Abstract
Cortical growth and remodeling continues from birth through youth and adolescence to stable adult levels changing slowly into senescence. There are critical periods of cortical development when specific experiences drive major synaptic rearrangements and learning that only occur during the critical period. For example, visual cortex is characterized by a critical period of plasticity involved in establishing visual acuity. Adolescence is defined by characteristic behaviors that include high levels of risk taking, exploration, novelty and sensation seeking, social interaction and play behaviors. In addition, adolescence is the final period of development of the adult during which talents, reasoning and complex adult behaviors mature. This maturation of behaviors corresponds with periods of marked changes in neurogenesis, cortical synaptic remodeling, neurotransmitter receptors and transporters, as well as major changes in hormones. Frontal cortical development is later in adolescence and likely contributes to refinement of reasoning, goal and priority setting, impulse control and evaluating long and short term rewards. Adolescent humans have high levels of binge drinking and experimentation with other drugs. This review presents findings supporting adolescence as a critical period of cortical development important for establishing life long adult characteristics that are disrupted by alcohol and drug use.
Keywords: Alcohol, Adolescence, Cortical development, Binge drinking, Critical period
1. Introduction: Adolescence; a unique period of development
Adolescence is a critical period of development during the transition from childhood to adulthood. The ages associated with adolescence are commonly considered in humans to be approximately 12 to 20–25 years of age, and postnatal days (PND) 28 to 42 (Spear, 2000) in rodents. Adolescence is best defined by characteristic adolescent behaviors that include high levels of risk-taking, high exploration, novelty and sensation seeking, social interaction, high activity and play behaviors that likely promote the acquisition of the necessary skills for maturation and independence (Spear, 2000). Adolescent behaviors are shared across species, for example, high social interactions are found in human adolescents (increased communication with peers and increased number of conflicts with parents) (Csikszentmihalyi et al., 1977; Steinberg, 1989) as well as in adolescent rodents (increased peak level of play behavior and affiliative behaviors like huddling, grooming etc.) (Fassino and Campbell, 1981; Ehardt and Bernstein, 1987). These behaviors have been suggested to help adolescents develop the social skills needed when they become independent from their family or become senior adults in their group. In rodents, increased social interaction helps guide their food choices (Galef, 1977) and other adult behaviors such as sexual and aggressive behaviors (Fagen, 1976; Smith, 1982). Unfortunately, these high levels of novelty/sensation-seeking behaviors are also strong predictors of drug and alcohol use among adolescents (Baumrind, 1987; Andrucci et al., 1989; Wills et al., 1994). This review will cover brain maturation of neuroanatomy, neurotransmission and behavior during adolescence and present the postulate that the adolescent brain is a critical period of vulnerability for disruption of brain regions important for individual development.
2. Adolescent brain remodeling
The adolescent brain is in a unique state of transition as it undergoes both progressive and regressive changes providing a biological basis for the unique adolescent behaviors and the associated changes in behavior during maturation to adulthood. Human magnetic resonance imaging (MRI) studies have demonstrated an inverted U-shape change in the gray matter volume during adolescent period, with a pre-adolescent increase followed by a post-adolescent decrease (Giedd et al., 1999; Giedd, 2004). At the cellular levels, these changes correspond with the marked overproduction of axons and synapses in early puberty, and rapid pruning in later adolescence (Giedd et al., 1999; Andersen et al., 2000; Andersen and Teicher, 2004). Although the exact mechanisms of such synaptic changes are not well known, it is speculated that such remodeling is the biological basis of developmental plasticity where the neurological circuits are effectively shaped to adapt to the environmental needs leading to mature adult behavior. Such a period of remodeling could also make the adolescents more vulnerable to external insults and other psychiatric disorders.
The prefrontal cortex (PFC) and the limbic system, which includes the hippocampus, amygdala, nucleus accumbens (NAc), prefrontal, frontal and orbital frontal cortices and the hypothalamus, undergo prominent reorganization during adolescence. Absolute PFC volume declines in adolescence in humans (Sowell et al., 1999, 2001) as well as in rats (van Eden et al., 1990). Substantial loss of synapses, especially the excitatory glutamatergic inputs to the PFC, occurs during the adolescent period in humans and nonhuman primates (Huttenlocher, 1984; Zecevic et al., 1989). In contrast to such adolescent-associated pruning, dopamine and serotinin (5-HT) inputs to PFC increase during adolescence to peak levels well above those seen earlier or later in life (Kalsbeek et al., 1988; Rosenberg and Lewis, 1994). Cholinergic innervation of PFC also increases in adolescence to reach mature levels in rats (Gould et al., 1991) and humans (Kostovic, 1990). In the hippocampus, the exuberant outgrowth of excitatory axon collaterals and synapses during youth are morphologically remodeled and branches within dendritic arbors are pruned during adolescent maturation (Swann et al., 1999). Similarly, significant dendritic pruning and synaptic regression occur in medial amygdala (Zehr et al., 2006), nucleus accumbens (NAc) (Teicher et al., 1995; Tarazi et al., 1998b) and the hypothalamus (Choi and Kellogg, 1992; Choi et al., 1997) during adolescence. Although most synaptic pruning is likely glutamatergic, dopamine receptor expression peaks in early adolescence (PND28) followed by a one-third loss of receptors during PND35 to PND60 (Tarazi et al., 1998a). In terms of hypothalamic function, adolescent rats often exhibit more prolonged stress-induced increases in cortisol than adults (Walker et al., 2001). In addition, rats at PND 28 were found to show less stress-induced Fos-like immunoreactivity in cortical and amygdaloid nuclei than adult rats (Kellogg et al., 1998), but higher novelty-induced Fos activation in hippocampus during this period (Waters et al., 1997). Thus, environmental alterations in gene transcription are unique during adolescence and likely impact the active remodeling of synaptic connections. The following paragraphs review neurochemical markers of adolescent brain remodeling to illustrate the high plasticity of normal brain development. The remodeling of the adolescent brain may represent a critical period of development during which alcohol and drugs may become significant environmental factors modulating brain development.
