Abstract
Coastal estuaries globally, including the San Francisco Estuary (SFE), are experiencing significant degradation, often resulting in fisheries collapses. The SFE has undergone profound modifications due to population growth, industrialization, urbanization and increasing water exports for human use. These changes have significantly altered the aquatic ecosystem, favouring invasive species and becoming less hospitable to native species such as the longfin smelt (Spirinchus thaleichthys). With longfin smelt abundance declining to <1% of historical numbers, there is a pressing need for laboratory-based experiments aimed at investigating the effects of varying environmental conditions on their stress response and physiology. This study explored the impact of temperature (11 and 14°C) and turbidity maintained with algae (1, 4 and 11 nephelometric turbidity units (NTU)) on the physiological condition of juvenile longfin smelt. Fish were sampled after 2 and 4 weeks in experimental conditions and analysed for whole-body cortisol, glucose, lactate and protein. Condition factor was calculated using length and weight measurements. Critical thermal maximum trials were conducted to assess how prior rearing conditions affected upper thermal tolerance. Cortisol levels were significantly higher in fish held in low-turbidity conditions, whilst glucose levels were significantly greater at lower temperatures and higher turbidities. Protein-to-mass ratios were significantly greater in higher turbidity conditions, with a significant interaction between temperature and turbidity further influencing these ratios. Moreover, 14°C led to diminished condition factors but increased upper thermal tolerances (26.3 ± 0.05 vs 24.6 ± 0.18) compared to longfin smelt at 11°C, highlighting a potential trade-off between the induction of defense mechanisms and subsequent reductions in energy and growth. Data suggest that cooler temperatures (11°C) and elevated turbidities (11 NTU) can benefit juvenile longfin smelt by reducing stress and enhancing growth and energy. These findings hold significant implications for informing and optimizing future endeavours in the culturing and conservation of this species.
Keywords: stress physiology, San Francisco Estuary, longfin smelt, climate change
Graphical Abstract
Graphical Abstract.
1. Introduction
The global issue of fisheries collapses is escalating in both frequency and severity, particularly in coastal regions (Jackson et al., 2001; Worm et al., 2006). The San Francisco Estuary (SFE), the largest estuary on the US Pacific coast, known for its diverse habitats and rich biodiversity, exemplifies this troubling trend (Myers et al., 2000; Healey et al., 2016). Significant alterations within the SFE began in the 19th century during California’s Gold Rush, rendering it one of the most modified and controlled estuaries in the world (Nichols et al., 1986). Continued population growth, urbanization and increased water demands prompted extensive infrastructure, channelization and dredging (Moyle et al., 2010). These modifications irreversibly transformed the SFE, removing 95% of historical wetlands, disrupting salinity gradients, decreasing turbidity and fostering a pronounced increase in invasive species (Cohen and Carlton, 1998; Kimmerer, 2004; Cloern and Jassby, 2012). For decades, state and federal agencies have collaborated under the Interagency Ecological Program (IEP) to conduct continuous fish population monitoring in the SFE, resulting in one of the longest and most comprehensive fish abundance datasets globally (Honey et al., 2004). Beginning in 2000, declines in four major fisheries within the estuary, including delta smelt (Hypomesus transpacificus) and longfin smelt (Spirinchus thaleichthys), were observed by the IEP (Baxter et al., 2008). The term ‘Pelagic Organism Decline’ (POD) was coined in 2004 to describe the escalating population declines as delta smelt and longfin smelt reached unprecedented low abundances (Sommer et al., 2007). This POD has been attributed to multiple interacting stressors, adding complexity to conservation efforts aimed at protecting these fisheries from the threat of extinction.
The SFE is home to a genetically distinct population of longfin smelt, a planktivorous forage fish native to coastal and estuarine waters in the Northeastern Pacific and distributed from SFE to the Aleutian Islands in Alaska (Garwood, 2017; Sağlam et al., 2021). Longfin smelt are semelparous, typically living for ~2 years (Moyle, 2002). Exhibiting a semi-anadromous life history, longfin smelt inhabit the coastal Pacific Ocean and migrate to low-salinity tidal habitats in the upper estuary to spawn during the winter months (Moyle, 2002; Rosenfield and Baxter, 2007). Once one of California’s most abundant fish species, longfin smelt supported a small commercial fishery prior to the 1970s (Skinner, 1962; Lewis et al., 2020). However, over the past few decades, the longfin smelt population in the SFE has plummeted to <1% of pre-1980 levels, leading to its classification as ‘threatened’ under the California Endangered Species Act in 2009 (CDFG, 2009; Nobriga and Rosenfield, 2016). These population declines culminated in a near-complete collapse in 2015 when only three longfin smelt were captured in the Fall Midwater Trawl (FMWT) in December, during their peak spawning period (Hobbs et al., 2017). This decline is particularly worrisome given that the longfin smelt population in the SFE likely serves as a source of genetic diversity for adjacent populations (Sağlam et al., 2021).
Declines in longfin smelt populations, along with other Osmeridae species, are believed to result from a combination of interacting factors. These include habitat degradation, changing environmental conditions such as elevated temperatures, reduced turbidity, and decreased freshwater outflows, increased water pollution, and alterations to the food web due to invasive species introductions (Kimmerer, 2002; Brown et al., 2016; Fong et al., 2016; Hammock et al., 2019). In response to significant reductions in longfin smelt abundance, the University of California Davis has undertaken efforts to establish a captive culture program aimed at advancing research and conservation initiatives for this species. Protocols for longfin smelt rearing and maintenance are modelled after methodologies developed for delta smelt (Baskerville-Bridges et al., 2005; Lindberg et al., 2013). However, longfin and delta smelt, whilst similar, have distinct differences that necessitate further study for the development of an optimal rearing program tailored specifically to the unique requirements of longfin smelt (Aghbolaghi et al., 2024). They exhibit different life histories, such that the delta smelt exclusively inhabits and completes its life cycle in freshwater and low-salinity habitats, whereas only young longfin smelt rear in these conditions. Juvenile longfin smelt migrate to marine habitats in the San Francisco Bay and coastal Pacific Ocean and return to the upper SFE for spawning as adults (Yanagitsuru et al., 2021). Furthermore, longfin smelt exhibit a lower upper thermal tolerance compared to delta smelt, potentially limiting their suitable habitat in natural environments (Jeffries et al., 2016).
As aquatic ectotherms, fish conform to ambient water temperatures, rendering them highly susceptible to sudden and substantial temperature fluctuations (Beitinger et al., 2000). Increases in temperatures due to climate change induce multi-faceted and complex consequences in fish, leading to alterations across all levels of biological organization, ranging from cellular- to ecosystem-level effects, ultimately impacting distribution and survival (Perry et al., 2005; Graham and Harrod, 2009). Over the last 50 years, water temperature in the upper SFE has increased by an average of 0.017°C per year (Bashevkin et al., 2022). As a migratory species, longfin smelt are particularly vulnerable to the impacts of climate change and increased temperatures (Robinson et al., 2009). Their reliance on suitable habitat across multiple locations and life stages makes them highly sensitive to environmental shifts. Elevated temperatures can significantly influence crucial life history events, including the timing and challenges associated with seaward migration (Taylor, 2008). Despite considerable research into the upper thermal tolerance and physiological response of early life stages of longfin smelt to increased temperature, there remains a data gap regarding later life stages, particularly the juvenile stage (Jeffries et al., 2016; Yanagitsuru et al., 2021, 2024). Physiological lab-based experiments are essential for predicting the sensitivity of longfin smelt to climate change, by providing insights into their response to temperature change at later life stages as well as their capacity to adjust thermal tolerance through acclimation. As temperatures continue to rise and anomalous climatic events continue to intensify, this knowledge will be invaluable for implementing cost-effective and proactive conservation management actions (Cooke et al., 2022).
