. In a similar manner, decreased CRT levels correlated with a decrease in ER Ca2+ accumulation in vitro. Treatment with the Ca2+ ionophore ionomycin showed that the ER Ca2+-buffering capacity was CRT dependent. We also examined how an altered level of CRT affects plant responses to stress. Heat shock-induced production of CRT in transformed Arabidopsis plants enhanced the survival of plants transferred from Ca2+-containing medium to Ca2+-depleted medium, compared with wild-type controls. These results suggest that CRT plays a key role in the regulation of the Ca2+ status of the plant ER and that the ER, in addition to the vacuole, is an important Ca2+ store in plant cells.
RESULTS
Selection of Transgenic Tobacco Cell Lines
We used a transgenic approach to determine if ER calcium levels could be modulated by altered expression of the calcium-binding protein CRT. Because calcium is sequestered in various cellular compartments, we used an inducible promoter to control the CRT transgene to guard against long-term compensatory mechanisms that might reestablish normal Ca2+ levels. By perturbing one component of the Ca2+ storage/regulatory network, we hoped to begin to determine how cytoplasmic calcium levels are regulated.
Tobacco suspension culture cells (NT1) are readily transformed (An, 1985), and provide ample material for isolating ER microsomes. NT1 cells were transformed with Agrobacterium tumefaciens binary vectors carrying sense or antisense CRT cDNA sequences, or mgfp5 (encoding ER-targeted green fluorescent protein [GFP]; Haseloff et al., 1997), all under the control of an Arabidopsis heat shock promoter. Twelve independent kanamycin-resistant cell cultures were isolated for each construct. All twelve cell lines showed HS-inducible changes in CRT expression. Three cell lines transformed with sense CRT cDNA were selected and labeled Nt CRT:1, Nt CRT:5, and Nt CRT:7, one cell line transformed with the CRT cDNA in antisense orientation was selected and labeled Nt CRT-A:3, and one cell line transformed with mgfp5 was selected and labeled Nt GFP:4. Levels of gene induction were tested by immunoblots of total protein (Fig. 1C), or by monitoring GFP fluorescence (data not shown) of cells, harvested 16 h after a 2-h heat shock.
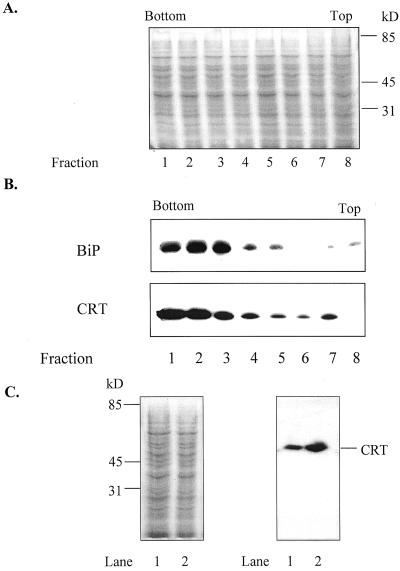
Figure 1.
CRT and BiP, an ER-localized chaperone, show similar distribution on Suc gradients. Microsomes were isolated from the wild-type cell line and layered onto a discontinuous Suc gradient (45%, 38%, and 22% [w/v] Suc). One-milliliter fractions were collected and equal amounts of protein were analyzed by 10% (w/v) SDS-PAGE (10 μg protein lane−1). The bottom and top of the gradient are indicated. A, Equal amounts of protein from collected gradient-fractions of a wild-type cell line, visualized with Gelcode staining. B, Immunostaining of gradient fractions from a wild-type cell line with polyclonal antibodies against maize CRT (1:5,000) and polyclonal antibodies against BiP (1:10,000), an ER marker (Denecke et al., 1991). C, Comparison of CRT in ER-enriched fraction 2 from gradient-fraction of wild-type and CRT overproducing cell line 7 (Nt CRT:7). Gelcode-stained gel is on the left and immunostained western blot is on the right. Lane 1, Heat-shocked wild type; Lane 2, heat-shocked CRT sense line 7 (Nt CRT:7). Migrations of standards and CRT are indicated.
To investigate the effects of altered CRT levels on ER Ca2+ fluxes in vitro, microsomes were isolated from wild-type, CRT transgenic cell lines, and mgfp5 transgenic cell lines and were fractionated by discontinuous Suc gradients (45%, 38%, and 22% [w/v] Suc) to isolate ER-enriched fractions. One-milliliter fractions were collected from the Suc gradients and equal amounts of protein per fraction were analyzed by SDS-PAGE, blotted, and immunostained with polyclonal antibodies against either CRT or BiP, an ER-localized chaperone. CRT showed a similar fractionation pattern as BiP (Fig. 1A and B). The highest amounts of both proteins were recovered at approximately 40% (w/v) Suc (fraction 2). This fraction was designated the ER-enriched fraction. The fractionated microsomes from all the above-mentioned cell lines showed a similar distribution of BiP and CRT based on western-blot analysis (data not shown). Our results are consistent with other studies in which the ER was recovered in the range of 30% to 40% (w/v) Suc using similar isolation conditions (Ahmed et al., 1997).
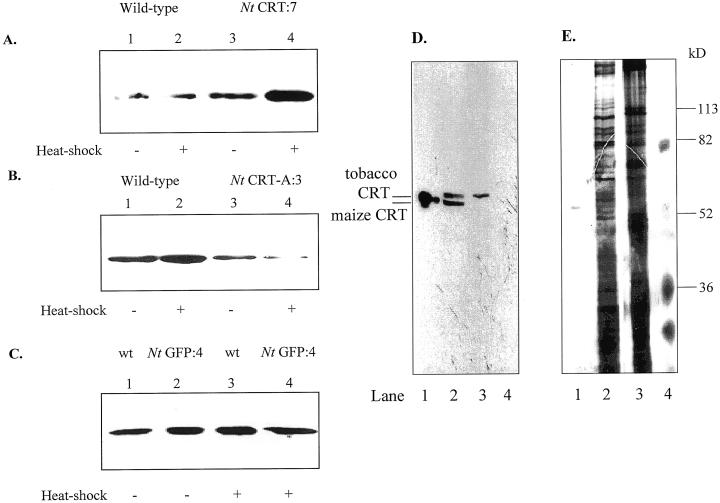
The levels of CRT for both the wild-type and the transgenic cell lines, containing the maize CRT gene in sense (Nt CRT:7), antisense (Nt CRT-A:3), or mgfp5 gene (Nt GFP:4), were investigated by immunoblot analysis of ER-enriched membrane fractions. Typical expression patterns are shown in Figure 2. In three individual experiments, the overexpressing line (Nt CRT:7) showed a 2- to 3-fold increase and the antisense line (Nt CRT-A:3) a 1.5- to 2-fold decrease in CRT expression levels following heat shock. The fold increase of CRT production in the Nt CRT:7 cell line is consistent with the fold increase observed in mammalian CRT-overproducing cells (Bastianutto et al., 1995; Mery et al., 1996). The induced Nt GFP:4 transgenic line and the wild-type line both showed similar levels of CRT (Fig. 2C).
Figure 2.
Altered CRT expression in ER-enriched vesicles from transgenic cell lines. ER-enriched vesicles from CRT, GFP transgenic, and wild-type cell lines were separated on Suc gradients. Fraction 2, containing ER-enriched membrane vesicles (Fig. 1), was analyzed by 10% (w/v) SDS-PAGE (15 μg protein lane−1), blotted, and immunostained with polyclonal antibodies against maize CRT (1:5,000). A, Increased production of CRT in CRT sense lines. Lane 1, Non-heat-shocked wild type. Lane 2, Heat-shocked wild type. Lane 3, Non-heat-shocked CRT sense line (Nt CRT:7). Lane 4, Heat-shocked CRT sense line (Nt CRT:7). B, Lowered production of CRT in CRT antisense lines. Lane 1, Non-heat-shocked wild type. Lane 2, Heat-shocked wild type. Lane 3, Non-heat-shocked antisense CRT (Nt CRT-A:3). Lane 4, Heat-shocked antisense CRT (Nt CRT-A:3). C, No significant changes in CRT production in mgfp5 transgenic cell lines (Nt GFP:4). Lane 1, Non-heat-shocked wild type. Lane 2, Non-heat-shocked Nt GFP:4 transgenic line. Lane 3, Heat-shocked wild type. Lane 4, Heat-shocked Nt GFP:4 transgenic line. D, Expression of a maize CRT in the transformed line Nt CRT:7. Lane 1, Purified maize CRT. Lane 2, Heat-shocked Nt CRT:7. Lane 3, Non-heat-shocked wild type. Lane 4, Standard. E, Protein visualized with silver staining. Lane 1, Purified maize CRT. Lane 2, Heat-shocked Nt CRT:7. Lane 3, Non-heat-shocked wild type. Lane 4, Standard.
