Abstract
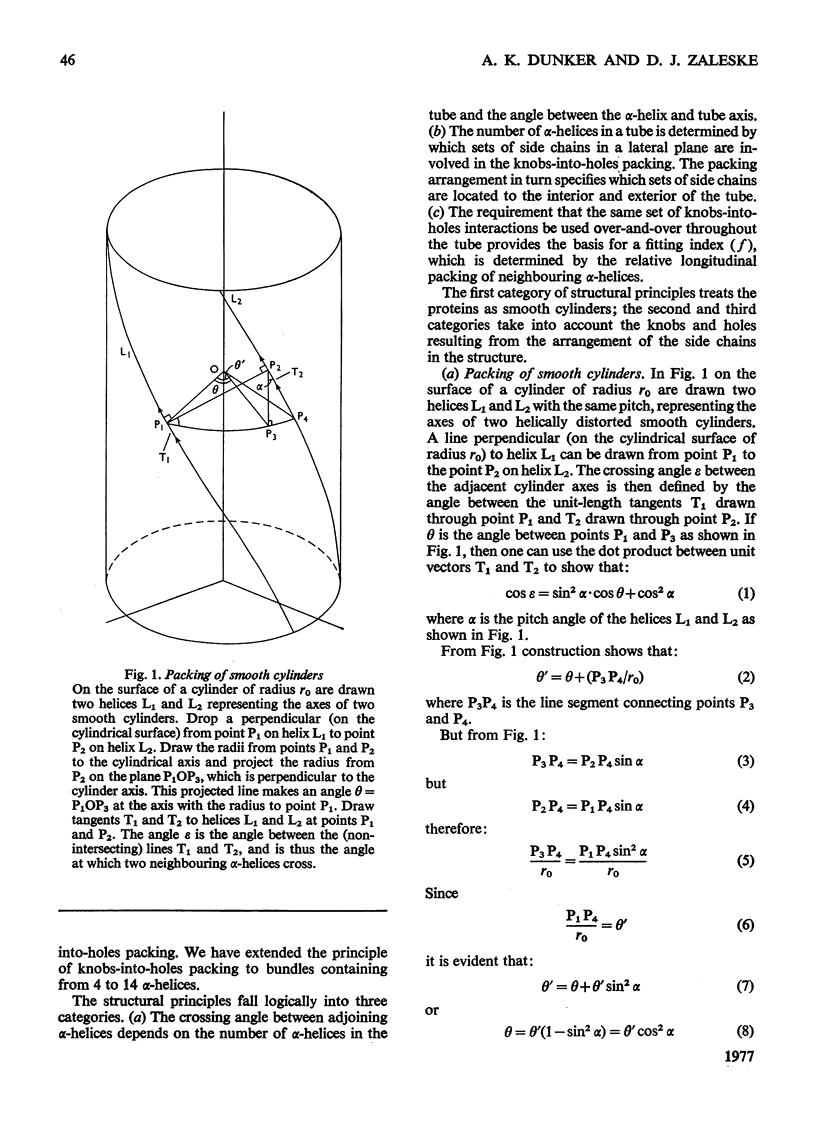
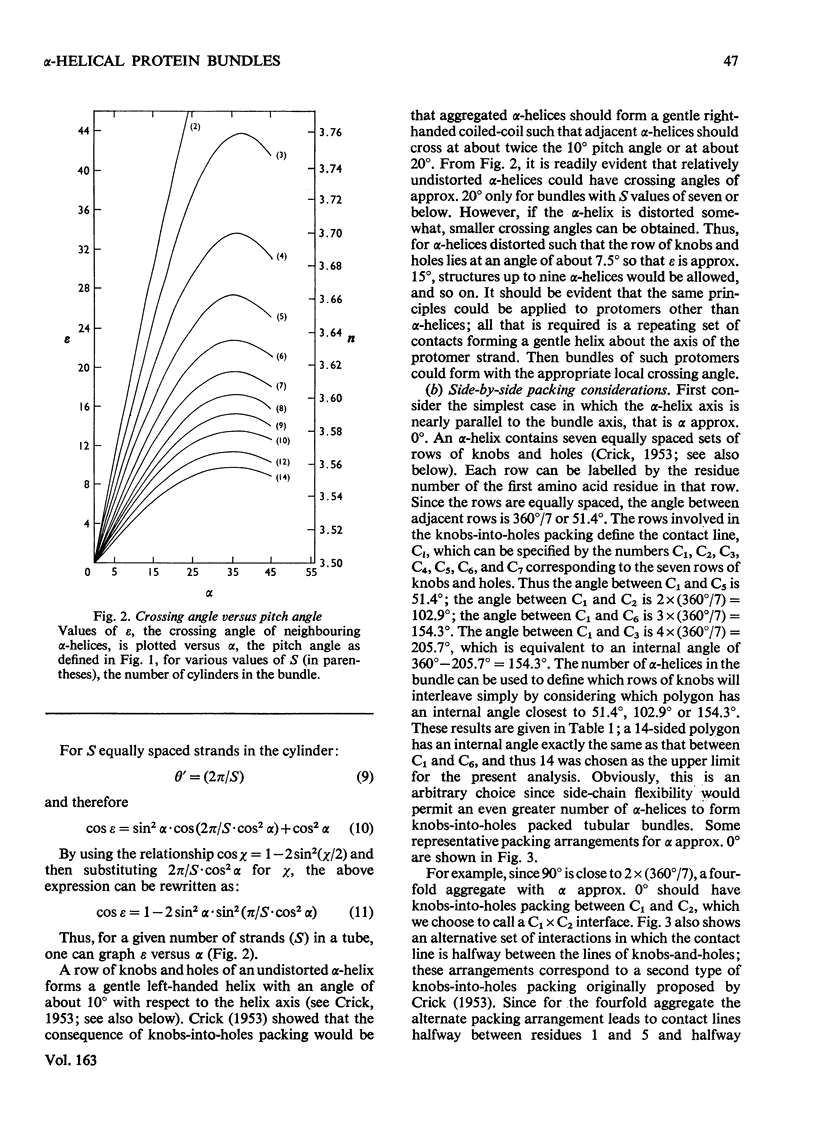
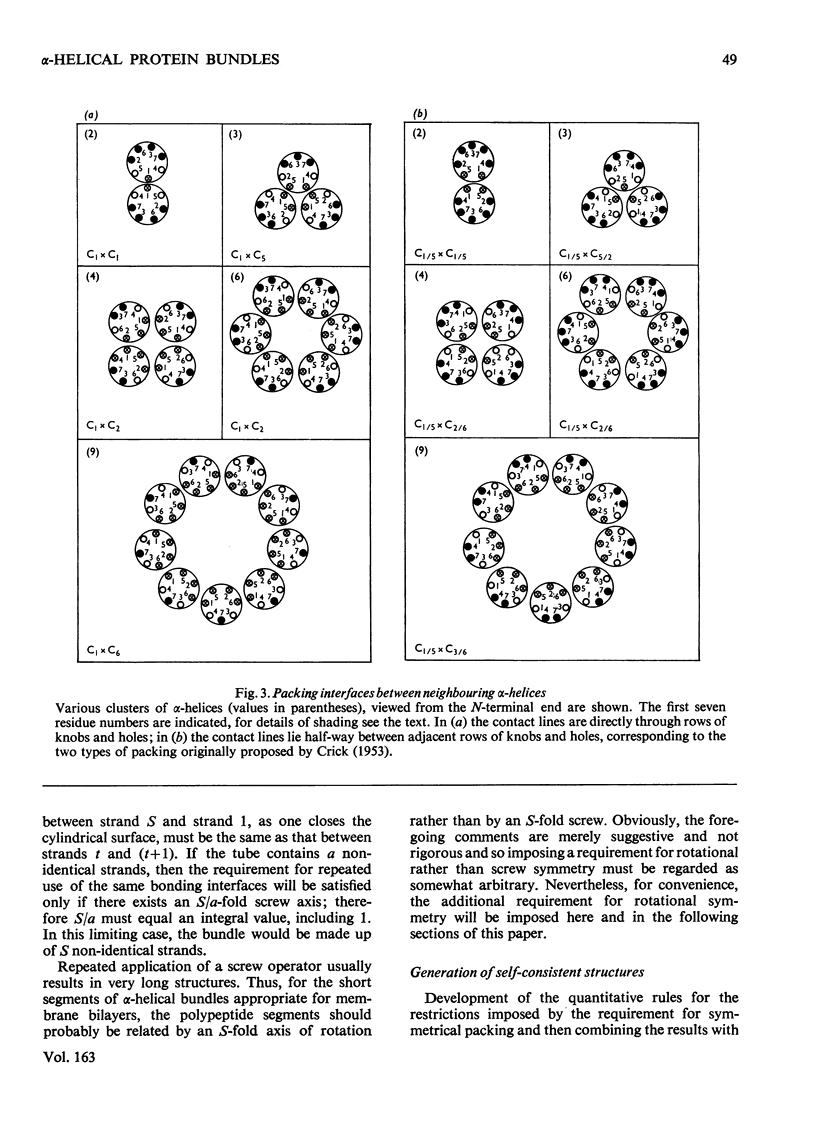
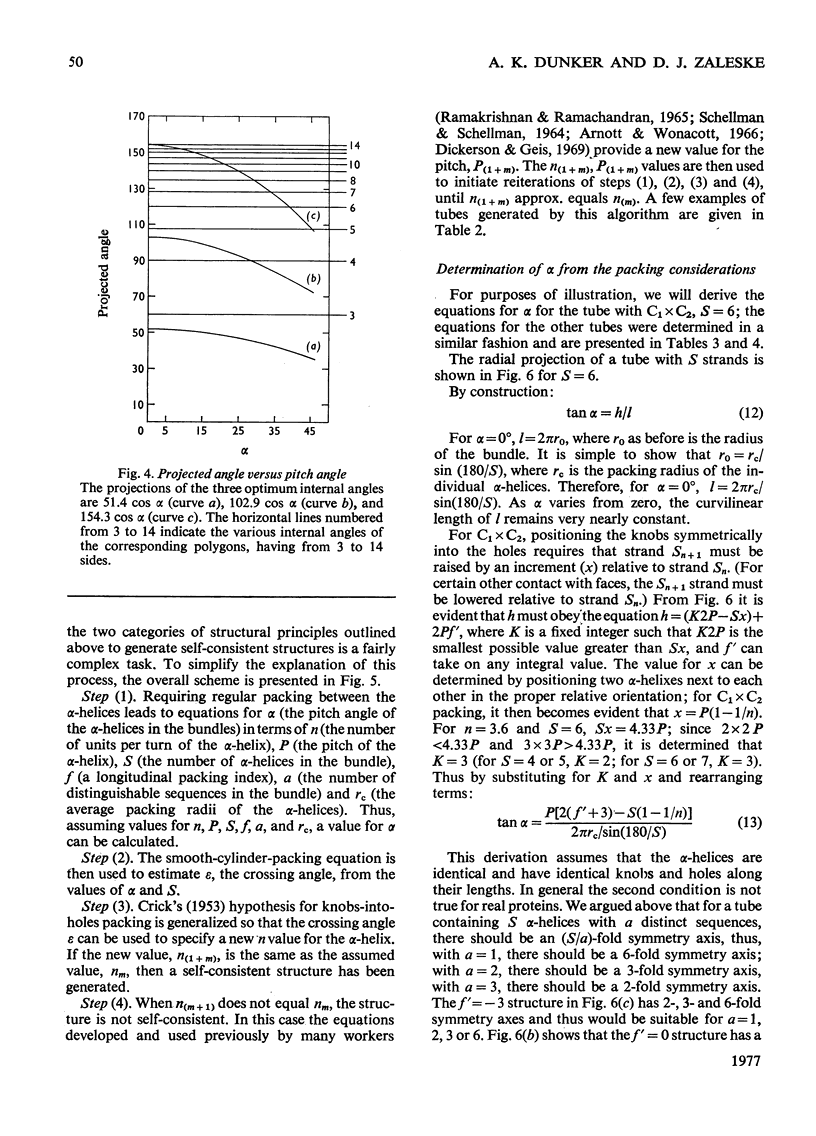
The stereochemical constraints originally used to construct two- and three-stranded alpha-helical coiled-coils were generalized for aggregates of alpha-helices containing from 4 to 14 alpha-helices in tubular bundles. Certain features of bacteriorhodopsin show excellent correlations with these stereochemical constraints.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Wonacott A. J. Atomic co-ordinates for an alpha-helix: refinement of the crystal structure of alpha-poly-l-alanine. J Mol Biol. 1966 Nov 14;21(2):371–383. doi: 10.1016/0022-2836(66)90105-7. [DOI] [PubMed] [Google Scholar]
- Edsall J. T., Flory P. J., Kendrew J. C., Liquori A. M., Némethy G., Ramachandran G. N., Scheraga H. A. A proposal of standard conventions and nomenclature for the description of polypeptide conformations. J Mol Biol. 1966 Jan;15(1):399–407. doi: 10.1016/s0022-2836(66)80240-1. [DOI] [PubMed] [Google Scholar]