Turbidity, defined as the quantification of light scattered and absorbed by suspended particles within the water column, can have significant impacts on fish health and abundance (Kirk, 1985). Factors, such as water depth, light intensity and the physical characteristics of suspended materials, collectively influence the level of turbidity in a body of water (Davies-Colley and Smith, 2001; Davies-Colley and Nagels, 2008). Turbidity levels can naturally increase through processes like soil erosion and sediment transport following precipitation events, leading to suspended sediment or algal growth driven by increased nutrient availability (Henley et al., 2000; Kang et al., 2013). Anthropogenic activities, such as deforestation, mining and urbanization can lead to sudden increases in turbidity by accelerating sediment transport (Rodrigues et al., 2023). The source of turbidity can cause varied physiological effects on fish. For example, suspended sediments may interfere with gill function, causing epithelial damage and increased mucus production, which could impair respiratory efficiency over time (Sutherland and Meyer, 2007). Elevated turbidity can also negatively impact fish by causing diminished visual acuity, reduced feeding and growth, limited mobility and altered diets and habitats (Gardner, 1981; Hecht and Van der Lingen, 1992; Ortega et al., 2020; Rodrigues et al., 2023). Conversely, low turbidity levels may induce heightened stress, increased predation and reduced feeding and growth (Boehlert and Morgan, 1985; Rieger and Summerfelt, 1997; Pasparakis et al., 2023). Turbidity’s effects on fish are complex and diverse, depending on factors such as life history traits, life stages, feeding strategies, physiologies and interacting environmental variables (Utne-Palm, 2002; Hasenbein et al., 2016; Rodrigues et al., 2023). Consequently, many fish species exhibit an optimal turbidity range, above or below which they begin to encounter adverse consequences.
Turbidity levels in the SFE and Delta vary significantly across different locations, with values ranging from 1 to 220 nephelometric turbidity units (NTU), with peaks often associated with storm events (Werner et al., 2010). The SFE has experienced a notable decrease in turbidity over recent decades, with a 36% reduction in suspended sediment observed between water years 1991–98 and 1999–2007, attributed in part to the depletion of erodible sediment from hydraulic mining practices and sediment entrapment in reservoirs and dams (Wright and Schoellhamer, 2004; Schoellhamer, 2011). This decrease in turbidity is considered one of several contributing factors to the POD, with longfin smelt populations exhibiting a notable decline in abundance in the SFE when turbidity levels drop to lower values (Sommer et al., 2007; Grimaldo et al., 2017). Turbidity in the SFE is composed of a dynamic combination of suspended particles, including sediments, organic matter and algae. Although our treatments do not replicate the full range of environmental combinations, they align with conditions used for culturing and rearing smelt. In this study, turbidity was maintained using Nannochloropsis algae, as outlined in established protocols (Tigan et al., 2020; Hung et al., 2024).
The interactive effects of multiple stressors, such as temperature and turbidity, can have significant implications for fish physiology and overall health. For example, a recent study on adult pugnose shiner (Miniellus anogenus) found that fish exposed to both warmer and more turbid water exhibited a lower critical thermal maximum (CTMax) and reduced thermal safety margin when compared to those held in warmer, clear water, indicating that turbidity can exacerbate thermal stress responses (Fortin-Hamel and Chapman, 2024). Similarly, research from our group on juvenile delta smelt demonstrated that both elevated temperatures and lower turbidity levels led to diminished whole-body free glucose, with the highest available energy observed in fish reared under cooler temperatures and higher turbidity (Pasparakis et al., 2023). These findings highlight the need to consider the combined effects of multiple, ecologically relevant stressors when evaluating fish species’ physiological resilience, growth and survival in rapidly changing environments.
The primary aim of this study was to investigate how varying environmental conditions affect the physiology and upper thermal tolerance of juvenile longfin smelt, aiming to provide insights for aquaculture and future population supplementation practices. Juvenile longfin smelt were reared at fixed temperatures of 11 and 14°C, and under three turbidity levels (1, 4 and 11 NTU) for a period of 5 weeks. Our first objective was to evaluate how varying temperature and turbidity conditions impacted the stress response, physiological condition and growth of juvenile longfin smelt. Fish were sampled after two and four weeks in treatment conditions, with subsequent measurements of whole-body cortisol, glucose, lactate, protein-to-mass ratios and condition factor to assess overall health and growth. Our second objective was to determine the ability of longfin smelt to increase their upper thermal tolerance through acclimation to warmer water, using CTMax trials conducted after five weeks in treatment conditions. Given their natural preference for cooler waters and higher turbidities, we predicted that longfin smelt would show reduced stress and increased growth and energy at 11°C and 11 NTU. Additionally, we anticipated that rearing in warmer water would increase CTMax through thermal acclimation. Considering the limited research on the juvenile stage in longfin smelt, the data from this study addresses critical knowledge gaps and has the potential to significantly contribute to the development of effective conservation strategies for this endangered species.
2. Materials and Methods
2.1. Study species and maintenance
Longfin smelt (49.4 ± 0.3 mm fork length, 0.68 ± 0.01 g) were acquired from the University of California Davis Fish Conservation and Culture Laboratory (FCCL) located in Byron, CA. Methodologies employed for rearing and spawning longfin smelt are similar to those utilized for delta smelt and can be found in Lindberg et al. (2013), Tigan et al. (2020), and Hung et al. (2021). Juvenile longfin smelt (~170 days post-hatch (dph)) were transferred from FCCL to UC Davis Putah Creek Aquaculture Facility (PCF) in early July 2020. Upon arrival, fish were immediately introduced into 24 15-gallon black polyethylene tubs (hereafter, sub-tanks), with 30 fish per sub-tank. The PCF consists of a recirculating aquaculture system with 8 400-l tanks (hereafter, holding tanks) that serve as experimental water baths and are maintained by external temperature control units. Each holding tank contained three sub-tanks (Supp. Fig. 1). To optimize holding conditions and maintain turbidity and salinity, sub-tanks received running flow-through treatment water from outdoor reservoir tanks via the recirculating system. Excess water overflowed through a 1-inch hole in sub-tanks covered in mesh to prevent fish from escaping. In addition, each sub-tank included its own external biofiltration unit, where sub-tank water was channeled through the unit filled with k1 biomedia (Evolution Aqua Ltd) and returned via an airlift mechanism. The airlift mechanism also ensured the maintenance of sufficient dissolved oxygen.
Figure 1.