3. Critical periods of cortical development
Critical periods are specific windows during development when both genetic driven processes and environmental processes, e.g. nature and nurture, interact to establish functional characteristics. These interactions correspond to structural rearrangements of the cerebral cortex that occur during this specific developmental window. As described above, cortical development in humans occurs over the first 3 decades of life with grey matter changes correlating with post-mortem findings of brain regionally different synaptic pruning and myelination during the transitions from childhood to adolescence to adulthood. The human visual cortex reaches a peak of synaptic overproduction around the 4th month after birth followed by synaptic elimination starting after that and continuing until preschool age at which time synaptic density stabilizes to adult levels (Toga et al., 2006). Other cortical areas develop at different ages with dorsal parietal and primary sensorimotor regions showing grey matter loss at ages 4–8, and parietal areas of language and spacial orientation changing around ages 11–13 and prefrontal areas involved in integrating information from senses, reasoning and other “executive functions” maturing last during late adolescence (Gogtay et al., 2004; Toga et al., 2006). These age-related changes in cortical structure involve improved function. Cortical thinning in the left dorsal frontal and parietal lobes correlates with improved performance on a test of general verbal intellectual functioning between the ages of 5 to 11 (Sowell et al., 2004). Other studies following individuals from age 6 through 19 found that individuals with superior intelligence show the greatest changes in frontal cortical thickness compared to individuals with high or average intelligence (Shaw et al., 2006). These changes likely are a combination of environment and genetic regulation of cortical development and overall function. Environmental experiences and training are known to induce changes in cerebral cortex including neurochemical, altered cortical thickness, size of synaptic contacts and dendritic structure as well as improving performance on learning tests (Rosenzweig and Bennett, 1996). Learning in humans during studying for exams (Draganski et al., 2006) or practicing juggling (Draganski et al., 2004) alters cortical structure consistent with environment contributing to structural changes in brain. These developmental processes are thought to underlie time-limited windows when specific experiences can drive development, e.g. critical periods of plasticity or learning that can only occur during these critical period windows (Munakata et al., 2004). For example, learning a second language is thought to be optimal during a critical period of development (Johnson and Newport, 1989). The complexity of higher brain functions makes it difficult to relate synaptic rearrangements to alterations in function. The synaptic rearrangements and increased myelination of frontal cortical areas in mid to late adolescence could be involved in altered executive functions. Behavioral studies show that performance on tasks including inhibitory control, decision making and processing speed continues to develop during adolescence. During adolescence tasks of selective attention, working memory and problem solving improve consistently correlating with frontal cortical synaptic pruning and myelination (Blakemore and Choudhury, 2006). Inhibitory control involves executive functions that improve from adolescence to adulthood. Studies measuring behavioral inhibition on a Go-No-Go task and fMRI data reveal greater activation of dorsolateral frontal cortex and orbitofrontal cortexes in children, than adolescence, and greater in adolescence than adults with the adults showing the lowest dorsolateral, but equal orbitofrontal activation and greater inhibitory control performance (Casey et al., 1997; Tamm et al., 2002). These studies support the concept that the immature brain with excess synapses causes more extensive and less efficient frontal activation and lower performance compared to adults that have a more pruned and myelinated frontal cortex that results in more focused, lower overall activation and faster reaction times and better performance (Blakemore and Choudhury, 2006). Taken together these studies suggest that remodeling of the cortex during the transitions from youth to adolescence to adulthood has functional implications for the entire adult stages of life.
Environmental plasticity of visual cortex development has been extensively studied. Hubel and Wiesel found that depriving an eye of light altered cortical responses to light only if the deprivation occurred during a “critical period” of cortical development. The critical period for visual cortical plasticity is defined as the period during which monocular deprivation (covering one eye) results in a shift in cortical neuronal spiking responses away from the covered eye and increased spiking responses to the active eye. The spiking responses are shifted to the open eye only if the deprivation occurs during the critical period. The critical period for visual cortex plasticity in rodents overlaps with early adolescence being between PD19 and PD 32 (Gordon and Stryker, 1996). Studies indicate that an activity dependent synaptic plasticity occurs during this critical period that allows cortical adjustments to environmental factors. Dark rearing delays the critical period of visual cortex development into adulthood likely by decreasing BDNF expression and GABA synaptic strength (Hensch, 2005). Genetic BDNF overexpression or benzodiazepines administration can accelerate the appearance of the critical period of visual cortical development. Environment and genetic programming interact to regulate synaptic organization during the critical period resulting in a mature cortex. The mature cortex is stable and does not undergo the dramatic shift in cortical synaptic plasticity with monocular deprivation or dark rearing. Thus, early adolescence is a critical period for visual cortical maturation.
Ethanol treatment has been shown to lead to a permanent impairment of visual neocortex plasticity and to be particularly toxic to adolescent brain. Studies in ferrets have found that ethanol treatment before the critical period prevents ocular dominance from occurring in neurons during the critical period (Medina et al., 2003; Medina and Ramoa, 2005). Electrophysiological single unit recording indicates that alcohol exposure weakened neuronal orientation selectively while preserving robust visual responses (Medina et al., 2005). Further, optical imaging maps of intrinsic synaptic signaling from the eyes to the visual cortex are highly contrasted in controls following the critical period of synaptic ocular dominance, but have little contrast or no ocular dominant columns after ethanol administration. Disruption of ocular dominant columns in visual cortex is known to reduce visual acuity and monocular deprivation during the critical period essentially creates a blind eye due to altered synaptic connections (Prusky and Douglas, 2003). These studies indicate that alcohol exposure preceding and during critical periods of cortical development disrupt cortical development resulting in reduced function permanently. Studies of binge drinking induced brain damage in rats have found that adolescent forebrain is particularly sensitive to ethanol induced neurodegeneration (Crews et al., 2004). Further, adolescent brain has been found to be particularly sensitive to ethanol induced inhibition of neurogenesis, the formation of new neurons (Crews et al., 2006). These studies indicate that ethanol disrupts cortical remodeling during critical periods and is particularly neurotoxic to the adolescent brain.
Ethanol is known to interact with glutamatergic NMDA receptors, GABAa receptors, DA pathways, CREB transcription, and neurogenesis. Each of these systems is undergoing extensive remodeling in adolescence. The following sections review the remodeling of these neurochemical markers known to be sensitive to ethanol.
4. Neurotransmitter systems and adolescent development
4.1. Glutamate and NMDA receptor systems
The binding of cortical glutamate to its NMDA receptor subtype peaks in early adolescence, and declines significantly thereafter, with a loss of one-third of NMDA receptors by PND60 (Insel et al., 1990; Guilarte, 1998). Such synaptic pruning contributes to the loss of excitatory glutamate input to NAc (Frantz and Van Hartesveldt, 1999a) and a reduction in accumbal NMDA receptors (Frantz and Van Hartesveldt, 1999b) during adolescent brain maturation. Interestingly, long-term potentiation (LTP), a measurable increase in synaptic strength and a form of neuroplasticity, is more frequently found in NAc of adolescent mice compared to adults consistent with adolescence being a highly plastic period of mesolimbic brain development (Schramm et al., 2002). This plasticity was also demonstrated in other limbic regions that are believed to be involved in drug addiction, including amygdala (Ungless et al., 2001), VTA (Thomas et al., 2001) and hippocampus (Adriani et al., 2004). Alcohol and other drugs are known to modulate glutamatergic transmission and alter limbic brain development (Crews et al., 2002). Therefore, glutamate and NMDA receptor systems play a crucial role in the neurochemical remodeling in adolescents, especially in limbic brain regions that are highly plastic and actively undergoing remodeling. The impact of alcohol and other illicit drugs on the development of glutamatergic synapses in these brain regions is critical for understanding the particular vulnerability of adolescents to drug addiction.