To ensure that the maize CRT transgene was being expressed, ER-enriched fractions from wild type and Nt CRT:7 were analyzed by a 10% (w/v) SDS-PAGE large-gel (20 cm) system, blotted, and probed with polyclonal antibodies against a castor bean CRT (1:10,000) (Fig. 2D). As can be seen from Figure 2D, the maize CRT is expressed in the heat-shocked Nt CRT:7 line, and the maize CRT and tobacco CRT could be resolved using a 10% (w/v) SDS-PAGE 20-cm gel. One of the two bands detected in the Nt CRT:7 line correlated with a purified maize CRT (lane 1), and one band correlated with the band that was recognized by the antibody in the wild-type line (lane 3). Our normal minigel system did not resolve maize and tobacco CRT, and therefore was used to monitor changes in total CRT. For each experiment, CRT production was monitored in one aliquot of the ER-enriched fractions to ensure that expression levels were within ranges indicated above for each transgenic cell line.
Alterations of CRT Levels Affect the Ca2+ Uptake Capacity in Vitro
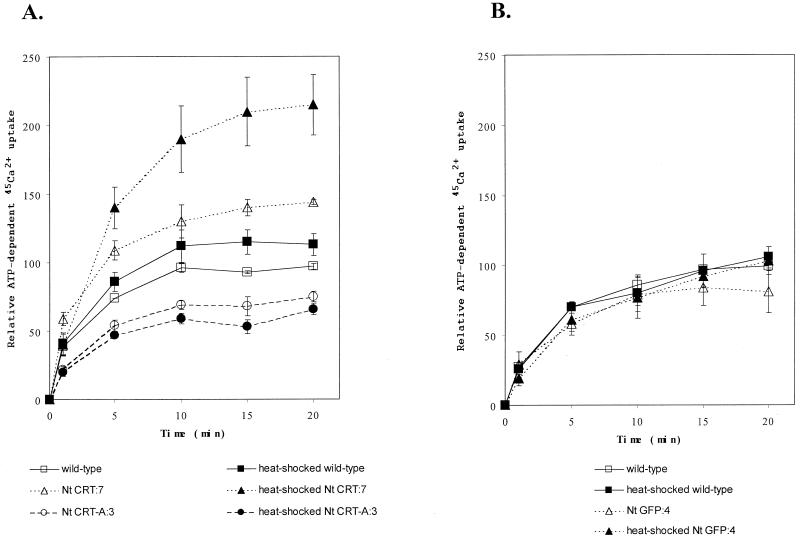
To investigate the effects of altered CRT levels on ATP-dependent Ca2+ uptake, ER-enriched vesicles were assayed for 45Ca2+ uptake in the presence and absence of 3 mm ATP (Table I; Fig. 3). After 20 min of ATP-dependent 45Ca2+ uptake, the ER-enriched membrane fractions from the heat-shocked Nt CRT:7 (sense) line had accumulated 22.2 ± 2.2 nmol 45Ca2+ mg−1 protein, whereas the heat-shocked wild-type line had accumulated 11.1 ± 0.7 nmol 45Ca2+ mg−1 protein (Table I). The ATP-dependent 45Ca2+ uptake for the non-heat-shocked wild-type line was 10.0 ± 0.2 nmol 45Ca2+ mg−1 protein. These values are comparable to values obtained when the ER and plasma membrane Ca2+-ATPases from carrot suspension culture cells were characterized (Hwang et al., 1997). ER-enriched membrane vesicles from the CRT-overproducing line showed a 2.0 ± 0.3-fold increase (mean ± sd of duplicate values from three experiments) in ATP-dependent Ca2+ uptake after about 20 min of incubation compared with the heat-shocked wild-type line (Fig. 3A). In addition, the ER from the antisense line showed a 40% decrease in Ca2+ uptake at 20 min when compared with the heat-shocked wild-type cell line. These values showed a positive correlation in ATP-dependent Ca2+ uptake and relative levels of CRT (compare Figs. 2 and 3).
Table I.
An increase in CRT levels increases ATP-dependent 45Ca2+ accumulation of ER-enriched membranes in vitro
| Cell Type | Recovered 45Ca2+ |
|---|---|
| nmol 45Ca2+ mg protein−1 | |
| Wild type | 10.0 ± 0.2 |
| Nt CRT:7 | 14.1 ± 0.2 |
| Heat-shocked wild type | 11.1 ± 0.7 |
| Heat-shocked Nt CRT:7 | 22.2 ± 2.2 |
ER-enriched membranes vesicles (fraction 2, Fig. 1), were obtained from heat-shocked and non-heat-shocked CRT transgenic and wild-type cell lines as indicated in “Materials and Methods.” ATP-dependent 45Ca2+ uptake was performed on the ER-enriched vesicles and terminated after a 20-min incubation. The ATP-dependent 45Ca2+ uptake was measured in the presence and absence of 3 mM ATP, and is shown as the amount accumulated of 45Ca2+ per milligram protein after subtraction of control values (absence of ATP). Data (mean of two values ± range) are shown from one experiment. The experiment has been repeated at least three times with consistent results.
Figure 3.
Increased levels of CRT increase Ca2+ uptake capacity in vitro. ER-enriched membrane vesicles (fraction 2, Fig. 1), were obtained from CRT transgenic, Nt GFP:4 transgenic, and wild-type cell lines as indicated in “Materials and Methods.” ATP-dependent 45Ca2+ uptake was performed on the ER-enriched vesicles (see “Materials and Methods”). The ATP-dependent Ca2+ uptake was measured in presence and absence of 3 mm ATP, and is shown as Δ ATP. Black symbols denote heat-shocked cells and white symbols denote non-heat-shocked cells. A, ER-enriched membrane vesicles from CRT overproducing (triangles), wild-type (squares), and CRT antisense (circle) cell lines were assayed for ATP-dependent 45Ca2+ uptake (10 μg protein aliquot−1 see “Materials and Methods”). The 45Ca2+ recovered was 11.1 ± 0.7 nmol 45Ca2+ mg protein−1 at 20 min for heat-shocked wild type. B, ER-enriched membrane vesicles from GFP expressing (triangles) and wild-type (squares) cell lines were assayed for ATP-dependent 45Ca2+ uptake (10 μg protein aliquot−1). Data (mean of two values ± the range) are shown from one experiment. The experiment has been repeated at least three times with consistent results. The increase in 45Ca2+ uptake in the CRT sense lines compared with heat-shocked wild type was 2.0 ± 0.3-fold (mean ± sd) for three experiments.
Because stress, e.g. heat shock, can affect the expression levels of chaperones such as CRT and BiP, it was important to compare heat-shocked with non-heat-shocked controls for each of the cell lines (Conway et al., 1995; Nguyen et al., 1996; Leborgne-Castel et al., 1999). As predicted, heat-shocked wild-type cells showed an increase in CRT compared with non-heat-shocked wild type (Fig. 2); however, the CRT increase in the wild-type cells was small relative to that in heat-shocked cells carrying the inducible CRT construct. It is important that in all instances, there was a positive correlation between CRT production and ATP-dependent Ca2+ uptake (compare Figs. 2 and 3).
When we compared CRT production in non-heat-shocked wild type and Nt CRT:7, we observed higher levels of CRT in the uninduced Nt CRT:7 cell lines (Fig. 2A) and correlating higher Ca2+ uptake (Fig. 3A), suggesting that the heat shock promoter in that cell line was leaky. To determine whether enhanced ER protein accumulation per se would alter Ca2+ transport, we analyzed ATP-dependent 45Ca2+ uptake in transgenics expressing an ER-targeted GFP. Both the heat-shocked and the non-heat-shocked ER-GFP-expressing cell line showed no significant deviations in Ca2+ uptake compared with the wild-type line (Fig. 3B). These data indicate that a general increase in a non-Ca2+-binding ER protein, such as GFP, does not affect Ca2+ uptake.