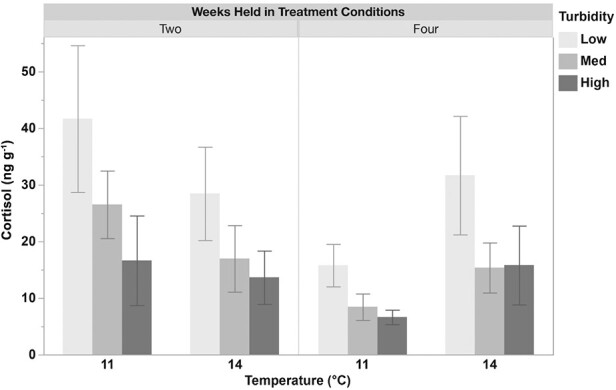
Whole-body cortisol measurements (ng g−1) in juvenile longfin smelt held at two temperatures (11 or 14°C) and three turbidities (low (1), med (4) or high (11) NTU) for two and four weeks. Cortisol levels were significantly greater in fish exposed to lower turbidity conditions (1 NTU) compared to high turbidity (11 NTU) and decreased significantly over time. No significant effect of temperature was observed. Data (n = 13–20) are presented as mean ± SEM.
Longfin smelt were acclimated to ambient conditions of 12.5°C, 7 psu and 1.4 NTU for two weeks prior to the start of experimentation. Due to known light sensitivities of longfin smelt, care was taken to ensure low-light conditions throughout the acclimation period. Mesh lids were placed over sub-tanks, and additional lids made from thick light-blocking Styrofoam covered holding tanks. Lights in the building were kept off at all times. The only light in the room came from a small window on the top of the ceiling under a natural photoperiod. Fish were fed twice daily to satiation with freshly hatched Artemia franciscana (Argent Chemical Laboratories, WA).
To monitor water quality, temperature (°C), dissolved oxygen (mg O2/l) and salinity (psu) were measured daily using a handheld YSI 556 MPS meter (YSI Inc., Yellow Springs, OH). Ammonia, nitrite and nitrate concentrations, as well as pH, were measured biweekly. pH was measured using a pinpoint pH monitor (American Marine Inc., Ridgefield, CT) or commercial pH strips. Ammonia concentrations were determined only in control groups after the acclimation period, as the colour of Nannochloropsis algae used to make treatment turbidities interfered with the measurement method. Ammonia measurements were conducted using a Hach pocket colorimeter (Hach Company, Loveland, CO), whilst a marine care multi-test kit (Red Sea, Houston, TX) was utilized for ammonia, nitrite and nitrate measurements. Mortality was quantified daily, with any dead fish immediately removed from sub-tanks. All handling, care and experimental procedures used were reviewed and approved by the UC Davis Institutional Animal Care and Use Committee (IACUC Protocol #16591).
2.2. Temperature and turbidity treatments
Juvenile longfin smelt were held at one of two temperatures (11 and 14°C) and one of three turbidities (1, 4 and 11 NTU) for a duration of five weeks. There were four holding tanks per experimental temperature, and within each holding tank, three sub-tanks were randomly assigned to each turbidity level. This set-up resulted in four replicates per treatment (Supp. Fig. 1). Prior to the start of the experimental period, acclimation temperatures and turbidities were gradually transitioned to treatment levels over a three-day period, with increments of 0.5°C and 3 NTU per day. Each of the 12 Styrofoam lids covering the holding tanks contained embedded LED light bulbs. Light fixtures were activated (12:12 photoperiod) at the beginning of the experimental period and once the desired temperature and turbidity treatments were achieved. Light intensity within the sub-tanks was measured using a portable digital light meter (LX1330B; Dr meter) and ranged from 7 to 120 lux, indicating the potential for fish to behaviourally avoid light by favouring areas with lower light conditions. Temperatures were maintained using a recirculating water system, with each row of holding tanks connected to a separate chiller/heater. Nannochloropsis algae (Nanno 3600—High-yield grow-out feed; Reed Mariculture Inc., USA) were added to individual sub-tanks to achieve the desired turbidity levels. This is the same algal suspension used by the FCCL to rear larval longfin and delta smelt in increased turbidities (Tigan et al., 2020; Hung et al., 2024). To maintain treatment turbidity throughout the experimental period, sub-tanks were connected to three outdoor reservoir tanks held at 1, 4 and 11 NTU, respectively. These turbidity levels were selected for their ecological relevance in the SFE and for their alignment with rearing conditions (Werner et al., 2010; Tigan et al., 2020). Continuous flow of fresh algae-spiked water was ensured by connecting the reservoir tanks to individual sub-tanks via PFA standard tubing (inner diameter = 0.5 cm) connected to a standpipe located in the middle of each sub-tank. Salinity (7 psu) was maintained in a similar manner, by adding Instant Ocean (Aquarium Systems, Mentor, OH) to reservoir tanks every 2–3 days, ensuring that sub-tanks received fresh saline water via the same PFA standard tubing. Turbidity was measured daily using a Hach 2100q portable turbidimeter (Hach Company, Loveland, CO). A nephelometric turbidimeter is a standard instrument employed for turbidity measurement, determining scattered light at a 90-degree angle from the incident light beam through a water sample (Henley et al., 2000; Rohan et al., 2021). These findings are expressed in nephelometric turbidity units (NTU). In addition to daily temperature measurements, eight HOBO temperature loggers (Onset Computer Corporation, Bourne, MA) were swapped between sub-tanks every couple of days and recorded temperatures every 15 min. Temperature and turbidity conditions during acclimation and experimental periods are reported as mean ± standard error of the mean (SEM) in Supplementary Tables 1 & 2.
2.3. Sampling
Sampling occurred at two and four weeks following exposure of juvenile longfin smelt to their respective treatments. Prior to sampling, longfin smelt were fasted for 24 h. Individual fish were netted from their respective sub-tanks and immediately euthanized with an overdose of tricaine methanesulfonate (MS-222; Finquel) buffered to a neutral pH with sodium bicarbonate. Longfin smelt were weighed (g), measured for fork length (mm), and snap-frozen in liquid nitrogen for later analysis of whole-body cortisol, glucose, lactate and protein. The entire sampling process for all six fish from each treatment was completed in <3 min to ensure handling stress did not affect cortisol levels. Care was taken to catch fish as efficiently and quietly as possible to minimize disturbance to surrounding sub-tanks.
2.4. Upper thermal tolerance trials
The upper thermal tolerance of juvenile longfin smelt reared in different temperatures and turbidities was determined using critical thermal maximum (CTMax) methodology as previously described in Lapointe et al. (2018) and Davis et al. (2019). CTMax trials were conducted over a consecutive three-day period on the remaining 132 fish, resulting in 69–93 fish per treatment. Fish were randomly netted from sub-tanks and transferred to one of 12 glass mason jars, which were painted black on the outside to reduce visual stress. Mason jars contained 900 ml of treatment water, were held in a water table for temperature control and contained individual air stones to ensure adequate oxygen supply throughout trials. Longfin smelt were given 30 min in individual mason jars at their respective treatment temperatures to recover from handling stress before the start of trials. Temperature was increased at a rate of 0.3°C per min using four Finnex submersible heaters and water pumps for circulation. Loss of equilibrium (LOE), a common endpoint to assess upper thermal tolerance, was determined by reporting the temperature at which fish first lost equilibrium continuously for 10 s (Beitinger et al., 2000; Komoroske et al., 2014). Temperature at LOE was measured using a calibrated immersion thermometer, and fish were immediately transferred to recovery tanks at their respective rearing temperatures. In general, recovery for CTMax trials is determined after 24 h (Davis et al., 2019); however, due to the extreme sensitivities of longfin smelt, recovery time was reduced in our trials. We opted to assess the survival of fish at three different recovery times: 3, 6 and 18 h post-trial. This approach allowed us to investigate whether acclimation temperature also influenced the recovery potential from heat stress.