4.2. GABAergic systems
GABAergic (γ-aminobutyric acid) neurotransmission, as the major inhibitory neurotransmitter in the brain, that likely makes a major contribution of cortical remodeling. In adolescent monkeys significant changes occur in pre- and post-synaptic markers of GABAergic synapses in the prefrontal cortex during adolescence (Lewis et al., 2004). Generally, basal levels of GABAA receptor-mediated chloride uptake are greater in the cortex of adolescents than that of adults (Kellogg et al., 1993) and the responsiveness of cortical GABAA neurotransmission to stressors decreases from adolescence to adulthood (Kellogg, 1998). In hippocampus, the expression of GABA transporter 1 (GAT-1) and glutamic acid decarboxylase (GAD), e.g. GABA synthase, peak around early infancy (Hachiya and Takashima, 2001), although hippocampal GABAB receptor regulation of synaptic transmission does not mature until adolescence (PND35) (Nurse and Lacaille, 1999). GABAA receptors are heterogeneous group of receptors composed of two alpha, one beta and two delta or other subunits. There are multiple alpha subunits that can contribute to GABAA receptors and varied subunits modulate GABAA receptor function and responsiveness to GABA, anti-anxiety drugs, neurosteroids, alcohol and other modulators of GABA transmission. In fact, the subunit composition of GABAA receptor subunits largely determines the pharmacological and electrophysiological properties of GABA neurotransmission (Brooks-Kayal et al., 2001; Mohler et al., 2001). GABAA subunits undergo dramatic postnatal reorganization (Yu et al., 2006). The alpha1 subunit that contributes to sedative, amnestic and anticonvulsant actions of GABA undergoes a dramatic increase in the frontal cortex between youth (10 PND) and adolescence (30 PND) followed by a significant decline in the transition to adulthood (90 PND) becoming relatively stable at later ages (Yu et al., 2006). This likely represents remodeling of GABAergic synapses. Alpha2 and Alpha3 subunits show a similar increase from youth to adolescence, in frontal cortex, however, alpha2 stays elevated through adulthood and alpha3 GABAA subunits decline slowly reaching statistical significance at 9 months. Alpha5 GABAA subunits are high in youth and in different brain regions decline through adolescence into adulthood. Alpha2, 3 and 5 subunits contribute to anxiolytic and other properties of GABA modulators (Fritschy and Brunig, 2003). Interestingly, the alpha2 subunit of the GABAA receptor complex, whose expression steadily increases across the brain from early youth throughout adolescence (Yu et al., 2006) has been implicated in the genetics of alcoholism. A sequence difference in the alpha2 subunit of the GABAA receptor has been found to be about twice as common in alcohol dependent German patients than matched controls consistent with contributing to genetic predisposition to alcoholism (Fehr et al., 2006). Interestingly, chronic alcohol in adults decreases cerebral cortical alpha2 and alpha3 subunits resulting in loss of benzodiazepine recognition sites and likely changing overall GABA transmission (Mehta and Ticku, 2005). Studies in adolescents have not been done, however, alcohol induced changes in GABA subunits during the active period of cortical development might result in differential stabilization of adult GABAA receptor synaptic organization that remain disrupted for long periods in adulthood. The maturation of GABAergic neurotransmission from infancy to adolescence to adulthood likely contributes to inhibitory interneuron fine-tuning of synaptic inputs improving discrimination of signals and more efficient processing. Disruption by alcohol and other addictive drugs during the adolescent remodeling of GABA-mediated inhibitory control of neuronal circuitry could alter susceptibility to alcohol dependence and other drug addiction in adulthood.
4.3. Dopaminergic systems
Dopaminergic transmission contributes to attention, reward, movement, hormone regulation and multiple other important physiological processes. Postnatal reorganization of dopaminergic neurotransmission is brain regional and receptor subtype-specific. In rat frontal cortex, entorhinal cortex and hippocampus, dopamine D1, D2 and D4 receptors rise several fold from PND7 to PND35, e.g. adolescence, and then stabilize to adulthood (Tarazi and Baldessarini, 2000). In striatum and nucleus accumbens (NAc) dopamine receptors are overproduced with subsequent pruning of approximately one-third during adolescent suggesting maturational remodeling of motor and reward pathways (Teicher et al., 1995; Tarazi and Baldessarini, 2000). Furthermore, dopamine D3 receptors do not reach peak levels until adulthood (PND60) in striatum, NAc and olfactory tubercle (Stanwood et al., 1997). In contrast, dopamine transporters increase 6–7 fold steadily throughout brain from PD7 to PD60 in striatum in contrast to the remodeling of dopamine receptors in adolescent striatum (Tarazi et al., 1998b). This substantial postnatal remodeling of dopamine neurotransmission during adolescence may contribute to a stabilization of behaviors established during adolescence. Alcohol and drug taking may alter the maturation of dopamine neurotransmission during adolescence contributing to altered development of attitudes, actions and social rewards.
4.4. Serotonergic systems
Serotonergic neurotransmission undergoes reorganization during postnatal development and is important for mood, sleep, anxiety and many other complex behaviors. In humans and rats, 5-HT neurons are generated prenatally (Lauder and Bloom, 1974; Lauder, 1990) with brain 5-HT levels peaking early in life, then decreasing to adult levels (Hedner et al., 1986; Toth and Fekete, 1986). Postnatal reorganization of developing serotonergic projections is exemplified by fluctuations of the number of serotonergic synapses during this period, 5HT synapses reach adult levels at PND14 and then in rat basal forebrain drop to significantly lower levels during early adolescence (Dinopoulos et al., 1997; Dori et al., 1998). The reorganization of 5-HT receptor expression is also pronounced during development, likely relating to reorganization of serotonergic innervation patterns. For example, 5-HT2A receptors reach cortical peak expression just before adolescence and then progressively decline to adult levels correlated with increased innervation and pruning of 5-HT axons in rat and monkey (Morilak and Ciaranello, 1993). Similarly, 5-HT7 receptors exhibit transient expression patterns in striatum and hippocampus (Vizuete et al., 1997). 5-HT1A receptors are highly expressed in humans, cats and rodents at birth, but decline dramatically during adolescence (Daval et al., 1987; Bar-Peled et al., 1991; Dillon et al., 1991; Dyck and Cynader, 1993; Burnet et al., 1994; del Olmo et al., 1998). 5-HT turnover in NAc is reported to be approximately 4-fold lower in adolescent rats (PND30–40) than either younger rats (PND10–15) or mature adults (PND60–80) (Teicher, 1999). Interestingly, low 5-HT activity in adolescence has been suggested contribute to common adolescent behaviors such as hypersensitivity to mild stressors, increased anxiety and alcohol drinking (Depue and Spoont, 1986). In contrast to the alteration in serotonin receptors during adolescence, serotonin transporters steadily increase from PND7 to adulthood without significant pruning in striatum and NAc (Tarazi et al., 1998b). Studies modeling adolescence binge drinking in rats have found marked increases in adult levels of serotonin transporters (Monti et al., 2005). Thus, serotonin neurotransmission undergoes dramatic remodeling from youth through adolescence into adulthood and it is sensitive to alcohol and drug disruption.