To determine whether the enhanced Ca2+ accumulation in the ER-localized CRT-overproducing line was due to vanadate-sensitive, P-type ATPase activity, Ca2+ uptake was measured either in the presence or absence of 200 μm vanadate. Vanadate has been shown to inhibit P-type ATPases and hence would inhibit the P-type ER Ca2+-ATPases (Cantley et al., 1977; O'Neal et al., 1979). In the presence of vanadate, there was no increase in 45Ca2+ uptake over time, indicating that uptake was dependent on a P-type ATPase. In the absence of vanadate, CRT overproducing lines showed a 2-fold increase in 45Ca2+ accumulation, and CRT antisense lines showed a lowered 45Ca2+ uptake compared with wild type similar to data shown in Figure 3A. The difference in 45Ca2+ uptake did not result from a variation in the specific activity of the ATPase because there was no significant difference in the specific activity of the vanadate-sensitive ATPase in wild-type and CRT-overproducing lines (data not shown).
Alterations of CRT Levels Facilitate an Increase in Ca2+ Storing Ability in Vitro
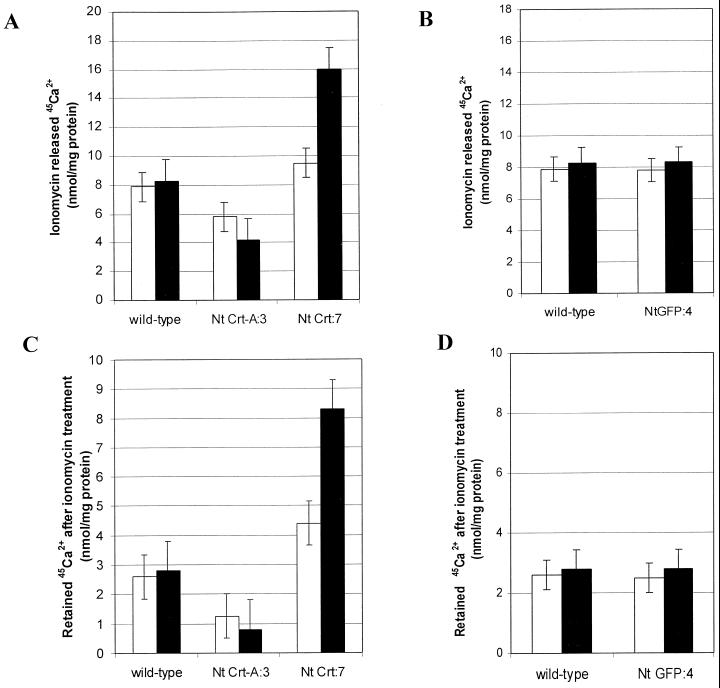
We investigated whether or not the ER-accumulated Ca2+ pool was released by a Ca2+ ionophore and, if so, how this release was affected by an alteration in ER-localized CRT. Ionomycin (1.5 μm), a Ca2+/H+ ionophore, was added to the 45Ca2+ uptake assay when equilibrium was reached (after 22 min incubation, see Fig. 3). Addition of the ionophore resulted in a decrease of the 45Ca2+ in the Ca2+-loaded vesicles (Fig. 4A). A low, stable steady-state level was attained almost immediately after addition of ionomycin (data not shown). Figure 4C shows that the CRT-overproducing cell line retained approximately 2.5 times more 45Ca2+ in the ER than the heat-shocked wild-type ER after treatment with the ionophore. Furthermore, when the Ca2+ contents from wild-type and CRT antisense cell lines were compared, the ER-enriched vesicles from wild type contained almost three times more 45Ca2+ than the CRT antisense lines (Fig. 4C). No significant difference in the rate of 45Ca2+ released between the induced and noninduced transgenic cell lines and the wild-type control line could be detected. Overproducing GFP in the ER had no significant effect on ionomycin-induced 45Ca2+ release or retention (Fig. 4B). These data indicate that the remaining 45Ca2+ is sequestered, i.e. bound, and that overproducing CRT increases a potential Ca2+ storage pool in the ER.
Figure 4.
CRT affects the amount of released and retained ER Ca2+ after treatment with ionomycin in vitro. ER-enriched membrane vesicles (fraction 2, Fig. 1), were collected from CRT transgenic, Nt GFP:4 transgenic, and wild-type cell lines. Membranes were incubated with ATP in the presence of 45Ca2+ for 22 min (see “Materials and Methods”). The Ca2+ ionophore ionomycin (1.5 μm) was added and membrane vesicles were analyzed for 45Ca2+ after 5 min. White bars, vesicles from non-heat-shocked cells; black bars, vesicles from heat-shocked cells. A, ER-enriched vesicles from CRT over- and under-producing cell lines were assayed for ATP-dependent 45Ca2+ uptake (10 μg protein aliquot−1), and compared with wild type. Data are shown as amount 45Ca2+ released after addition of ionomycin. The heat-shocked CRT sense lines showed a 2 ± 0.5-fold higher amount of released 45Ca2+ than heat-shocked wild type (8.1 ± 1.8 nmol 45Ca2+ mg protein−1 for heat-shocked wild type, and 16 ± 1.6 nmol 45Ca2+ mg protein−1 for the CRT sense line). B, ER-enriched vesicles from the Nt GFP:4 cell line were assayed for ATP-dependent 45Ca2+ uptake (10 μg protein aliquot−1), and compared with wild type. Data are shown as amount 45Ca2+ released after addition of ionomycin. C, ER-enriched vesicles from CRT over- and under-producing cell lines were assayed for ATP- dependent 45Ca2+ uptake (10 μg protein aliquot−1), and compared with wild type. Data are shown as amount 45Ca2+ associated with the ER vesicles after ionomycin treatment. The CRT sense lines had a 2.5- ± 1-fold higher amount of retained 45Ca2+ after ionomycin treatment, compared with heat-shocked wild type (2.9 ± 1.0 nmol 45Ca2+ mg protein−1 for heat-shocked wild type, and 8.2 ± 1 nmol 45Ca2+ mg protein−1 for the CRT sense line). D, ER-enriched vesicles from Nt GFP:4 cell line were assayed for ATP-dependent 45Ca2+ uptake (10 μg protein aliquot−1), and compared with wild type. Data are shown as amount 45Ca2+ associated with the ER vesicles after ionomycin treatment. Data are shown from one experiment. All experiments have been repeated at least three times with similar trends.
It has been suggested that InsP3 might trigger a Ca2+ release from the ER in plant cells (Muir and Sanders, 1997). Therefore, InsP3 was added to the ER-enriched membrane vesicles and Ca2+ release was measured. No significant 45Ca2+ release was observed after InsP3 treatment (data not shown). Either InsP3 is not an agonist for Ca2+ release from the isolated ER fraction or essential components involved in an InsP3-mediated release are absent in this in vitro study (Dasgupta et al., 1997).