2.5. Whole-body homogenization
A total of 232 longfin smelt (four replicates per treatment and four to six fish per replicate) were sampled for whole-body cortisol, glucose, lactate and total protein analysis. The head of each frozen fish was removed using a sterile razor blade and the remaining body was ground to a fine powder, using a mortar and pestle over liquid nitrogen. Whole-body fish powder was weighed prior to homogenization. Using a handheld homogenizer (PRO Scientific, Oxford, CT), each sample was homogenized in 4 ml ice-cold 1 × phosphate-buffered saline (PBS buffer: 137 mM sodium chloride, 2.7 mM potassium chloride, 10 mM disodium phosphate and 1.8 mM monopotassium phosphate (pH 7.4)) with the addition of protease inhibitors (Roche Molecular Systems, Inc). Samples were then divided into four equal volumes for whole-body cortisol, glucose, lactate and total protein measurements. Glucose, lactate and protein homogenates (1 ml) were centrifuged for 30 min at 14 500 g at 4°C and the supernatant was extracted and stored at −80°C for later analysis. Cortisol homogenates were added to a 9-ml Pyrex glass tube for same-day extraction.
2.6. Cortisol extraction and analysis
Cortisol extraction followed methods outlined in Pasparakis et al., 2022, 2023. Briefly, cortisol homogenates were spiked with 2.5 ml of diethyl ether, vortexed for 1 min and then centrifuged for 7 min at 3200 g at 4°C. The resulting supernatant was carefully transferred to a new 9-ml Pyrex glass tube. To ensure maximal cortisol extraction, this process was repeated two more times, and the supernatant from all three washes was combined. Samples were left in the hood overnight for complete diethyl ether evaporation, resuspended the following day in 200-μl 1 × PBS, vortexed and stored at −80°C until later analysis. For whole-body cortisol quantification, an enzyme immunoassay (EIA) kit (Salivary Cortisol Immunoassay, Salimetrics LLC) was employed. Samples were run in duplicate, concentrations (μg dl−1) were calculated using a four-parameter sigmoidal standard curve and values were normalized to fish mass (ng g−1).
2.7. Glucose and lactate assays
For the analysis of whole-body glucose and lactate, frozen tissue homogenates were first thawed on ice. Samples were analysed using commercial test kits and following the manufacturer’s instructions for glucose (glucose assay kit, Sigma-Aldrich) and lactate (lactate assay kit II, Sigma-Aldrich). Samples were run in duplicate, concentrations (ng μl−1) were calculated using a linear standard curve and values were normalized to fish mass (μg g−1).
2.8. Protein-to-mass ratios
The bicinchoninic acid method (BCA; Pierce, Thermo Fisher Scientific Inc.) was employed to quantify whole-body protein concentrations in juvenile longfin smelt. Samples were run in duplicate and concentrations (μg ml−1) were calculated using a linear standard curve. Total protein was divided by mass of fish (g) to calculate protein-to-mass ratios.
For all four assays, if samples fell outside the range of standard curves, they were diluted and rerun. Those samples that remained outside the range of standard curves after dilution were eliminated from analysis. Standard curves of all assays had r2 values ≥0.98.
2.9. Condition factor
Longfin smelt mass and fork lengths were measured on both days of sampling, as well as on the day of CTMax trials. To calculate Fulton’s condition factor, the following equation was employed: CF = (WB/FL3) × 100, where WB represents the body mass (g) and FL denotes the fork length (cm).
2.10. Statistical analysis
Statistical analyses were conducted using R version 4.3.2 (R Core Team, 2023), with the package ‘nlme’ (Pinheiro et al., 2023). Non-parametric tests were employed to account for the random effects of sub-tanks. Linear mixed effect models (LMEs) were used to analyze whole-body cortisol, glucose and lactate concentrations, protein-to-mass ratios, condition factors and CTMax data in juvenile longfin smelt. Sub-tank was included as a random effect in all models. Whole-body cortisol, glucose, lactate and protein-to mass ratios were normalized to fish mass. Fixed effects in these models were temperature (11 and 14°C), turbidity (1, 4 and 11 NTU) and timing of exposure (two vs four weeks). Condition factor was analyzed at two and four weeks to account for changes in fish size due to growth, with temperature and turbidity as the fixed effects. CTMax was measured at five weeks, with temperature and turbidity as the fixed effects. Multiple LMEs using singular, combined and interactive effects of biological relevance were run and Akaike information Criteria (AICc) were calculated to determine the model of best fit for the data. Statistical output for these models can be found in Supplementary Table 3, and AICc scores are reported in Supplementary Table 4. The full model provided the best fit for cortisol and glucose data, whereas the interactive effects of temperature and turbidity offered the most robust explanation for lactate and protein-to-mass ratios. For condition factor and CTMax, temperature alone was identified as the strongest predictor. Detailed post hoc results, using the Tukey method for the most parsimonious LME models, are available in Supplementary File 2. Data were tested for normality and homogeneity using the ‘shapiro.test’ function from the ‘stats’ package and the ‘leveneTest’ function from the ‘car’ package, respectively. The Kruskal–Wallis rank sum test was employed to test the effect of water conditions (temperature and turbidity) on longfin smelt mortality. The cortisol immunoassay yielded a few outliers that were found to lack biological relevance. Employing a quantile range outlier test (Tail Quantile = 0.1; Q = 3) through JMP® (Version 17.2., SAS Institute) led to the exclusion of four data points, evenly distributed across various turbidity and temperature treatments. Data are presented as means ± SEM and differences between means were deemed significant at P < 0.05.
3. Results
3.1. Mortality
Temperature and turbidity treatments had no effect on longfin smelt survival after either two (H(5) = 3.2861, P = 0.656) or four (H(5) = 3.2336, P = 0.664) weeks. This suggests that treatment conditions resulted only in sub-lethal effects during our study.
3.2. Cortisol
There was no effect of temperature on whole-body cortisol levels in juvenile longfin smelt. However, cortisol levels were significantly influenced by turbidity and the duration of exposure. Specifically, fish exposed to low-turbidity conditions (1 NTU) exhibited significantly higher cortisol levels compared to those in high-turbidity conditions (11 NTU; P < 0.01). Timing of exposure also had a significant effect, with cortisol levels decreasing over time (P < 0.05) (Fig. 1; Supplementary Table 3).
3.3. Glucose and lactate
Glucose levels in juvenile longfin smelt were significantly influenced by temperature, turbidity and time. Fish exposed to lower temperatures exhibited significantly higher glucose compared to those at higher temperatures (P < 0.001). Turbidity also had a significant effect, with fish in higher turbidity conditions (11 NTU) displaying elevated glucose levels compared to those in lower turbidity treatments (P < 0.05). Additionally, glucose levels increased significantly over time, with higher values observed after four weeks of exposure compared to two weeks (P < 0.01) (Fig. 2; Supplementary Table 3).
Figure 2.