In summary, major neurotransmitter systems are not mature at birth and postnatal brain development continues through adolescence, with remodeling most pronounced in frontal and limbic regions.
5. Transcription factor CREB and growth factor BDNF in adolescence
The cAMP-response element binding protein (CREB) is an important mediator for the differentiation and maturation of CNS neurons. CREB is also critical for induction of trophic factors such as BDNF, for neuronal vitality and for learning and memory (Bender et al., 2001; Zou and Crews, 2006). Through phosphorylation, CREB is activated to propagate signals from synapses to the nucleus to the expression of genes necessary for synaptic plasticity (Martin and Kandel, 1996; Silva et al., 1998; Bender et al., 2001). As such a key transcriptional factor for neuronal growth, it is not surprising that the expression of CREB can play a critical role in postnatal neurochemical remodeling. Phospho-CREB (pCREB), the transcriptionally active form of CREB, is highly expressed in early postnatal development (PND7) and declines during adolescence to adult levels in both hippocampus and cortex (Toscano et al., 2003). Additionally, CREB activation occurs prior to the expression of BDNF (brain-derived neurotrophic factor) and neurotrophin-3 (NT3) (Bender et al., 2001), consistent with CREB being upstream and activating transcription of these neurotrophic factors. BDNF is involved in the regulation of neuronal differentiation, survival and neuroplasticity as well as being linked with a variety of neurological and psychiatric disorders (epilepsy, mood disorder, bipolar depression) in children, adolescents as well as adults (Zhu and Roper, 2001; Geller et al., 2004; Strauss et al., 2004). CREB and BDNF interact in a variety of brain regions and are known to play a critical role in addition (Carlezon et al., 2005). Thus, the developmental alterations in CREB-BDNF contribute to continuous modeling of brain through youth, adolescence into adulthood and are vulnerable to alcohol and drug induced disruption of development.
6. Neurogenic processes in adolescent brains
Although neurogenesis is primarily an early developmental process with most neurons being formed in the prenatal and early postnatal periods, it continues into adulthood within specific adult brain regions including the forebrain subventricular zone (SVZ) and hippocampal dentate gyrus (DG) where neurogenesis continues into senescence. Generating and integrating new neurons into preexisting neuronal circuits is believed to enable the hippocampus to adapt to novel and more complex situations (Kempermann, 2002). The contribution of adult hippocampal neurogenesis to learning and memory (Shors et al., 2001), as well as mood and affective state (Malberg et al., 2000) is supported by many studies. Adolescent neurogenesis and its role in the brain remodeling and unique adolescent behaviors have not been investigated. Studies have indicated that adolescents have higher levels of neurogenesis in the hippocampus (He and Crews, in press) and adolescent brain neurogenesis is very sensitive to alcohol-induced inhibition (Crews et al., 2006). Disruption of neurogenesis by drugs and alcohol during adolescence could produce long-lasting changes in adulthood.
7. Adolescent behavior: Risky, motivated, and vulnerable
As just noted, the adolescent brain undergoes remodeling in a variety of structural and functional regions, particularly corticolimbic and frontal regions known to regulate emotional as well as analytical and executive processes. Simultaneous with these changes, adolescents demonstrate new behaviors that are associated with acquisition of adult cognitive and emotional repertoires (Yates, 1996). These are normal adaptive changes that help usher the adolescent into adulthood. However, adolescents also exhibit increased health-risk behaviors that represent the leading causes of morbidity and mortality among the adolescent age group (Merrick et al., 2004). Disruption of adolescent development by environmental factors, particularly alcohol and other abused drugs might produce subtle changes other than the known pronounced mortality that have a delayed impact on the quality of adult life. For this reason, it is important to understand adolescent behavior and neurobiology in the context of the developing brain in order to appreciate the adaptive changes within a critical period of acquisition of adult cognitive and emotional repertoires.
One of the most pronounced changes in adolescent behavior is the characteristic increased risk taking. Epidemiological studies show that human adolescents engage in more risky behavior, which includes hazardous driving, unprotected sex, and substance abuse as compared to adults (Berndt, 1979; Arnett, 1992). These behaviors are associated with low levels of anxiety regarding the potential for harm (Wilson and Daly, 1985). One explanation for age-related differences in risk taking is the characteristic reduction in reward sensitivity that leads adolescents to seek higher levels of novelty and external stimulation. Changes in reward sensitivity may reflect maturational differences in mesolimbic neural circuits (Spear, 2000; Kelley et al., 2004), which regulate the translation of motivation into action (Le Moal and Simon, 1991). Increased adolescent reward sensitivity, motivation and action is likely related to the synaptic remodeling of striatal, limbic and frontal brain regions as the final stage of development to adulthood.
In addition to reward circuits, a variety of other neural systems also exhibit developmental changes during adolescence that lead to behavioral and cognitive alterations. For example, evidence suggests that a variety of self-regulatory executive functions are still maturing during adolescence. For this reason, adolescents are in the sometimes unfortunate situation of having poor judgment and lack of impulse control even though they are driven to seek increasing levels of novelty and external stimulation. A variety of brain systems mediates impulse control (Chambers et al., 2003) but maturation of prefrontal cortical systems appears to track development of executive functions (Keating, 2004).
Adolescence is also characterized by the appearance of strong emotional states where some individuals experience striking changes in mood that are sometimes difficult to distinguish from clinical syndromes, such as depression (Golombek and Kutcher, 1990). An understanding of mood changes during adolescence may be gained from evaluating neurobiological systems that regulate adult mood disorders. Along these lines, one potentially important outcome of developmental changes in gene transcription (e.g., CREB) and growth factor (e.g., BDNF) expression is altered neurogenesis (see above). It has been hypothesized that decreased neurogenesis in the hippocampus is a mechanism underlying mood changes, such as depression (Duman et al., 2000; Jacobs et al., 2000), which are common during adolescence. Although the functional consequences of altered neurogenesis to adolescent behavioral changes remain to be studied, some noteworthy linkages exist. For example, stress and elevated stress hormones, which are hallmarks of adolescence, alter neurogenesis and precipitate changes in mood (Cameron and Gould, 1994; Tanapat et al., 2001; Malberg and Duman, 2003; Gregus et al., 2005). Antidepressant drugs that target serotonin and norepinephrine systems, both of which change during adolescence, increase hippocampal neurogenesis (Malberg and Duman, 2003; Santarelli et al., 2003). When taken together with evidence that antidepressants also increase serotonin, CREB and BDNF activity (Malberg and Blendy, 2005), these data suggest that developmental changes in these pathways may underlie some of the mood alterations that characterize adolescence. In addition, evidence shows that enriched environment and exercise are associated with increased mood and neurogenesis, whereas stress is associated with depression and decreased neurogenesis (Gould, 1999; Gage, 2000; Nixon and Crews, 2002). The adolescent trait of impulsivity is also normalized by enriched rearing during adolescence (Adriani et al., 2006). Moreover, adolescents who have enriched environments in the form of socially active friends and engaged parents exhibit fewer behavioral problems and engage in less risky behavior (Biglan et al., 1990). Thus, it is plausible that both developmentally and environmentally regulated changes in neurogenesis may underlie alterations in mood and behavior that accompany adolescence.