Phenotypic Analysis of CRT Transgenic Arabidopsis Lines
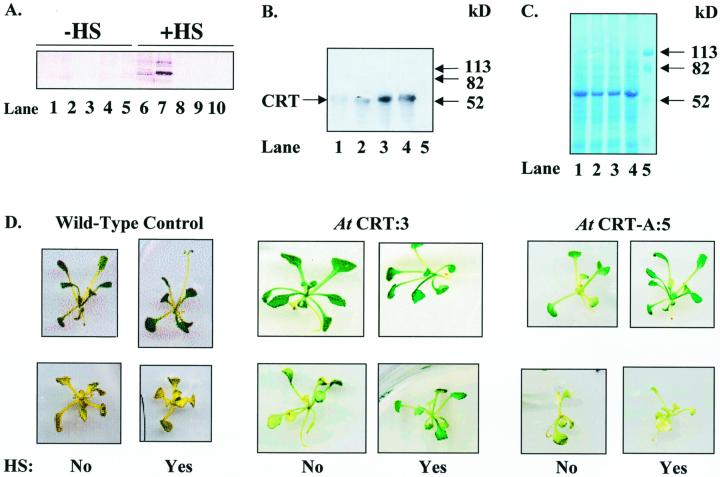
To investigate if increased production of CRT had any physiological effects in Arabidopsis plants, we generated 40 transgenic lines of Arabidopsis, 20 with the sense cDNA construct, denoted At CRT:1-20, and 20 with the antisense construct, denoted At CRT-A:1-20. All 40 T2 generation lines showed alterations in CRT expression after heat shock. Three plant lines, At CRT:3, At CRT:7, and At CRT-A:5, were selected for further study based on immunoblot analysis of CRT levels (Fig. 5A) following heat shock. To increase transgene expression and potential physiological effects, At CRT:3 seedlings were given repeated heat shock treatments. As shown in Figure 5, B and C, 2-h heat shock treatments given on each of three consecutive days resulted in the largest increase in CRT. This 2-h, 3-d heat shock regime was used on seedlings for all subsequent physiological studies. Heat shock induction of the CRT transgene throughout development was not tested because of potential secondary effects.
Figure 5.
Increased transient expression of CRT decreases chlorosis of Arabidopsis plants transferred to calcium-depleted medium. Seeds from At CRT:3 and At CRT-A:5 were germinated on nutrient medium. Sixteen days after germination, plants were incubated at 35°C for 2 h (heat shock) and allowed to recover at 21°C overnight. The heat shock procedure was repeated for 3 consecutive d. Heat-shocked plants were either placed on fresh calcium-depleted medium on d 4 or harvested, homogenized, and analyzed by 10% (w/v) SDS-PAGE (2.5 μL homogenate lane−1). Proteins were blotted and immunostained with polyclonal antibodies against CRT. A, Immunostaining of CRT in homogenized transgenic plant lines with polyclonal antibodies against maize CRT (1:5,000). Lanes 1 through 5, Non-heat-shocked plants; Lanes 6 through 10, heat-shocked plants. Lanes 1 and 6, At CRT:7; lanes 2 and 7, At CRT:3; lanes 3 and 8, At CRT-A:3; lanes 4 and 9, At CRT-A:4; lanes 5 and 10, At CRT-A:5. B, Immunostaining of CRT in homogenized At CRT:3 with polyclonal antibodies against castor bean CRT (1:10,000). Lane 1, Non-heat-shocked At CRT:3. Lane 2, At CRT:3 heat shocked 1 d. Lane 3, At CRT:3 heat-shocked 2 d. Lane 4, At CRT:3 heat-shocked 3 d. Lane 5, Standard. C, Total protein visualized with Gelcode staining. Lanes as indicated in B. D, Upper, photographs show non-heat-shocked and heat-shocked wild type (left), At CRT:3 (center), and At CRT-A:5 (right) plants 16 h after induction; lower, photographs show a non-heat-shocked and heat-shocked wild type (left), At CRT:3 (center), and At CRT-A:5 (right) plants transferred after induction to calcium-depleted medium (Arabidopsis [AT] medium containing 10 mm EGTA) for 9 d. Twelve seedlings for each transgenic line and medium treatment were germinated, one-half were induced for transgene expression, and one-half were maintained as non-induced controls. (Note: the wild-type plants shown were from a separate experiment.) The experiment has been repeated three times with similar results. In subsequent experiments, 20 to 50 seedlings for each line were assessed. In all experiments, a minimum of 80% of the plants showed the phenotypes represented.
Plants carrying the CRT transgene but not exposed to the inductive heat shock displayed no obvious phenotypic differences in appearance, growth rate, time to flower, or seed production when grown to maturity in soil (data not shown). There also were no apparent phenotypic differences when seeds from the CRT transgenic line At CRT:3 were germinated on AT medium, which contains 2 mm calcium, and heat treated to induce transgene expression. However, when heat shock-induced transgenic seedlings were transferred to calcium-depleted medium (AT medium containing 10 mm EGTA), phenotypic variations appeared (Fig. 5D). By d 9 after transfer to calcium-depleted medium, the heat-shocked At CRT:3 plant line showed less chlorosis when compared with the non-heat-shocked At CRT:3. In addition, the heat-shocked At CRT-A:5 showed severe chlorosis on calcium-depleted medium (Fig. 5D). In similarly treated sibling plants, there was an inverse correlation between CRT levels and the extent of chlorosis after growth on calcium-depleted medium (compare CRT levels and chlorosis in Fig. 5). Greater than 80% of the heat-shocked At CRT:3 plants remained green a minimum of 5 and 8 d longer than the noninduced At CRT:3 plants and the heat-shocked At CRT-A:5, respectively. To determine whether the observed phenotype on the calcium-depleted medium was reversible, we transferred plants to calcium-depleted medium containing an additional 12 mm Ca2+. Chlorotic tissue did not regreen, but all lines except the heat shock-induced At CRT-A:5 recovered and grew new leaves when the medium was supplemented with Ca2+ (data not shown).
DISCUSSION
We altered the expression levels of CRT and investigated its potential role in ER Ca2+ homeostasis. We have shown that a 2.5-fold increase in ER CRT levels led to a 2-fold enhancement of ATP-dependent Ca2+ accumulation in isolated ER vesicles. These observations are consistent with earlier studies in mammalian systems where an increase in CRT expression resulted in an increased accumulation of cellular Ca2+ in vivo (Bastianutto et al., 1995; Mery et al., 1996). In all instances, the altered ER Ca2+ accumulation correlated positively with ER-associated CRT levels and was shown to be ATP dependent.
A CRT-mediated alteration of the ER Ca2+ pool could potentially make Ca2+ more readily accessible for release into the cytosol. CRT has been suggested to be associated with agonist-triggered Ca2+ channels and might be important for regulating ER-derived Ca2+ signals (Michalak et al., 1998; Corbett and Michalak, 2000). Alterations in CRT levels thus might affect both the Ca2+-holding capacity in the ER and be involved in the regulation of Ca2+ release. To investigate the buffering capacity in the CRT-overproducing lines, 45Ca2+-loaded ER vesicles were treated with ionomycin, an ionophore that will allow passive transport of free and/or loosely bound Ca2+ across membrane vesicles. The amount of Ca2+ released by ionomycin from the ER of the CRT-overproducing lines was higher than both the wild-type and antisense lines. In addition, CRT- over-producing lines showed higher levels of retained Ca2+ after ionomycin treatment. Although the Ca2+ released when calculated as percentage of the total Ca2+ taken up is similar for the wild-type and the CRT-overproducing line, the 2-fold increase in the total amount of 45Ca2+ released and retained in CRT overexpressors indicates that overproduction of CRT would lead to an enhanced Ca2+-buffering capacity in the ER and thereby could affect the availability of Ca2+ for an ER Ca2+-signaling pool.
In Xenopus laevis oocytes, CRT interacts with a SERCA 2b isoform, an ER/SR Ca2+-ATPase and decreases its activity (Camacho and Lechleiter, 1995; John et al., 1998). Overexpression of both CRT and SERCA 2b resulted in an inhibition of repetitive InsP3-induced Ca2+ oscillations in the oocytes. The SERCA 2b isoform contains a glycosylation site, which was shown to be essential for an interaction between the ATPase and CRT. Two Ca2+-ATPases have been reported in plant ER, one similar to the classical ER-type pump and one that is activated by calmodulin (Liang et al., 1997; Hwang et al., 2000). Although we cannot rule out a possible regulation of ER Ca2+-ATPases by CRT, no obvious glycosylation sites were present in the sequence for Eca1, an ER Ca2+-ATPase from Arabidopsis (Liang et al., 1997), and no significant decrease in ATPase activity was detected when CRT overproducing lines were compared with wild-type controls.