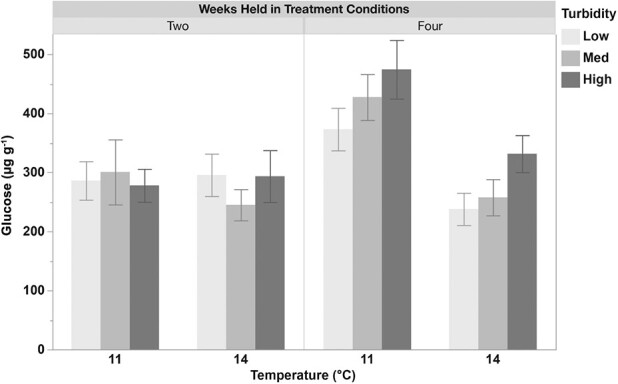
. Whole-body glucose measurements (μg g−1) in juvenile longfin smelt held at two temperatures (11 or 14°C) and three turbidities (low (1), med (4), or high (11) NTU) for two and four weeks. Glucose levels were significantly greater at lower temperatures and higher turbidity conditions (11 NTU compared to 1 NTU). Glucose also increased significantly over time. Data (n = 10–20) are presented as mean ± SEM.
No significant effects of temperature, turbidity or their interaction were observed for lactate levels in juvenile longfin smelt (Fig. 3; Supplementary Table 3).
Figure 3.
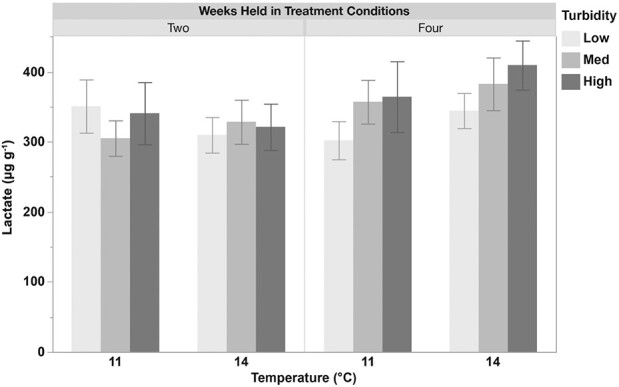
Whole-body lactate measurements (μg g−1) in juvenile longfin smelt held at two temperatures (11 or 14°C) and three turbidities (low (1), med (4), or high (11) NTU) for two and four weeks. Lactate levels were not significantly affected by temperature, turbidity or their interaction. Data (n = 16–20) are presented as mean ± SEM.
3.4. Protein-to-mass ratio
Turbidity, but not temperature or timing of exposure, had a significant effect on protein-to-mass ratios, with higher ratios observed in high-turbidity conditions (11 NTU) compared to low-turbidity conditions (1 NTU; P < 0.05). Significant interactions between temperature and turbidity were also detected. Specifically, fish held at 11°C in high turbidity (11 NTU) had significantly greater protein-to-mass ratios compared to those held at 14°C in either low (1 NTU) or medium turbidity (4 NTU) (P < 0.05) (Fig. 4; Supplementary Table 3).
Figure 4.
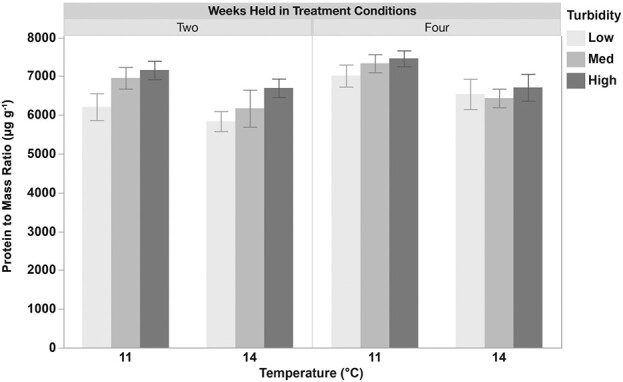
Protein-to-mass ratios (μg g−1) in juvenile longfin smelt held at two temperatures (11 or 14°C) and three turbidities (low (1), med (4), or high (11) NTU) for two and four weeks. Turbidity had a significant effect on protein-to-mass ratios, with fish held at higher turbidity (11 NTU) exhibiting greater ratios compared to those at lower turbidity (1 NTU). Significant interactions between temperature and turbidity were also observed. Data (n = 17–21) are presented as mean ± SEM.
3.5. Condition factor
There was no effect of temperature on juvenile longfin smelt condition factor after two weeks of exposure. However, after four weeks, a significant effect of temperature was observed, with fish held at cooler temperatures (11°C) exhibiting significantly higher condition factor compared to those at 14°C (P < 0.01) (Table 1; Supplementary Table 3).
Table 1.
Condition factor data for juvenile longfin smelt held at different temperatures (11 or 14°C) and turbidities (1 (low), 4 (med), or 11 (high) NTU) for either two or four weeks. After four weeks in treatment conditions, fish had significantly greater condition factor when held at lower temperature of 11°C compared to 14°C. Values are presented as mean ± SEM
| Treatment conditions | Weeks held in treatment conditions | ||
|---|---|---|---|
| Temperature | Turbidity | Two | Four |
| 11 | Low | 0.50 ± 0.01 | 0.53 ± 0.01 |
| 11 | Med | 0.49 ± 0.01 | 0.57 ± 0.03 |
| 11 | High | 0.50 ± 0.01 | 0.52 ± 0.01 |
| 14 | Low | 0.43 ± 0.01 | 0.47 ± 0.01 |
| 14 | Med | 0.48 ± 0.02 | 0.49 ± 0.01 |
| 14 | High | 0.50 ± 0.01 | 0.50 ± 0.01 |
3.6. CTMax
The upper temperature tolerance (CTMax) of juvenile longfin smelt was measured after five weeks in treatment conditions. Temperature had a significant effect on CTMax, with fish held at higher temperatures (14°C) exhibiting greater CTMax values compared to those held at 11°C (P < 0.001). The CTMax for juvenile longfin smelt was 26.3 ± 0.05 for fish held at 14°C and 24.6 ± 0.18 for fish held at 11°C. This resulted in a gain in CTMax of 1.7 for a 2.75°C (13.8–11.05°C) increase in temperature (Table 2; Supplementary Table 3). Additionally, fish held at higher temperatures had greater survival after recovery periods (Supplementary Table 5).
Table 2.
CTMax data for juvenile longfin smelt held at different temperatures (11 or 14°C) and turbidities (low (1), med (4), or high (11) NTU). Longfin smelt had significantly higher upper thermal tolerance when held at 14°C for five weeks compared to fish held at 11°C. Values are presented as mean ± SEM
| Temperature | Turbidity | CTMax |
|---|---|---|
| 11 | Low | 24.5 ± 0.25 |
| 11 | Med | 24.4 ± 0.44 |
| 11 | High | 25 ± 0.24 |
| 14 | Low | 26.4 ± 0.12 |
| 14 | Med | 26.2 ± 0.12 |
| 14 | High | 26.6 ± 0.16 |
Detailed post hoc comparisons for all treatment interactions from the LME models are provided in Supplementary File 2.