Overall, adolescents exhibit a variety of behavioral changes that reflect normal development of brain systems. Paradoxically, these developmental changes also create conflicts in behavioral repertoires that mark the unique vulnerability of this developmental period. A few of these have been discussed above in terms of specific systems that may regulate specific behaviors. Ultimately, however, a more complete understanding of the paradoxical properties of adolescent behavior is likely to require a multidisciplinary systems approach that integrates developmental, behavioral, and neural analyses.
8. Adolescent alcohol abuse is common
Alcohol use among adolescents is common. As described earlier, adolescent high risk-taking, thrill and novelty-seeking behaviors promote heavy drinking and other drug experimentation. Individuals in their teens and early twenties are among the heaviest episodic drinkers. For example, among U.S. high school students 12% of 8th graders (13–14 years of age), 22% of 10th graders and 28% of 12th grade seniors reported heavy episodic drinking within the past 2 weeks (Johnston et al., 2004). According to the National Institute of Drug Abuse, 82% of adolescents have tried alcohol by the time they reach their senior year in high school. For college students 44% binge drink every two weeks and 19% are frequent binge drinkers, having more than 3 binge drinking episodes per week (Wechsler et al., 1995). Thus, adolescents are often drinking large quantities of alcohol.
9. Binge drinking during critical periods in cortical development may lead to life long changes of executive function
The effects of alcohol on adolescent brains are different from those on adults. Adolescents are less sensitive to the sedative effects of alcohol (Silveri and Spear, 1998), which allows them to binge drink, however, they are more vulnerable to alcohol-induced neurotoxicity (Monti et al., 2005). The increase in sensitivity of the adolescent brain to toxicity and the dynamic synaptic remodeling of the maturing adolescent brain may enhance the strong learning components of heavy drinking behaviors and the loss of important self-control and goal setting components of the maturing brains executive centers. Individuals who start drinking before the age of 15 are four times more likely to become alcohol dependent at some time in their life (Grant and Dawson, 1998). Current studies indicate that 25–35% of high school students began drinking before the age of 13 (CESAR, 2006). Studies of adolescent individuals with alcohol use disorder have found smaller prefrontal grey and white matter volumes than age matched controls. Lower prefrontal volumes correlated with a higher maximum number of drinks per drinking episode (De Bellis et al., 2005). It is likely that both genetics and environment (heavy drinking) contribute to the alcohol use disorder and lower prefrontal volumes in adolescence with alcohol use disorder. Studies of social drinkers have found that the heaviest binge drinkers have more negative moods and performed worse on executive function tasks (Townshend and Duka, 2003; Weissenborn and Duka, 2003). Alcoholics have been found to see more fear in facial expressions and animal studies have suggested these alterations in fear response are the result of alcohol induced deficits in associative learning (Duka et al., 2004). Other studies have found perseverative relearning deficits following a rat model of binge drinking that relates to damage to association cortex (Obernier et al., 2002). None of these studies directly show a critical period of executive function development during adolescence that is disrupted by ethanol. However, the findings of ethanol disruption of the critical period of visual cortical development, ethanol induced cortical neurotoxicity and ethanol induced alterations in executive function support the theory that disruption of frontal cortical development and executive function maturation occur in adolescent alcohol abusers (Fig. 1). It is possible that adolescent alcohol abuse by disrupting impulse inhibition, attention and motivation promotes adult alcohol dependence and underlies the high risk of lifetime alcohol dependence found among those who begin drinking as adolescents. In total the evidence does support a link between adolescent alcohol abuse during a critical period of executive function development and an increased risk of lifetime alcohol dependence and perhaps other psychopathology.
Fig. 1.
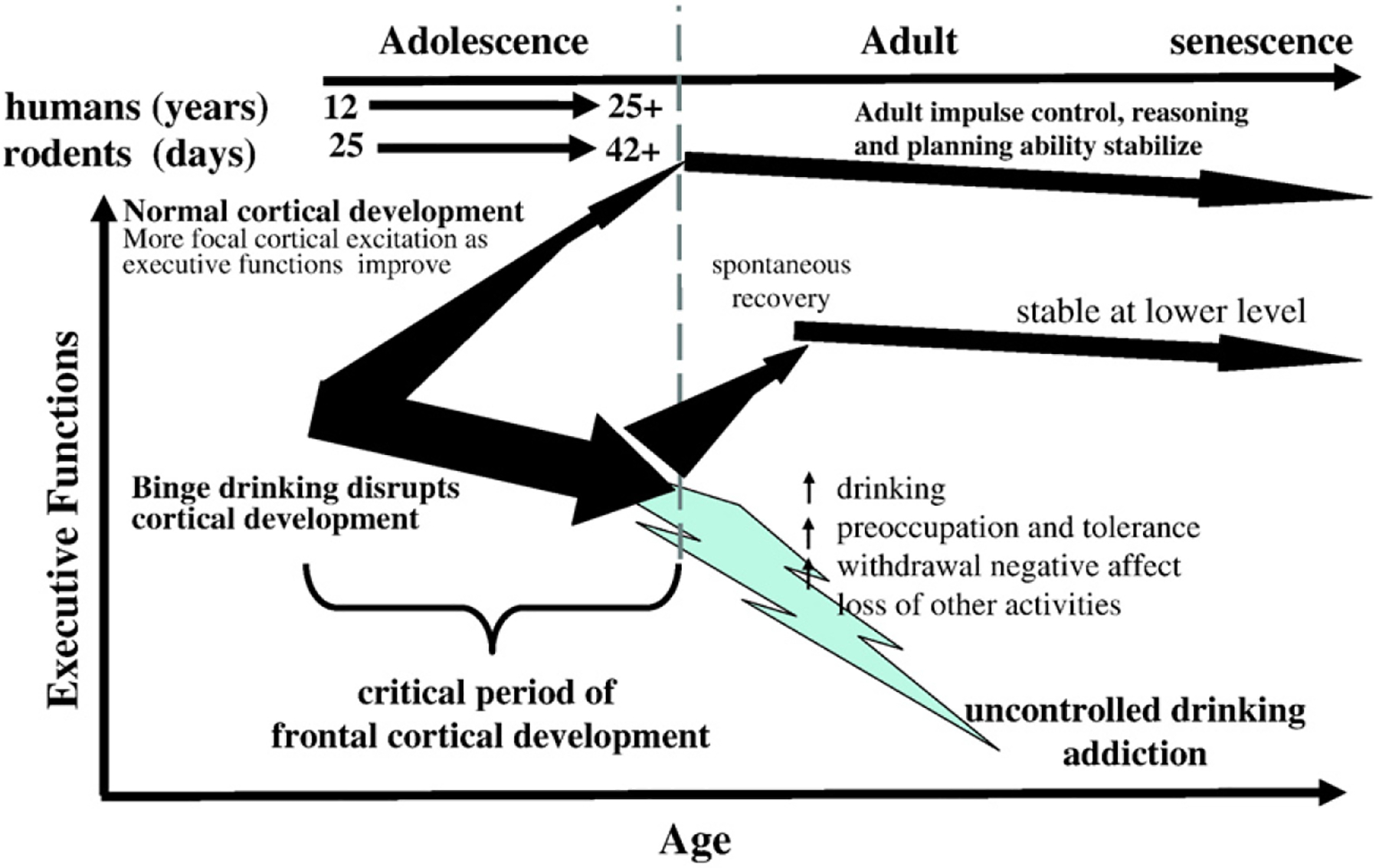
Adolescent alcohol abuse disrupts frontal cortical development and maturation of executive function. This schematic diagram emphasizes the normal focusing of cortical excitation during cerebral tasks that occurs during the transition from adolescent to adult as indicated by the upward narrowing arrow. Frontal cortical remodeling is associated with improved performance at tasks and stabilization of reasoning, impulse control, goal setting, maturation of risk taking, reward sensitivity, motivation, and emotional states. Adult executive functions stabilize after adolescence with a slow decline in senescence. Individuals who have talent (genetics) and training (environment) are most likely to achieve their best abilities at the juncture of adulthood. Individuals who binge drink during adolescence damage and disrupt forebrain cortical development during a critical period of behavioral and cortical maturation. Binge drinking interferes with cortical remodeling as illustrated by the large vertical slightly downward arrow that does not focus. Following adolescence many individuals spontaneously or through therapy reduce their drinking and partially recover executive functions, although they remain lower in ability than those who develop normally. Other individuals remain heavy drinkers continuing to drink. Through the life course many will escalate drinking due to stress, tolerance development, avoidance of negative withdrawal states and other factors that drive them to therapy in their mid-adult years. This model suggests that interventions to reduce adolescent drinking will greatly improve abilities of many individuals and reduce overall lifetime alcoholism and addiction.