In addition to CRT, other ER-localized chaperones, such as BiP, have been shown to affect Ca2+ homeostasis. Lièvremont et al. (1997) showed that an overexpression of BiP in HeLa cells resulted in an increased InsP3-sensitive Ca2+ pool. Both BiP and CRT are induced by stress, e.g. heat shock. In this study we observed that in wild-type cells both BiP and CRT increased in response to heat shock [1.5-fold (data not shown) and 1.2-fold, respectively (Fig. 2). BiP has been shown to bind 1 to 2 mol Ca2+ mol protein−1 and to account for about 25% of the total ER Ca2+ in HeLa cells (Lièvremont et al., 1997). Because CRT binds at least 25 mol Ca2+ mol protein−1 and is the primary Ca2+-binding protein in 45Ca2+ ligand overlay studies in peas (Hassan et al., 1995), it is unlikely that BiP would make a major contribution to the total ER Ca2+ in this study. Furthermore, the increase in a non-Ca2+-binding ER protein, such as GFP, did not affect the ER Ca2+ uptake or the ER-buffering capacity (Fig. 3 and 4).
It has been proposed that plants use different Ca2+ stores and signaling patterns in response to different signals (Knight et al., 1996, 1997; Sanders et al., 1999; Trewavas, 1999). Variations in location, amplitude, and frequency thereby can cause opposing responses, as in the closure and opening of stomata in response to abscisic acid and auxin-induced Ca2+ signals (Irving et al., 1992; Allen et al., 1999, 2000; Staxén et al., 1999; McAinsh et al., 2000). If the ER Ca2+ is important in plant signaling, then an increased level of CRT could affect the ER-Ca2+ signaling pool and thereby regulate Ca2+ homeostasis.
To investigate whether transient overproduction of CRT in the ER has any physiological effect in planta, CRT transformed Arabidopsis plants were grown on Ca2+-containing medium, induced for CRT transgene expression and subsequently transferred to Ca2+-depleted medium. Inducible overproduction of CRT delayed chlorosis on Ca2+-depleted medium, and further strengthening the notion that the increased Ca2+-buffering capacity generated by an overproduction of CRT was available for the cell to help maintain its Ca2+ homeostasis. In support of these data, we recently showed that expression of a heat shock-inducible ER-targeted CRT C domain in Arabidopsis enhanced survival on calcium-depleted medium (Wyatt et al., 2001). The CRT C-domain plants contained significantly higher levels of total Ca2+ (approximately 10%) per gram dry weight. Increasing intracellular Ca2+ levels alone, however, is not the key to withstanding lower levels of extracellular Ca2+ (Hirschi, 2001). For example, Hirschi (1999) showed that an overexpression of the vacuolar H+/Ca2+ antiporter (CAX1) in tobacco resulted in a 2-fold increase in Ca2+ per dry weight as compared with wild-type plants. In spite of the increased total Ca2+, the CAX1-overproducing plants, unlike the CRT-overproducing plants were more sensitive to lowered external Ca2+ levels than the wild-type plants.
In summary, the data reported here strongly suggest that ER Ca2+ is involved in maintaining Ca2+ homeostasis in the plant cell and that CRT has a key role as an ER Ca2+-sequestering protein in plants. The challenge is now to determine the various roles for different Ca2+ stores and to understand how the orchestration of intracellular Ca2+ signals is affected when levels and activities of Ca2+ storage and transporting proteins are altered.
MATERIALS AND METHODS
Plasmid Constructs
A full-length CRT cDNA was isolated from a maize (Zea mays) endosperm library (R.L. Wrobel and R.S. Boston, unpublished data). The cDNA was sequenced (GenBank accession no. AF190454), and was used in all CRT-derived constructs. The constructs used for transformation consist of a binary plasmid vector containing an Arabidopsis heat shock promoter (AtHSP), followed by the CRT gene in either sense (pBIN2101) or antisense (pBIN210A) orientation, or the GFP (pBIN2011). For these plasmids, the AtHSP was inserted into pUCAP (van Engelen et al., 1995) to generate a plasmid containing the AtHSP, a multiple cloning site, and the ocs terminator. The resulting plasmid, labeled pWY2000, was digested with XbaI. A 1,365-bp fragment containing the CRT coding sequence and 40 bp of DNA upstream of the start codon was isolated using XbaI, purified, and ligated into the pWY2000, creating both a sense, pWY2101, and antisense, pWY210A, CRT construct under control of the AtHS promoter. The DNA fragments consisting of the AtHSP-CRT-ocs sequence and the AtHSP-antisense CRT-ocs sequence were ligated into the AscI and PacI sites of the binary plasmid vector pBINPLUS (van Engelen et al., 1995), which contains a plant kanamycin resistance cassette. The resulting plasmids were labeled pBIN2101 and pBIN210A, respectively.
To serve as a control, an AtHSP-mgfp5 construct was designed. The mgfp5 gene encodes an ER-localized GFP (Haseloff et al., 1997). The 35S promoter was removed from plasmid pWY1011 (Scott et al., 1999), containing the 35S promoter-driving mgfp5, and the ocs terminator, by digestion with HindIII and BamHI. The promoter was replaced with the AtHSP that had been similarly removed from plasmid pWY2000. The DNA fragment consisting of the AtHSP-mgfp5-ocs sequence was then ligated into the AscI and PacI sites of the binary plasmid vector to produce pBIN2011.
Tobacco (Nicotiana tabacum) Tissue Cultures
Tobacco cell cultures (NT1 cells, An, 1985) were maintained in 50 mL of liquid culture medium (1× Murashige and Skoog salts; Gibco BRL, Bethesda, MD), 0.18 g L−1 KH2PO4, 0.1 g L−1 myo-inositol, 1 mg thiamine HCl, 0.2 mg L−1 2,4-D, and 30 g L−1 Suc, pH 5.7) at 27°C with gyratory shaking at 125 rpm, in darkness. Cells were subcultured weekly with a 6% (v/v) inoculum. Care was taken to use cell lines with similar growth rates (25–30 g, 50-mL flask were recovered after initial centrifugation of cells).
Culture Transformation and Selection
NT1 cells were transformed using Agrobacterium tumefaciens-mediated gene transfer (An, 1985). pBIN2101, pBIN210A, and pBIN2011 were electroporated into A. tumefaciens, strain LBA-4404, using a Gene Pulser system (Bio-Rad, Hercules, CA). A single transformant A. tumefaciens colony for each plasmid was cultured in 5 mL yeast extract broth (0.5% [w/v] beef extract, 0.5% [w/v] peptone, 0.5% [w/v] Suc, 0.1% [w/v] yeast extract, and 0.05% [w/v] MgCl2) containing 50 mg L−1 kanamycin, at 27°C and 250 rpm for 2 d. Wild-type NT1 were grown to logarithmic growth phase (Allen et al., 1993) in 100 mL NT1 culture medium for 4 d at 125 rpm. Four milliliters of this culture was gently mixed with 200 μL of 2-d A. tumefaciens cultures transformed with pBIN2101, pBIN210A, or pBIN2011. The NT1 cell-A. tumefaciens mix was incubated for 48 h at 27°C and suspended in an equal volume of NT1 culture medium. Approximately 0.5 mL of the resulting cell suspension was plated onto NT1 culture medium-0.8% (w/v) phytagar (Gibco BRL) containing 50 μg mL−1 kanamycin and 200 μg mL−1 timetin. Plates were incubated for 14 d at 27°C. For each transformation, at least 40 independent, transgenic microcalli were picked, each microcalli was suspended in 1 mL NT1 medium containing 50 μg mL−1 kanamycin and 200 μg mL−1 timetin, and incubated for 7 d at 27°C, at 190 rpm in darkness.
For each transformation, 12 of these cell lines were selected and screened for heat shock-inducible transgene expression. Each of the 12 cell lines showed heat shock-inducible transgene expression based on western-blot analysis. Three cell lines transformed with pBIN2101, one cell line from pBIN210-A, and one cell line from pBIN2011 were maintained for subsequent experiments. The selected cell lines were transferred to 4 mL NT1 culture medium containing 50 μg mL−1 kanamycin and 200 μg mL−1 timetin, and incubated for 7 d as described above. Three replicate cultures were established for each independent, transgenic NT1 line by subculture of 0.5 mL of cell suspension into 5 mL of NT1 culture medium containing 50 μg mL−1 kanamycin. One of these replicates was cultured for 7 d and subcultured as described above to maintain the line (Allen et al., 1993). The other two replicates were cultured for 4 d, to logarithmic growth phase. One replicate set was incubated at 35°C for 2 h with shaking at 190 rpm and then returned to 27°C for 12 h. The other replicate set was not heat shocked.