4. Discussion
This study investigates how varying temperatures (11 and 14°C) and turbidities (1, 4 and 11 NTU) influence survival, stress response and physiological condition of juvenile longfin smelt over time. Whilst these treatments did not impact survival, significant sub-lethal effects were observed from variations in both temperature and turbidity. Fish exposed to lower turbidity (1 NTU) experienced elevated stress, as indicated by increased cortisol levels, as well as reduced whole-body glucose and protein-to-mass ratios, compared to fish at higher turbidity (11 NTU). Higher temperatures resulted in significantly reduced whole-body glucose and condition factor. However, higher temperatures also yielded beneficial outcomes, including greater upper thermal tolerance and enhanced recovery post CTMax trials. Whole-body lactate remained unaffected by either temperature or turbidity treatments (Fig. 2). Interactive effects of temperature and turbidity were observed in juvenile longfin smelt. There was a significant reduction in cortisol from Week two to Week four driven largely by changes in fish held at 11°C, indicating potential acclimation to turbidity stress in fish in this group (Fig. 1). Additionally, significant interactive effects were observed in protein-to-mass ratios, with fish at lower temperatures and higher turbidity exhibiting higher ratios compared to those at higher temperatures and lower turbidity (Fig. 4). These results are consistent with our predictions and correlate closely with observed longfin smelt abundances in the wild (Grimaldo et al., 2017).
Investigating the generalized stress response in fish provides critical insight into optimal environmental and rearing conditions (Iwama et al., 1997). The stress response serves as an adaptive mechanism, allowing fish to maintain homeostasis in response to perceived stressors (Barton, 2002). Central to this process is the activation of the hypothalamic–pituitary–interrenal (HPI) axis, which regulates the production and release of cortisol, a steroid hormone (Wendelaar Bonga, 1997). However, chronic stress and prolonged elevation of cortisol levels can have severe secondary and tertiary effects, posing significant threats to fish populations, particularly those already endangered (Barton and Iwama, 1991; Somero, 2010). Juvenile longfin smelt displayed significantly elevated whole-body cortisol levels at low turbidity of 1 NTU compared to higher turbidity of 11 NTU (Fig. 1). These findings are consistent with prior research on juvenile delta smelt, which indicated significantly heightened stress levels at lower turbidities (1–2 NTU) compared to higher turbidities (10–11 NTU) after a two-week period, irrespective of the presence of a largemouth bass predator cue (Micropterus salmoides) (Pasparakis et al., 2023).
The reduced stress observed in smelt in turbid conditions may be attributed to their general preference for darker conditions, as smelt are known to be light-sensitive and actively avoid light in their natural habitats (Dembiński, 1971; Appenzeller and Leggett, 1995). Underscoring smelt’s preference for low-light conditions, strobe lights were proposed as an effective means to reduce rainbow smelt (Osmerus mordax) entrainment losses through Oahe Dam by inducing behavioural avoidance (Hamel et al., 2008). This behavioural preference for low-light likely influences their distribution and may contribute to habitat compression, as smelt seek out areas with higher turbidity that provide these preferred conditions (Heist and Swenson, 1983; Feyrer et al., 2007). Indeed, multiple studies have noted a strong correlation between turbidity and longfin smelt abundance in their natural habitat, suggesting that long-term declines in turbidity within the SFE may have contributed to the drastic population declines in this species (Grimaldo et al., 2017; Mahardja et al., 2017; Bever et al., 2018; Brennan et al., 2022).
Turbidity is also believed to alleviate stress in fish by improving predator avoidance and offering protection through visual cover (Gregory and Northcote, 1993; Sirois and Dodson, 2000; De Robertis et al., 2003). Despite the absence of predators or predator cues in our study, significant interactions occurred during feeding and maintenance, with longfin smelt being fed twice daily and water quality check and mortality removal taking place once a day. These human interactions closely resemble activities at an aquaculture facility and may have induced considerable stress in the fish. This could explain why longfin smelt in higher turbidities perceived greater protection, resulting in significantly lower whole-body cortisol levels compared to clearer waters (Fig. 1). These findings align with a study on late-larval delta smelt, which indicated minimal stress between 35 and 80 NTU and elevated cortisol at low turbidities (5, 12 and 25 NTU) after a 24-h exposure (Hasenbein et al., 2016).
Prolonged stress may redirect metabolic energy towards maintaining homeostasis, ultimately reducing energy for growth and other important biological functions (Wendelaar Bonga, 1997). This relocation of energetic reserves could explain the significantly reduced whole-body glucose and protein-to-mass ratios observed in juvenile longfin smelt (Fig. 2 and Fig. 4). Glycogen, which was not measured in this study, would provide valuable insight into the energetic reserves of the fish, as it represents carbohydrate stores. The whole-body glucose levels presented here reflect the immediate energy status of the fish. Elevated cortisol levels at lower turbidities suggest that longfin smelt were experiencing prolonged stress, which likely led to increased energy demands. Similarly, juvenile delta smelt, exhibiting significantly increased whole-body cortisol levels in lower turbidities, also displayed significantly reduced whole-body glucose levels (Pasparakis et al., 2023). The reduced protein-to-mass ratio observed in juvenile longfin smelt may suggest that energy was diverted from growth and protein synthesis towards stress-induced maintenance processes, reflecting physiological prioritization under prolonged stress.
Whilst feeding rate was not specifically evaluated in this study, it is plausible that the heightened stress and diminished energy observed in longfin smelt in clear waters could be attributed to increased difficulty and effort in locating artemia. Turbidity is believed to enhance visual acuity of small planktivorous or larval fish by increasing the contrast between prey and its background, thereby aiding in feeding (Utne-Palm, 2002). Longfin smelt are pelagic zooplanktivores, relying on their visual acuity to capture prey typically consisting of species such as copepods, cladocerans and mysid shrimp (Chigbu and Sibley, 1998; Barros et al., 2022). Field studies indicate that the interplay between turbidity and light alters predator–prey dynamics and interactions by influencing the depth of smelt and their prey (Horppila et al., 2004). In a laboratory setting, larval delta smelt displayed significantly reduced feeding in the absence of turbidity, with maximal feeding responses observed at 11 NTU, the highest tested turbidity level (Baskerville-Bridges et al., 2004; Tigan et al., 2020). Late-larval delta smelt displayed peak feeding rates between 25 and 80 NTU, with reduced feeding at lower (5 and 12 NTU) and higher (120 and 250) turbidities (Hasenbein et al., 2016). Juvenile delta smelt, on the other hand, exhibited reduced feeding >250 NTU, consistent feeding between 12 and 120 NTU, and the highest feeding rates <12 NTU, emphasizing the importance of investigating effects of turbidity at different life stages (Hasenbein et al., 2013).
The turbidity treatments implemented in our study do not precisely replicate the conditions found in the SFE, where turbidity is composed of a diverse and variable amalgamation of suspended sediment, dissolved organic matter and algae. However, our turbidity conditions closely resemble those applied at the FCCL for rearing both delta and longfin smelt. At the FCCL, turbidity is maintained using the same commercially preserved algae (Nannochloropsis) utilized in this study (Tigan et al., 2020). Larval longfin smelt are reared at ~10 NTU from 0 to 40 dph, after which they were transitioned to clear waters (Hung et al., 2024). Subsequently, due to poor survival rates, turbidity has been increased to 5.5 NTU up to 100 dph. Given the positive impacts of turbidity observed in juvenile longfin smelt in our study (~170–225 dph), our findings suggest that maintaining higher turbidity levels (~11 NTU) for extended periods when rearing cultured longfin smelt is advantageous, potentially reducing stress and increasing available energy. Whilst no current plans exist, population supplementation may become necessary for the sustainability of longfin smelt in the near future. Our results propose that transportation and release conditions with mid-range turbidities could enhance the success of these efforts by mitigating stress in this highly sensitive fish.