10. Summary
Adolescence represents an important period of brain development, particularly for the cerebral cortex. There are critical periods of development when the cortex is sensitive to environment induced changes in synaptic strength. Between the ages of 10 and 25 years there are major changes in synaptic receptors and density as well as myelination of frontal cortical areas important for impulse control, goal setting, motivation, interpersonal interactions, reasoning, assessment of rewards and punishments in evaluating actions and other complex brain functions. Studies support the possibility that alterations in adolescent synaptic remodeling create entrenchment of committed connections through altered pruning and focus of cortical networks that underlie adult behavior repertoires. Heavy alcohol consumption during adolescence disrupts cortical development altering higher executive functions in a manner that promotes continued impulsive behavior, alcohol abuse and risk of alcohol dependence.
Acknowledgements
This research is supported by NIAAA. We would also like to thank Melissa Mann for manuscript preparation.
References
- Adriani W, Granstrem O, Macri S, Izykenova G, Dambinova S, Laviola G. Behavioral and neurochemical vulnerability during adolescence in mice: studies with nicotine. Neuropsychopharmacology 2004;29:869–78. [DOI] [PubMed] [Google Scholar]
- Adriani W, Giannakopoulou D, Bokulic Z, Jernej B, Alleva E, Laviola G. Response to novelty, social and self-control behaviors, in rats exposed to neonatal anoxia: modulatory effects of an enriched environment. Psychopharmacology (Berl) 2006;184:155–65. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology 2004;29:1988–93. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse 2000;37:167–9. [DOI] [PubMed] [Google Scholar]
- Andrucci GL, Archer RP, Pancoast DL, Gordon RA. The relationship of MMPI and Sensation Seeking Scales to adolescent drug use. J Pers Assess 1989;53:253–66. [DOI] [PubMed] [Google Scholar]
- Arnett J Reckless behavior in adolescence: a developmental perspective. Dev Rev 1992;12:339–73. [Google Scholar]
- Bar-Peled O, Gross-Isseroff R, Ben-Hur H, Hoskins I, Groner Y, Biegon A. Fetal human brain exhibits a prenatal peak in the density of serotonin 5-HT1A receptors. Neurosci Lett 1991;127:173–6. [DOI] [PubMed] [Google Scholar]
- Baumrind D A developmental perspective on adolescent rick taking in contemporary America. In: Irwin CE Jr, editor. Adolescent social behavior and health. San Francisco, CA: Jossey-Bass; 1987. p. 93–125. [DOI] [PubMed] [Google Scholar]
- Bender RA, Lauterborn JC, Gall CM, Cariaga W, Baram TZ. Enhanced CREB phosphorylation in immature dentate gyrus granule cells precedes neurotrophin expression and indicates a specific role of CREB in granule cell differentiation. Eur J Neurosci 2001;13:679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt TJ. Developmental changes in conformity to peers and parents. Dev Psychol 1979;15:608–16. [Google Scholar]
- Biglan A, Metzler CW, Wirt R, Ary D, Noell J, Ochs L, et al. Social and behavioral factors associated with high-risk sexual behavior among adolescents. J Behav Med 1990;13:245–61. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry 2006;47:296–312. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Kelly ME, Coulter DA. gamma-Aminobutyric acid(A) receptor subunit expression predicts functional changes in hippocampal dentate granule cells during postnatal development. J Neurochem 2001;77:1266–78. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Eastwood SL, Harrison PJ. Detection and quantitation of 5-HT1A and 5-HT2A receptor mRNAs in human hippocampus using a reverse transcriptase-polymerase chain reaction (RT-PCR) technique and their correlation with binding site densities and age. Neurosci Lett 1994;178:85–9. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 1994;61:203–9. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci 2005;28:436–45. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Cohen JD, et al. A pediatric functional MRI study of prefrontal activation during performance of a Go-No-Go task. J Cogn Neurosci 1997;9:835–47. [DOI] [PubMed] [Google Scholar]
- CESAR. Despite declines in early initiation rates, many U.S. High School students still drink or smoke before age 13. Cesar Fax, vol. 15; 2006. [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry 2003;160:1041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Kellogg CK. Norepinephrine utilization in the hypothalamus of the male rat during adolescent development. Dev Neurosci 1992;14:369–76. [DOI] [PubMed] [Google Scholar]
- Choi S, Weisberg SN, Kellogg CK. Control of endogenous norepinephrine release in the hypothalamus of male rats changes over adolescent development. Brain Res Dev Brain Res 1997;98:134–41. [DOI] [PubMed] [Google Scholar]
- Crews F, JG R, Chandler LJ. Glutamate and alcohol-induced neurotoxicity. In: Herman BH, Frankenheim J, Litten R, Sheridan PH, Weight FF, Zukin SR, editors. Glutamate and addiction. Humana Press; 2002. p. 357–73. [Google Scholar]
- Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EJ, et al. Alcohol-induced neurodegeneration: when, where and why? Alcohol Clin Exp Res 2004;28:350–64. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience 2006;137:437–45. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, Prescott S. The ecology of adolescent activity and experience. J Youth Adolesc 1977;6:284–94. [DOI] [PubMed] [Google Scholar]
- Daval G, Verge D, Basbaum AI, Bourgoin S, Hamon M. Autoradiographic evidence of serotonin1 binding sites on primary afferent fibres in the dorsal horn of the rat spinal cord. Neurosci Lett 1987;83:71–6. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res 2005;29:1590–600. [DOI] [PubMed] [Google Scholar]
- del Olmo E, Lopez-Gimenez JF, Vilaro MT, Mengod G, Palacios JM, Pazos A. Early localization of mRNA coding for 5-HT1A receptors in human brain during development. Brain Res Mol Brain Res 1998;60:123–6. [DOI] [PubMed] [Google Scholar]
- Depue RA, Spoont MR. Conceptualizing a serotonin trait. A behavioral dimension of constraint. Ann N Y Acad Sci 1986;487:47–62. [DOI] [PubMed] [Google Scholar]
- Dillon KA, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain Res 1991;554:56–64. [DOI] [PubMed] [Google Scholar]
- Dinopoulos A, Dori I, Parnavelas JG. The serotonin innervation of the basal forebrain shows a transient phase during development. Brain Res Dev Brain Res 1997;99:38–52. [DOI] [PubMed] [Google Scholar]
- Dori IE, Dinopoulos A, Parnavelas JG. The development of the synaptic organization of the serotonergic system differs in brain areas with different functions. Exp Neurol 1998;154:113–25. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature 2004;427: 311–2. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci 2006;26:6314–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Gentry J, Malcolm R, Ripley TL, Borlikova G, Stephens DN, et al. Consequences of multiple withdrawals from alcohol. Alcohol Clin Exp Res 2004;28:233–46. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry 2000;48:732–9. [DOI] [PubMed] [Google Scholar]