- Frey T. G., Eisenberg D., Eiserling F. A. Glutamine synthetase forms three- and seven-stranded helical cables. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3402–3406. doi: 10.1073/pnas.72.9.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P., Hoyer B. H. Induction of cellular DNA synthesis in human leukocytes by Epstein-Barr virus. Nature. 1971 May 7;231(5297):46–47. doi: 10.1038/231046a0. [DOI] [PubMed] [Google Scholar]
- Harrison R. G., Lowey S., Cohen C. Assembly of myosin. J Mol Biol. 1971 Aug 14;59(3):531–535. doi: 10.1016/0022-2836(71)90317-2. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Inouye M. A three-dimensional molecular assembly model of a lipoprotein from the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2396–2400. doi: 10.1073/pnas.71.6.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Protein conformation in cell membrane preparations as studied by optical rotatory dispersion and circular dichroism. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1828–1835. doi: 10.1073/pnas.56.6.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine Y. K., Wilkins M. H. Structure of oriented lipid bilayers. Nat New Biol. 1971 Mar 17;230(11):69–72. doi: 10.1038/newbio230069a0. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Wachtel E. J. Structure and assembly of filamentous bacterial viruses. Nature. 1975 Jan 3;253(5486):19–23. doi: 10.1038/253019a0. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Circular dichroism of liver cell membrane organ specific protein. Biochem Biophys Res Commun. 1969 Jan 6;34(1):60–64. doi: 10.1016/0006-291x(69)90528-2. [DOI] [PubMed] [Google Scholar]
- PAULING L., COREY R. B., BRANSON H. R. The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proc Natl Acad Sci U S A. 1951 Apr;37(4):205–211. doi: 10.1073/pnas.37.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAULING L., COREY R. B. Compound helical configurations of polypeptide chains: structure of proteins of the alpha-keratin type. Nature. 1953 Jan 10;171(4341):59–61. doi: 10.1038/171059a0. [DOI] [PubMed] [Google Scholar]
- Pepe F. A. The myosin filament. I. Structural organization from antibody staining observed in electron microscopy. J Mol Biol. 1967 Jul 28;27(2):203–225. doi: 10.1016/0022-2836(67)90016-2. [DOI] [PubMed] [Google Scholar]
- RICH A., CRICK F. H. The molecular structure of collagen. J Mol Biol. 1961 Oct;3:483–506. doi: 10.1016/s0022-2836(61)80016-8. [DOI] [PubMed] [Google Scholar]
- RICH A., CRICK F. H. The structure of collagen. Nature. 1955 Nov 12;176(4489):915–916. doi: 10.1038/176915a0. [DOI] [PubMed] [Google Scholar]
- RICH A., CRICK F. H. The structure of collagen. Nature. 1955 Nov 12;176(4489):915–916. doi: 10.1038/176915a0. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan C., Ramachandran G. N. Stereochemical criteria for polypeptide and protein chain conformations. II. Allowed conformations for a pair of peptide units. Biophys J. 1965 Nov;5(6):909–933. doi: 10.1016/S0006-3495(65)86759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Zahler P. H. Protein conformations in cellular membranes. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1552–1559. doi: 10.1073/pnas.56.5.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]