Temperature is another key variable to consider in future rearing and conservation strategies, given its significant effects on fish physiology (Perry et al., 2005; Pörtner and Peck, 2010). Rising temperatures in the SFE likely contributed to the POD, highlighting the need to incorporate temperature into multi-stressor experiments to better inform management decisions (Sommer et al., 2007; Bashevkin et al., 2022). Longfin smelt are thought to be especially sensitive to temperature stress and less tolerant of high temperatures than closely related delta smelt, based on both field and laboratory-based studies. Field data indicate larval delta smelt abundance peaks between 14 and 18°C, whilst larval longfin smelt are most abundant between 8 and 12°C (Bennett, 2005; Grimaldo et al., 2017). Although both species exhibit a preference for lower temperatures, predictions suggest that young-of-year longfin smelt have reduced occupancy at higher temperatures compared to delta smelt, corroborating lab studies reporting lower upper thermal tolerances in larval longfin smelt (Jeffries et al., 2016; Mahardja et al., 2017). Indeed, juvenile longfin smelt in the current study displayed significantly reduced whole-body glucose and condition factor when held at 14°C compared to 11°C, supporting previous studies (Fig. 1 and Table 2). Water temperatures in the SFE regularly exceed 20°C during summer months, particularly in the Delta, where seasonal variations are most pronounced. Given their reduced thermal tolerance and high sensitivity to stress, such temperatures could potentially lead to reductions in energy reserves and overall fitness in longfin smelt (Vroom et al., 2017).
Warmer temperatures incur energetic costs for various reasons, including increased metabolic rates, stimulated physiological and behavioural processes, faster swimming activities and thermal acclimation mechanisms (Dell et al., 2011; Sandblom et al., 2014). Prolonged exposure to thermal stress may result in latent adverse impacts, such as reduced growth and compromised health, providing support for reduced condition factor observed in juvenile longfin smelt after four weeks at higher temperatures (Sokolova et al., 2012; Alfonso et al., 2021). Acclimation to warmer temperatures increased the upper temperature tolerance of longfin smelt, demonstrating their capacity to extend thermal limits. Longfin smelt reared at 11°C exhibited CTMax values ranging from 24.4 ± 0.44 to 25 ± 0.24°C, whilst smelt at 14°C displayed values ranging from 26.2 ± 0.12 to 26.6 ± 0.16°C (Table 2). This resulted in a CTMax increase of 1.7 for a 2.75°C temperature rise. These findings align with CTMax values of 24.8 ± 0.38°C in longfin smelt larvae (~50 dph) reared at 14°C in a previous study (Jeffries et al., 2016). Longfin smelt held at warmer temperatures exhibited higher survival rates following CTMax trials, providing additional evidence of reduced sensitivity to high temperatures with warm acclimation (Supplementary Table 5). This suggests that longfin smelt raised at higher temperatures in natural habitats are likely better equipped to endure heat shocks. However, this increase in thermal tolerance may entail a significant trade-off, as it can lead to reduced growth and energy levels, both of which are essential for coping with other demanding environmental conditions.
In the context of rearing longfin smelt for culture in conservation hatcheries, our data suggests that lower temperatures of 11°C are preferable for promoting growth and conserving energy. This recommendation is consistent with a previous study on recently hatched longfin smelt larvae, which advocated for culturing temperatures of 9 or 12°C over 15°C, as the latter temperature induced decreased hatch success, diminished growth rates and earlier mass mortality, resulting in fewer and smaller larvae with reduced endogenous reserves (Yanagitsuru et al., 2021). Conversely, if population supplementation becomes necessary for longfin smelt survival in the SFE, temperatures >11°C should be considered due to the benefits of enhancing thermal tolerance. Alternatively, supplementation could be strategically prioritized during winter months when the likelihood of thermal stress is reduced. Further research is necessary to gain a better understanding of the trade-offs involved in rearing longfin smelt at warmer temperatures to increase thermal tolerance and its subsequent impact on physiological performance.
Juvenile longfin smelt held at lower temperatures displayed signs of acclimation to turbidity stress, evidenced by a reduction in whole-body cortisol levels between the two- and four-week time points, with values decreasing by more than half at each turbidity level. In contrast, longfin smelt held at higher temperatures showed no indications of acclimation or reduction in stress markers, as their cortisol levels remained similar at both time points and, in fact, displayed minor increases in two of the three turbidity treatments at the four-week mark (Fig. 1). These findings suggest that elevated temperature and reduced turbidity may interact by imposing substantial stress on juvenile longfin smelt, hindering their capacity for recovery. Furthermore, a significant interaction between temperature and turbidity was observed for protein-to-mass ratios, with higher ratios in fish held at cooler temperature and higher turbidity compared to those at warmer temperature and lower turbidity (Fig. 4). These results suggest that fish in cooler, more turbid conditions may allocate resources more effectively towards growth, likely enhancing their physiological condition and resilience. Research investigating the interactive effects of temperature, turbidity and other relevant stressors in juvenile longfin smelt is essential for conservation and warrants further study.
5. Concluding Remarks
Juvenile longfin smelt demonstrate improved physiological condition at lower temperatures of 11°C compared to 14°C and elevated turbidities of 11 NTU compared to 1 NTU. These findings are consistent with field abundance data, suggesting that cooler, more turbid environments provide more favourable conditions for these fish (Moyle, 2002; Grimaldo et al., 2017). Prolonged exposure to stress and the reallocation of energy towards maintaining homeostasis likely contributed to observed reductions in energy and growth. Chronic stress in fish can lead to severe tertiary effects, including the suppression of immune function, increased susceptibility to disease and a reduction in overall fitness, fecundity and survival in their natural habitat (Wendelaar Bonga, 1997; Tort, 2011). Therefore, conditions that minimize stress and promote the growth and condition of fish should be prioritized in aquaculture settings and conservation initiatives. Results from our study indicate that lower temperatures and elevated turbidities enhance the physiological condition of the understudied juvenile stage of longfin smelt. Given the limited understanding of this critical developmental stage, the data presented herein fill an important knowledge gap, providing valuable insights for management-based conservation efforts.
Supplementary Material
Acknowledgements
We thank the hatchery staff at UC Davis Fish Conservation and Culture Lab for maintaining the captive population of longfin smelt used in this research. We also thank Jordan Colby, Sarah Baird and Anthony Tercero for their help with fish care, experimental set-up and sampling. And a special thanks to Richelle Tanner for her advice on statistics.
Contributor Information
Christina Pasparakis, Department of Environmental Toxicology, University of California Davis, 1 Shields Ave., Davis, CA 95616, USA; Bodega Marine Laboratory, University of California Davis, 2099 Westshore Rd., Bodega Bay, CA 94923, USA.
Felix Biefel, Department of Anatomy, Physiology and Cell Biology, School of Veterinary Medicine, University of California Davis, 1 Shields Ave., Davis, CA 95616, USA.
Francine De Castro, Department of Environmental Toxicology, University of California Davis, 1 Shields Ave., Davis, CA 95616, USA; Bodega Marine Laboratory, University of California Davis, 2099 Westshore Rd., Bodega Bay, CA 94923, USA.
Alexandra Wampler, Department of Wildlife, Fish and Conservation Biology, University of California Davis, 1 Shields Ave., Davis, CA 95616, USA .