- Dyck RH, Cynader MS. Autoradiographic localization of serotonin receptor subtypes in cat visual cortex: transient regional, laminar, and columnar distributions during postnatal development. J Neurosci 1993;13:4316–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehardt C, Bernstein I. Patterns of affiliation among immature rhesus monkeys (Macaca mulatta). Am J Primatol 1987;13:255–69. [DOI] [PubMed] [Google Scholar]
- Fagen R Exercise, play, and physical training in animals. Perspect Ethol 1976;2:189–219. [Google Scholar]
- Fassino M, Campbell B. The ontogeny of play in rats. Paper presented at the meeting of the Eastern Psychological Association, New York, NY; 1981. [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, et al. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet 2006;16:9–17. [DOI] [PubMed] [Google Scholar]
- Frantz K, Van Hartesveldt C. The locomotor effects of MK801 in the nucleus accumbens of developing and adult rats. Eur J Pharmacol 1999a;368: 125–35. [DOI] [PubMed] [Google Scholar]
- Frantz K, Van Hartesveldt C. Locomotion elicited by MK801 in developing and adult rats: temporal, environmental, and gender effects. Eur J Pharmacol 1999b;369:145–57. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science 2000;287:1433–8. [DOI] [PubMed] [Google Scholar]
- Galef JB. Mechanisms for the social transmission of food preferences from adult to weaning rats. In: Barker LM, Best M, Domjan M, editors. Learning mechanisms in food selection. Baylor University Press; 1977. p. 123–48. [Google Scholar]
- Geller B, Badner JA, Tillman R, Christian SL, Bolhofner K, Cook EH Jr. Linkage disequilibrium of the brain-derived neurotrophic factor Val66Met polymorphism in children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry 2004;161:1698–700. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci 2004;1021:77–85. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999;2:861–3. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 2004;101:8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek H, Kutcher S. Feeling states during adolescence. Psychiatr Clin North Am 1990;13:443–54. [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci 1996;16:3274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E Serotonin and hippocampal neurogenesis. Neuropsychopharmacology 1999;21:46S–51S. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolf NJ, Butcher LL. Postnatal development of cholinergic neurons in the rat: I. Forebrain. Brain Res Bull 1991;27:767–89. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSMIV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse 1998;10:163–73. [DOI] [PubMed] [Google Scholar]
- Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res 2005;156:105–14. [DOI] [PubMed] [Google Scholar]
- Guilarte T The N-methyl-D-aspartate receptor: physiology and neurotoxicology in the developing brain. In: Slikker W Jr, Chang LW, editors. Handbook of developmental neurotoxicology. San Diego, CA: Academic Press; 1998. p. 285–304. [Google Scholar]
- Hachiya Y, Takashima S. Development of GABAergic neurons and their transporter in human temporal cortex. Pediatr Neurol 2001;25:390–6. [DOI] [PubMed] [Google Scholar]
- He J, Crews F. Neurogenesis Decreases during Brain Maturation from Adolescence to Adulthood. Pharmacol Biochem Behav in press. [DOI] [PubMed] [Google Scholar]
- Hedner J, Lundell KH, Breese GR, Mueller RA, Hedner T. Developmental variations in CSF monoamine metabolites during childhood. Biol Neonate 1986;49:190–7. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci 2005;6:877–88. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic 1984;88:488–96. [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain-I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience 1990;35:31–43. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry 2000;5:262–9. [DOI] [PubMed] [Google Scholar]
- Johnson JS, Newport EL. Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cognit Psychol 1989;21:60–99. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future, national survey results on drug use, 1975–2004. NIH Pub No 05-5727 1 secondary school students; 2004. [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol 1988;269:58–72. [DOI] [PubMed] [Google Scholar]
- Keating D Cognitive and brain development. Handbook of adolescent psychology. 2nd ed.; 2004. p. 45–84. [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci 2004;1021:27–32. [DOI] [PubMed] [Google Scholar]
- Kellogg CK. Early developmental modulation of GABAA receptor function. Influence on adaptive responses. Perspect Dev Neurobiol 1998;5:219–34. [PubMed] [Google Scholar]
- Kellogg CK, Taylor MK, Rodriguez-Zafra M, Pleger GL. Altered stressor-induced changes in GABAA receptor function in the cerebral cortex of adult rats exposed in utero to diazepam. Pharmacol Biochem Behav 1993;44:267–73. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Awatramani GB, Piekut DT. Adolescent development alters stressor-induced Fos immunoreactivity in rat brain. Neuroscience 1998;83:681–9. [DOI] [PubMed] [Google Scholar]
- Kempermann G Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci 2002;22:635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I Structural and histochemical reorganization of the human prefrontal cortex during perinatal and postnatal life. Prog Brain Res 1990;85:223–39 [discussion 239–240]. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci 1990;600:297–313 [discussion 314]. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, Raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J Comp Neurol 1974;155:469–81. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev 1991;71:155–234. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann N Y Acad Sci 2004;1021:64–76. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Blendy JA. Antidepressant action: to the nucleus and beyond. Trends Pharmacol Sci 2005;26:631–8. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 2003;28:1562–71. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000;20:9104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Kandel ER. Cell adhesion molecules, CREB, and the formation of new synaptic connections. Neuron 1996;17:567–70. [DOI] [PubMed] [Google Scholar]
- Medina AE, Ramoa AS. Early alcohol exposure impairs ocular dominance plasticity throughout the critical period. Brain Res Dev Brain Res 2005;157:107–11. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Coppola DM, Ramoa AS. Neonatal alcohol exposure induces long-lasting impairment of visual cortical plasticity in ferrets. J Neurosci 2003;23:10002–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Ramoa AS. Early alcohol exposure induces persistent alteration of cortical columnar organization and reduced orientation selectivity in the visual cortex. J Neurophysiol 2005;93:1317–25. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Effect of chronic administration of ethanol on GABAA receptor assemblies derived from alpha2-, alpha3-, beta2- and gamma2-subunits in the rat cerebral cortex. Brain Res 2005;1031:134–7. [DOI] [PubMed] [Google Scholar]