Cells from putative CRT transgenic lines were purified by filtration and frozen in liquid N2. One-hundred milligrams of frozen cells was ground in 100 μL 2 × SDS-PAGE sample buffer, heated to 100°C for 3 min, and centrifuged for 1 min at 13,000 rpm. Proteins in these crude lysates were separated by SDS-PAGE, electroblotted, and probed with antibodies that recognize CRT. Cultures that showed high variation in CRT signal compared with the non-heat-shocked control were retained for further analysis. Cell lines from putative mgfp5 transgenic lines were screened for ER-targeted fluorescence under the microscope, using an excitation wavelength of 488 nm, and emission wavelength recording at 500 to 550 nm. Cells that showed GFP fluorescence were retained. Transgenic cell lines expressing pBIN2101, pBIN210A, and pBIN2011 were labeled Nt CRT, Nt CRT-A, and Nt GFP, respectively. Transgenic cell cultures were transferred to 5 mL NT1 medium containing 50 μg mL−1 kanamycin and incubated for 7 d at 27°C, at 190 rpm. Stock cultures were maintained in this manner by weekly subculture of 0.3 mL into 5 mL NT1 medium with 50 μg mL−1 kanamycin.
Plant Transformation and Selection
Arabidopsis plants were also transformed using A. tumefaciens-mediated gene transfer. Binary vectors pBIN2101, pBIN210A, and pBIN2011 were electroporated into A. tumefaciens, strain GV3101, using an electroporator according to the manufacture's instructions (Bio-Rad). Wild-type Arabidopsis var. Columbia plants (generation T0) were then transformed by vacuum infiltration as described (Bechtold and Pelletier, 1998). Seeds from these plants, labeled generation T1, were sterilized for 30 min in 30% (v/v) commercial bleach and plated onto AT medium {4.3 g L−1 Murashige and Skoog salts [Gibco BRL]; 1× B5 vitamins; 2% [w/v] Suc; 0.05% [w/v] MES [2-(N-morpholino)-ethanesulfonic acid], pH 5.8; 1% [w/v] phytagar [Gibco BRL]} containing 30 mg L−1 kanamycin. Plants were grown for 2 weeks at 21°C in constant light. Kanamycin-resistant plants were transferred to soil and cultivated at 21°C, under an 8-h-light/16-h-dark photoperiod. Plants were then transferred to a 16-h-light/8-h-dark photoperiod, allowed to self fertilize, and the resulting seed collected (T2 generation). T1 lines whose progeny segregated 3:1 for kanamycin resistance, as would be expected for a single locus event, were selected for further analysis. T2 plants from these lines were germinated on kanamycin-containing medium at 35°C for 2 h for induction of the HS promoter and then allowed to recover overnight at 21°C. Leaf samples were taken from all plants, weighed, frozen in liquid N2, and stored at −80°C for analysis of protein expression.
Preparation of ER-Enriched Microsomal Membrane Fraction
NT1 cells were harvested by centrifugation at 1,000g for 10 min at 4°C. All subsequent operations were performed at 4°C and/or on ice. Four grams of cells were homogenized in 5 mL homogenization buffer {200 mm Suc; 25 mm HEPES [4-(2-hydroxyethyl)]-1-piperazineethanesulfonic acid-KOH, pH 7.0; 3 mm EGTA; 1 mm MgSO4; 1 mm phenylmethylsulfonyl fluoride [PMSF]; and 1 mm dithiothreitol [DTT]} using a tightly fitting glass/glass tissue grinder. The homogenate was centrifuged at 1,000g for 10 min and the supernatant was centrifuged at 7,000g for 20 min. The 7,000g supernatant was loaded on a discontinuous Suc gradient and centrifuged at 100,000g for 2 h. The Suc gradient consisted of 2 mL each of 22%, 38%, and 45% (w/v) Suc in 25 mm HEPES-KOH (pH 7.0), 1 mm DTT, and 1 mm PMSF. Fractions (1.0 mL) were collected. Equal amounts of protein were analyzed on SDS-PAGE and western blots. The ER-enriched membrane fraction (fraction 2, based on western-blot analysis) was diluted with 5 mL of dilution buffer containing 200 mm Suc, 25 mm HEPES-KOH (pH 7.0), 1 mm DTT, and 1 mm PMSF and centrifuged at 40,000g for 90 min. The pellet was resuspended in dilution buffer to a final concentration of 1 μg protein μL−1.
Protein Determination
Protein concentrations was determined by the Bradford method (Bio-Rad protein assay) following the manufacturer's protocol. Bovine serum albumin was used as a standard.
45Ca2+ Uptake and Release
Ca2+ uptake and release were performed at 22°C and measured with 45CaCl2 as described by Hsieh et al. (1991) with some modifications. Transport was measured in an ATPase assay buffer containing 200 mm Suc, 25 mm HEPES-KOH (pH 7.0), 10 mm KCl, 100 μm 45CaCl2 (2 μCi mL−1), 100 μm EGTA (yielding a final approximate Ca2+ concentration of 0.5 μm; Bers et al., 1994), 3 mm MgSO4, 1 mm DTT, and 3 mm ATP. ATP-dependent 45Ca2+ transport into the ER microsome lumen was initiated by addition of 100 μL of the membrane fraction (100 μg protein) in a final volume of 1 mL. Aliquots (100 μL) from duplicate reactions were removed, filtered, and washed with 200 mm Suc, 25 mm HEPES-KOH (pH 7.0), 10 mm KCl, and 100 μm CaCl2 using a single millipore filter set. The 45Ca2+ retained on the filter was determined by liquid scintillation counting. Net active transport was determined as the difference in activity in presence and absence of ATP. Ca2+ release was triggered from 45Ca2+-loaded (22 min) vesicles by addition of the ionophore ionomycin (final concentration of 1.5 μm). Ionomycin was taken from a 660-μm stock solution in dimethyl sulfoxide. The net release was determined as the difference of 45Ca2+ recovered after the addition of ionomycin versus addition of dimethyl sulfoxide alone. Results shown were obtained from at least two replicate experiments run in parallel with a wild-type control from three individual Nt CRT-Sense lines, one Nt CRT-antisense line and, as a control, one Nt GFP line.
ATPase Assay
ATPase activity was measured at 22°C. Ten microliters of membrane (10 μg) was assayed in a buffer containing 200 mm Suc, 25 mm HEPES-KOH (pH 7.0), 10 mm KCl, 3 mm MgSO4, 3 mm ATP, 1 mm DTT, 100 μm CaCl2, and 100 μm EGTA (yielding a final approximate Ca2+ concentration of 0.5 μm; Bers et al., 1994) in a final volume of 200 μL. The reaction was incubated for 30 min and stopped by the addition of 2 mL NH4/FeSO4 reagent containing 1% (w/v) NH4Mo, 1 n H2SO4, and 0.18 m FeSO4-H2O. A660 was read and the generated inorganic phosphate was determined as the difference in activity in the presence or absence of 200 μm vanadate. KH2 PO4 (0–0.1 μmol) was used as a standard. Inorganic free phosphate was measured according to Taussky and Schorr (1953).
Phenotypic Analysis of Arabidopsis Lines
Seeds of each line T3 were germinated on normal AT growth medium (4.3 g L−1 Murashige and Skoog salts, Gibco BRL; 1× B5 vitamins, 2% [w/v] Suc, 0.05% [w/v] MES, pH 5.8, and 1% [w/v] phytagar, Gibco BRL). On d 16, 17, and 18 after germination, the plants were incubated at 35°C for 2 h each day to induce expression of the transgene. On d 19, Parafilm was removed from the plates for 6 h, and the plates opened in a laminar flow hood for 30 min to increase transpiration and calcium uptake. The plants were then transferred to fresh AT growth medium, calcium-depleted medium (AT growth medium with 10 mm EGTA), or calcium-depleted medium with 12 mm CaCl2. In the initial experiments, 12 seedlings for each transgenic line and medium treatment were germinated, one-half were induced for transgene expression, and half were maintained as noninduced controls. Images of these plants were taken at d 2, 4, 6, 9, and 12 d after transfer. In subsequent experiments, only At CRT:3 and AtCRT-A:5 were used and 20 to 50 seedlings for each line were assessed.