- Merrick J, Kandel I, Birnbaum L, Hyam E, Press J, Morad M. Adolescent injury risk behavior. Int J Adolesc Med Health 2004;16:207–13. [DOI] [PubMed] [Google Scholar]
- Mohler H, Crestani F, Rudolph U. GABA(A)-receptor subtypes: a new pharmacology. Curr Opin Pharmacol 2001;1:22–5. [DOI] [PubMed] [Google Scholar]
- Monti PM, Miranda R Jr, Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, et al. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res 2005;29:207–20. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Ciaranello RD. Ontogeny of 5-hydroxytryptamine2 receptor immunoreactivity in the developing rat brain. Neuroscience 1993;55:869–80. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Casey BJ, Diamond A. Developmental cognitive neuroscience: progress and potential. Trends Cogn Sci 2004;8:122–8. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem 2002;83:1087–93. [DOI] [PubMed] [Google Scholar]
- Nurse S, Lacaille JC. Late maturation of GABA(B) synaptic transmission in area CA1 of the rat hippocampus. Neuropharmacology 1999;38:1733–42. [DOI] [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol Biochem Behav 2002;72:521–32. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Developmental plasticity of mouse visual acuity. Eur J Neurosci 2003;17:167–73. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiatry 1994;36:272–7. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res 1996;78:57–65. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003;301:805–9. [DOI] [PubMed] [Google Scholar]
- Schramm NL, Egli RE, Winder DG. LTP in the mouse nucleus accumbens is developmentally regulated. Synapse 2002;45:213–9. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature 2006;440:676–9. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature 2001;410:372–6. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci 1998;21:127–48. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res 1998;22:670–6. [DOI] [PubMed] [Google Scholar]
- Smith P Does play matter? Functional and evolutionary aspects of animal and human play. Behav Brain Sci 1982;5:139–84. [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage 1999;9:587–97. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc 2001;7:312–22. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci 2004;24:8223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 2000;24:417–63. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, McElligot S, Lu L, McGonigle P. Ontogeny of dopamine D3 receptors in the nucleus accumbens of the rat. Neurosci Lett 1997;223:13–6. [DOI] [PubMed] [Google Scholar]
- Steinberg L Pubertal maturation and parent–adolescent distance: an evolutionary perspective. Advances in adolescent behavior and development. Sage Publications; 1989. p. 71–97. [Google Scholar]
- Strauss J, Barr CL, George CJ, King N, Shaikh S, Devlin B, et al. Association study of brain-derived neurotrophic factor in adults with a history of childhood onset mood disorder. Am J Med Genet B Neuropsychiatr Genet 2004;131:16–9. [DOI] [PubMed] [Google Scholar]
- Swann JW, Pierson MG, Smith KL, Lee CL. Developmental neuroplasticity: roles in early life seizures and chronic epilepsy. Adv Neurol 1999;79:203–16. [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry 2002;41:1231–8. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol 2001;437:496–504. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D (1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci 2000;18: 29–37. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparison with D2-like receptors. Brain Res Dev Brain Res 1998a;110:227–33. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neurosci Lett 1998b;254:21–4. [DOI] [PubMed] [Google Scholar]
- Teicher MH. Limbic serotonin turnover plunges during puberty. Poster presented at the meeting of the Society for Neuroscience, Miami Beach, FL; 1999. [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC Jr. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res 1995;89:167–72. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci 2001;4:1217–23. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci 2006;29:148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano CD, McGlothan JL, Guilarte TR. Lead exposure alters cyclic-AMP response element binding protein phosphorylation and binding activity in the developing rat brain. Brain Res Dev Brain Res 2003;145:219–28. [DOI] [PubMed] [Google Scholar]
- Toth G, Fekete M. 5-Hydroxyindole acetic excretion in newborns, infants and children. Acta Paediatr Hung 1986;27:221–6. [PubMed] [Google Scholar]
- Townshend JM, Duka T. Mixed emotions: alcoholics’ impairments in the recognition of specific emotional facial expressions. Neuropsychologia 2003;41:773–82. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 2001;411:583–7. [DOI] [PubMed] [Google Scholar]
- van Eden CG, Kros JM, Uylings HB. The development of the rat prefrontal cortex. Its size and development of connections with thalamus, spinal cord and other cortical areas. Prog Brain Res 1990;85:169–83. [DOI] [PubMed] [Google Scholar]
- Vizuete ML, Venero JL, Traiffort E, Vargas C, Machado A, Cano J. Expression of 5-HT7 receptor mRNA in rat brain during postnatal development. Neurosci Lett 1997;227:53–6. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Dev Psychopathol 2001;13:721–32. [DOI] [PubMed] [Google Scholar]
- Waters NS, Klintsova AY, Foster TC. Insensitivity of the hippocampus to environmental stimulation during postnatal development. J Neurosci 1997;17:7967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Dowdall GW, Davenport A, Castillo S. Correlates of college student binge drinking. Am J Public Health 1995;85:921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. Psychopharmacology (Berl) 2003;165:306–12. [DOI] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger’s theory. J Subst Abuse 1994;6:1–20. [DOI] [PubMed] [Google Scholar]
- Wilson M, Daly M. Competiveness, risk taking, and violence: the young male syndrome. Ethol Sociobiol 1985:59–73. [Google Scholar]
- Yates T Theories of cognitive development. In: Lewis M, editor. Child and adolescent psychiatry. Baltimore: Williams and Wilkins; 1996. p. 134–55. [Google Scholar]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res 2006;1099:73–81. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res 1989;50:11–32. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol 2006;66:578–90. [DOI] [PubMed] [Google Scholar]
- Zhu WJ, Roper SN. Brain-derived neurotrophic factor enhances fast excitatory synaptic transmission in human epileptic dentate gyrus. Ann Neurol 2001;50:188–94. [DOI] [PubMed] [Google Scholar]
- Zou J, Crews F. CREB and NFkB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cell Mol Neurobiol 2006;26:383–403. [DOI] [PMC free article] [PubMed] [Google Scholar]