SDS-PAGE and Immunoblotting
Protein was solubilized by addition of an equal volume of sample buffer (125 mm Tris-HCl [pH 6.8], 4% [w/v] SDS, 20% [v/v] glycerol, 10% [w/v] β-mercaptoethanol, and 0.02% [w/v] bromphenol blue). Equal amounts of solubilized proteins were separated on a 10% (w/v) SDS-polyacrylamide gel at pH 8.8. For Arabidopsis plants, 20- to 50-mg samples of young leaves were collected from each plant, weighed, and frozen in liquid nitrogen. Leaves were ground in sample buffer using 1 μL of buffer to 1 mg of plant material and equal amounts of plant tissue were loaded on a 10% (w/v) SDS-polyacrylamide gel at pH 8.8. Gels were either stained with Gelcode Blue Stain protocol (Pierce, Rockford, IL) or transferred to a hydrophobic polyvinylidene difluoride membrane (Gelman Sciences, Ann Arbor, MI). Proteins were wet blotted for 1 h at 100 V. After transfer, the polyvinylidene difluoride membranes were blocked with 1% (w/v) blocking reagent (Roche Biochemical, Indianapolis) in Tris-buffered saline with 0.2% (v/v) Tween 20 for 1 h at 22°C. The membranes were incubated with either an antiserum against CRT from maize diluted 1:5,000, an antiserum against CRT from castor bean diluted 1:10,000, or an antiserum against BiP from maize diluted 1:10,000. Antibodies were visualized with a chemiluminescent detection of horseradish peroxidase according to the Supersignal West Pico blotting protocol (Pierce), and microdensiometry of bands was analyzed using Imagequant software.
ACKNOWLEDGMENTS
The maize CRT cDNA was kindly provided by Dr. Rebecca S. Boston (North Carolina State University). Antisera against maize CRT and maize BiP were generously provided by Dr. Brian Larkins (University of Arizona, Tucson) and by Dr. Rebecca S. Boston, respectively. The pHSP5′ construct was kindly provided by Dr. Chi-Lien Cheng (University of Iowa, Iowa City). The purified maize CRT was kindly provided by Dr. Jeff Gillikan (North Carolina State University) and Dr. Rebecca S. Boston.
Footnotes
This work was supported in part by the North Carolina State University-National Aeronautics and Space Administration Specialized Center of Research and Training (grant no. NAGW–4984) and in part by funding from the North Carolina Agricultural Research Service (to W.F.T., D.R., and W.F.B.).
LITERATURE CITED
- Ahmed SU, Bar-Peled M, Raikhel NV. Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein-sorting receptors of eukaryotic cells. Plant Physiol. 1997;114:325–336. doi: 10.1104/pp.114.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science. 2000;289:2338–2342. doi: 10.1126/science.289.5488.2338. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 1999;19:735–747. doi: 10.1046/j.1365-313x.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- Allen GS, Hall GE, Childs LC, Weissinger AK, Thompson WF. Scaffold attachment regions increase reporter gene expression in stably transformed plant cells. Plant Cell. 1993;5:603–613. doi: 10.1105/tpc.5.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G. High efficiency transformation of cultured tobacco cells. Plant Physiol. 1985;79:568–570. doi: 10.1104/pp.79.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianutto C, Clementi E, Codazzi F, Podini P, De Giorgi F, Rizzuto R, Meldolesi J, Pozzan T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J Cell Biol. 1995;130:847–855. doi: 10.1083/jcb.130.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N, Pelletier G. In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol. 1998;82:259–266. doi: 10.1385/0-89603-391-0:259. [DOI] [PubMed] [Google Scholar]
- Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 1994;40:3–29. doi: 10.1016/s0091-679x(08)61108-5. [DOI] [PubMed] [Google Scholar]
- Burns K, Duggan B, Atkinson EA, Famulski KS, Nemer M, Bleackley RC, Michalak M. Modulation of gene expression by calreticulin binding to the glucocorticoid receptor. Nature. 1994;367:476–480. doi: 10.1038/367476a0. [DOI] [PubMed] [Google Scholar]
- Bush D. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1993;46:95–122. [Google Scholar]
- Bush D. Regulation of cytosolic calcium in plants. Plant Physiol. 1995;103:7–13. doi: 10.1104/pp.103.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho P, Lechleiter JD. Calreticulin inhibits repetitive intracellular Ca2+ waves. Cell. 1995;82:765–771. doi: 10.1016/0092-8674(95)90473-5. [DOI] [PubMed] [Google Scholar]
- Cantley LC, Jr, Josephson L, Warner R, Yanagisawa M, Lechene C, Guidotti G. Vanadate is a potent (Na, K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem. 1977;252:7421–7423. [PubMed] [Google Scholar]
- Cessna SG, Low PS. An apoplastic Ca2+ sensor regulates internal Ca2+ release in aequorin-transformed tobacco cells. J Biol Chem. 2001;276:10655–10662. doi: 10.1074/jbc.M006989200. [DOI] [PubMed] [Google Scholar]
- Chen F, Hayes PM, Mulrooney DM, Pan A. Identification and characterization of cDNA clones encoding plant calreticulin in barley. Plant Cell. 1994;6:835–843. doi: 10.1105/tpc.6.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway EM, Liu L, Nowakowski B, Steiner-Mosonyi M, Ribeiro SP, Michalak M. Heat shock-sensitive expression of calreticulin. J Biol Chem. 1995;270:17011–17016. doi: 10.1074/jbc.270.28.17011. [DOI] [PubMed] [Google Scholar]
- Coppolino MG, Woodside MJ, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997;386:843–847. doi: 10.1038/386843a0. [DOI] [PubMed] [Google Scholar]
- Corbett EF, Michalak M. Calcium, a signaling molecule in the endoplasmic reticulum? Trends Biochem Sci. 2000;25:307–311. doi: 10.1016/s0968-0004(00)01588-7. [DOI] [PubMed] [Google Scholar]
- Crofts AJ, Leborgne-Castel N, Hillmer S, Robinson DG, Phillipson B, Carlsson LE, Ashford DA, Denecke J. Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell. 1999;11:2233–2247. doi: 10.1105/tpc.11.11.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Dasgupta D, Chatterjee A, Biswas S, Biswas BB. Conformational changes in plant Ins(1,4,5) P3 receptor on interaction with different myo-inositol trisphosphates and its effect on Ca2+ release from microsomal fraction and liposomes. Biochem J. 1997;321:355–360. doi: 10.1042/bj3210355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S, Rennie PS, Shago M, Leung Hagesteijn C-Y, Yang H, Filmus J, Hawley RG, Bruchovsky N, Cheng H, Matusik RJ. Inhibition of nuclear hormone receptor activity by calreticulin. Nature. 1994;367:480–483. doi: 10.1038/367480a0. [DOI] [PubMed] [Google Scholar]
- Denecke J, Carlsson LE, Vidal S, Hoglund A-S, Ek B, van Zeijl MJ, Sinjorgo KMC, Palva ET. The tobacco homolog of mammalian calreticulin is present in protein complexes in vivo. Plant Cell. 1995;7:391–406. doi: 10.1105/tpc.7.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J, Goldman MH, Demolder J, Seurinck J, Botterman J. The tobacco luminal binding protein is encoded by a multigene family. Plant Cell. 1991;3:1025–1035. doi: 10.1105/tpc.3.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasolato C, Pizzo P, Pozzan T. Delayed activation of the store-operated calcium current induced by calreticulin overexpression in RBL-1 cells. Mol Biol Cell. 1998;9:1513–1522. doi: 10.1091/mbc.9.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A-M, Wesson C, Trumble WR. Calreticulin is the major Ca2+ storage protein in the endoplasmic reticulum of the pea plant (pisum sativum) Biochem Biophys Res Commun. 1995;211:54–59. doi: 10.1006/bbrc.1995.1777. [DOI] [PubMed] [Google Scholar]
- Haseloff J, Siemering K, Prasher D, Hodge S. Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA. 1997;94:2122–2127. doi: 10.1073/pnas.94.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza hemagglutinin in microsomes. EMBO J. 1996;15:2961–2968. [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD. Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell. 1999;11:2113–2122. doi: 10.1105/tpc.11.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD. Vacuolar H+/Ca2+ transport: who's directing the traffic? Trends Plant Sci. 2001;6:100–104. doi: 10.1016/s1360-1385(00)01863-x. [DOI] [PubMed] [Google Scholar]
- Hsieh WL, Pierce WS, Sze H. Calcium-pumping ATPases in vesicles from carrot cells. Stimulation by calmodulin or phophatidylserine and formation of a 120 kilodalton phosphoenzyme. Plant Physiol. 1991;97:1535–1544. doi: 10.1104/pp.97.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Harper JF, Liang F, Sze H. Calmodulin activation of an endoplasmic reticulum-located calcium pump involves an interaction with the N-terminal autoinhibitory domain. Plant Physiol. 2000;122:157–68. doi: 10.1104/pp.122.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Ratterman DM, Sze H. Distinction between endoplasmic reticulum-type and plasma membrane-type Ca2+ pumps. Plant Physiol. 1997;113:535–548. doi: 10.1104/pp.113.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving HR, Gehring CA, Parish RW. Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proc Natl Acad Sci USA. 1992;89:1790–1794. doi: 10.1073/pnas.89.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John LM, Lechleiter JD, Camacho P. Differential modulation of SERCA2 isoform by calreticulin. J Cell Biol. 1998;142:963–973. doi: 10.1083/jcb.142.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusener B, Boheim G, Liss H, Engelberth J, Weiler EW. Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J. 1995;14:2708–2714. doi: 10.1002/j.1460-2075.1995.tb07271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. Calcium signaling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997;12:1067–1078. doi: 10.1046/j.1365-313x.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- Krause K-H, Michalak M. Calreticulin. Cell. 1997;88:439–443. doi: 10.1016/s0092-8674(00)81884-x. [DOI] [PubMed] [Google Scholar]
- Leborgne-Castel N, Jelitto-Van Dooren EPWM, Crofts AJ, Denecke J. Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. Plant Cell. 1999;11:459–469. doi: 10.1105/tpc.11.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Cunningham KW, Harper JF, Sze H. ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:8579–8584. doi: 10.1073/pnas.94.16.8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lièvremont J-P, Rizzuto R, Hendershot L, Meldolesi J. BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+ J Biol Chem. 1997;272:30873–30879. doi: 10.1074/jbc.272.49.30873. [DOI] [PubMed] [Google Scholar]
- Llewelyn RH, Llewelyn DH, Campbell AK, Kendall JM. Role of calreticulin in regulating intracellular Ca2+ storage and capacitative Ca2+ entry in HeLa cells. Cell Calcium. 1998;24:253–262. doi: 10.1016/s0143-4160(98)90049-5. [DOI] [PubMed] [Google Scholar]
- Marshner H. Mineral Nutrition in Higher Plants. London: Academic Press; 1986. [Google Scholar]
- McAinsh MR, Gray JE, Hetherington AM, Leckie CP, Ng C. Ca2+ signalling in stomatal guard cells. Biochem Soc Trans. 2000;28:476–481. [PubMed] [Google Scholar]
- Mery L, Mesaeli N, Michalak M, Opas M, Lew DP, Krause K-H. Overexpression of calreticulin increases intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J Biol Chem. 1996;271:9332–9339. doi: 10.1074/jbc.271.16.9332. [DOI] [PubMed] [Google Scholar]
- Michalak M, Mariani P, Opas M. Calreticulin, a multifunctional Ca2+ binding chaperone of the endoplasmic reticulum. Cell Biol. 1998;76:779–785. doi: 10.1139/bcb-76-5-779. [DOI] [PubMed] [Google Scholar]
- Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. Biochem J. 1999;344:281–292. [PMC free article] [PubMed] [Google Scholar]
- Miseta A, Kellermayer R, Aiello DP, Fu L, Bedwell DM. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 1999;451:132–136. doi: 10.1016/s0014-5793(99)00519-0. [DOI] [PubMed] [Google Scholar]
- Muir SR, Sanders D. Inositol 1,4,5-trisphophate-sensitive Ca2+ release across non-vacuolar membranes in cauliflower. Plant Physiol. 1997;114:1511–1521. doi: 10.1104/pp.114.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Bossy-Wetzel E, Burns K, Fadel MP, Lozyk M, Goping IS, Opas M, Bleackley RC, Green DR, Michalak M. Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J Cell Biol. 2000;150:731–740. doi: 10.1083/jcb.150.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef WM, McCormick SJ, Clark RA. Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase. J Biol Chem. 1995;270:4741–4747. doi: 10.1074/jbc.270.9.4741. [DOI] [PubMed] [Google Scholar]
- Nguyen TQ, Capra DJ, Sontheimer RD. Calreticulin is transcriptionally upregulated by heat shock, calcium and heavy metals. Mol Immunol. 1996;33:379–386. doi: 10.1016/0161-5890(95)00149-2. [DOI] [PubMed] [Google Scholar]
- O'Neal SG, Rhoads DB, Racker E. Vanadate inhibition of sarcoplasmic reticulum Ca2+-ATPase and other ATPases. Biochem Biophys Res Commun. 1979;89:845–850. doi: 10.1016/0006-291x(79)91855-2. [DOI] [PubMed] [Google Scholar]
- Opas M, Szewczenko-Pawlikowski M, Jass GK, Mesaeli N, Michalak M. Calreticulin modulates cell adhesiveness via regulation of vinculin expression. J Cell Biol. 1996;135:1913–1923. doi: 10.1083/jcb.135.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteken A, Moss B. Calreticulin interacts with newly synthesized human immunodeficiency virus type 1 envelope glycoprotein, suggesting a chaperone function similar to that of calnexin. J Biol Chem. 1996;271:97–103. doi: 10.1074/jbc.271.1.97. [DOI] [PubMed] [Google Scholar]
- Saito Y, Ihara Y, Leach MR, Cohen-Doyle MF, Williams DB. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J. 1999;18:6718–6729. doi: 10.1093/emboj/18.23.6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Wyatt SE, Tsou P-L, Robertson D, Allen NS. A model system for plant cell biology: GFP imaging in living onion epidermal cells. Biotechniques. 1999;26:1127–1132. doi: 10.2144/99266st04. [DOI] [PubMed] [Google Scholar]
- Simon EW. The symptoms of calcium deficiency in plants. New Phytol. 1978;80:1–15. [Google Scholar]
- Staxén I, Pical C, Montgomery LT, Gray JE, Hetherington AM, McAinsh MR. Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc Natl Acad Sci USA. 1999;96:1779–1784. doi: 10.1073/pnas.96.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi J, Fujii A, Fujino Y, Tsujioka Y, Takahashi M, Tsuboi Y, Wada I, Yamada T. Different expression of calreticulin and immunoglobulin binding protein in Alzheimer's disease brain. Acta Neuropathol. 2000;100:153–160. doi: 10.1007/s004019900165. [DOI] [PubMed] [Google Scholar]
- Taussky HH, Schorr E. A microcolorimetric method for determination of inorganic phosphorus. J Biol Chem. 1953;202:675–685. [PubMed] [Google Scholar]
- Trewavas A. Le calcium, c'est la vie: calcium makes waves. Plant Physiol. 1999;120:1–6. doi: 10.1104/pp.120.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engelen FA, Molthoff JW, Conner AJ, Nap JP, Pereira A, Stiekema WJ. pBINPLUS: an improved plant transformation vector based on pBIN19. Transgenic Res. 1995;4:288–290. doi: 10.1007/BF01969123. [DOI] [PubMed] [Google Scholar]
- Wyatt SE, Tsou P-L, Robertson D (2001) The high capacity calcium-binding domain of calreticulin increases viability of seedlings on calcium-depleted medium. Transgenic Res (in press) [DOI] [PubMed]
- Xu W, Longo FJ, Wintermantel MR, Jiang X, Clark RA, DeLisle S. Calreticulin modulates capacitative Ca2+ influx by controlling the extent of inositol 1,4,5-trisphophate-induced Ca2+ store depletion. J Biol Chem. 2000;275:36676–36682. doi: 10.1074/jbc.M002041200. [DOI] [PubMed] [Google Scholar]