Dennis E Cocherell, Department of Wildlife, Fish and Conservation Biology, University of California Davis, 1 Shields Ave., Davis, CA 95616, USA .
Evan W Carson, U.S. Fish and Wildlife Service, San Francisco Bay-Delta Fish and Wildlife Office, 650 Capitol Mall, Sacramento, CA 95814, USA .
Tien-Chieh Hung, Fish Conservation and Culture Laboratory, Department of Biological and Agricultural Engineering, University of California Davis, 1 Shields Ave., Davis, CA 95616, USA.
Richard E Connon, Department of Anatomy, Physiology and Cell Biology, School of Veterinary Medicine, University of California Davis, 1 Shields Ave., Davis, CA 95616, USA.
Nann A Fangue, Department of Wildlife, Fish and Conservation Biology, University of California Davis, 1 Shields Ave., Davis, CA 95616, USA .
Anne E Todgham, Department of Animal Science, University of California Davis, 1 Shields Ave., Davis, CA 95616, USA.
Author Contributions
This manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. C.P., F.B., D.E.C., R.E.C., N.A.F. and A.E.T. designed the experiment. C.P., F.B., F.D. and A.W. collected the data. E.W.C. contributed biological expertise and expertise in the management of longfin smelt. T-C.H. provided experimental fish. C.P. wrote the manuscript. R.E.C., N.A.F. and A.E.T. secured the funding that supported the work.
Conflicts of Interest
The authors have no conflict of interest to declare.
Funding
This research was made possible by funding from the USFWS # F19AC00943 to A.E.T., R.E.C., T-C.H. and N.A.F., and the University of California, Davis Agricultural Experiment Station (CA-D-ASC-2252-H to A.E.T. and CA-D-ASC-2098-H to N.A.F.). USBOR, R20AC00027 funding to T-C.H. was used for longfin smelt rearing and maintenance. Funding for F.B. was provided by the Bayerische Forschungsstiftung Scholarship (DOK-181-19, Geist). The findings and conclusions in this article are those of the authors and do not necessarily represent the view of the US Fish and Wildlife Service.
Data availability
The data underlying this article are available in the online supplementary material.
Supplementary material
Supplementary material is available at Conservation Physiology online.
References
- Aghbolaghi MA, Maloy AP, Coombs JA, Hung TC, Carson EW (2024) Phylogenetic relationships and introduction history inferred from complete mitochondrial genomes of four smelts (Osmeridae) of the modern San Francisco Estuary. Zool Anz 308: 66–70. 10.1016/j.jcz.2023.11.008. [DOI] [Google Scholar]
- Alfonso S, Gesto M, Sadoul B (2021) Temperature increase and its effects on fish stress physiology in the context of global warming. J Fish Biol 98: 1496–1508. 10.1111/jfb.14599. [DOI] [PubMed] [Google Scholar]
- Appenzeller AR, Leggett WC (1995) An evaluation of light-mediated vertical migration of fish based on hydroacoustic analysis of the diel vertical movements of rainbow smelt (Osmerus mordax). Can J Fish Aquat Sci 52: 504–511. 10.1139/f95-051. [DOI] [Google Scholar]
- Barros A, Hobbs JA, Willmes M, Parker CM, Bisson M, Fangue NA, Rypel AL, Lewis LS (2022) Spatial heterogeneity in prey availability, feeding success, and dietary selectivity for the threatened longfin smelt. Estuar. Coast. 45: 1766–1779. 10.1007/s12237-021-01024-y. [DOI] [Google Scholar]
- Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42: 517–525. 10.1093/icb/42.3.517. [DOI] [PubMed] [Google Scholar]
- Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1: 3–26. 10.1016/0959-8030(91)90019-G. [DOI] [Google Scholar]
- Bashevkin SM, Mahardja B, Brown LR (2022) Warming in the upper San Francisco Estuary: patterns of water temperature change from five decades of data. Limnol Oceanogr 67: 1065–1080. 10.1002/lno.12057. [DOI] [Google Scholar]
- Baskerville-Bridges B, Lindberg JC, Doroshov SI (2004) The effect of light intensity, alga concentration, and prey density on the feeding behavior of delta smelt larvae. In American Fisheries Society Symposium 39: 219–227. [Google Scholar]
- Baskerville-Bridges B, Lindberg JC, Doroshov SI (2005) Manual for the Intensive Culture of Delta Smelt (Hypomesus transpacificus). University of California-Davis, Sacramento (CA), Report to CALFED Bay-Delta Program. ERP-02-P31. [Google Scholar]
- Baxter R, Breuer R, Brown L, Chotkowski M, Feyrer F, Gingras M, Herbold B, Mueller-Solger A, Nobriga M, Sommer Tet al. (2008) Pelagic organism decline progress report: 2007 synthesis of results. In Technical Report227. Interagency Ecological Program for the San Francisco Estuary. State of California Department of Water Resources, Sacramento, California, USA. [Google Scholar]
- Beitinger TL, Bennett WA, McCauley RW (2000) Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fishes 58: 237–275. 10.1023/A:1007676325825. [DOI] [Google Scholar]
- Bennett WA (2005) Critical assessment of the delta smelt population in the San Francisco Estuary, California. San. Franc. Estuary Watershed Sci 3. 10.15447/sfews.2005v3iss2art1. [DOI] [Google Scholar]
- Bever AJ, MacWilliams ML, Fullerton DK (2018) Influence of an observed decadal decline in wind speed on turbidity in the San Francisco Estuary. Estuaries Coast 41: 1943–1967. 10.1007/s12237-018-0403-x. [DOI] [Google Scholar]
- Boehlert GW, Morgan JB (1985) Turbidity enhances feeding abilities of larval Pacific herring, Clupea harengus pallasi. Hydrobiologia 123: 161–170. 10.1007/BF00018978. [DOI] [Google Scholar]
- Brennan CA, Hassrick JL, Kalmbach A, Cox DM, Sabal MC, Zeno RL, Grimaldo LF, Acuña S (2022) Estuarine recruitment of longfin smelt (Spirinchus thaleichthys) north of the San Francisco Estuary. San. Franc. Estuary Watershed Sci 20. 10.15447/sfews.2022v20iss3art3. [DOI] [Google Scholar]
- Brown LR, Komoroske LM, Wagner RW, Morgan-King T, May JT, Connon RE, Fangue NA (2016) Coupled downscaled climate models and ecophysiological metrics forecast habitat compression for an endangered estuarine fish. PloS One 11: e0146724. 10.1371/journal.pone.0146724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Department of Fish and Game (CDFG) (2009) A Status Review of the Longfin Smelt (Spirinchus Thaleichthys) in California, Sacramento. [Google Scholar]
- Chigbu P, Sibley TH (1998) Feeding ecology of longfin smelt (Spirinchus thaleichthys Ayres) in Lake Washington. Fish Res 38: 109–119. 10.1016/S0165-7836(98)00156-8. [DOI] [Google Scholar]
- Cloern JE, Jassby AD (2012) Drivers of change in estuarine-coastal ecosystems: discoveries from four decades of study in San Francisco Bay. Rev Geophys 50: 1–33. 10.1029/2012RG000397. [DOI] [Google Scholar]
- Cohen AN, Carlton JT (1998) Accelerating invasion rate in a highly invaded estuary. Science 279: 555–558. 10.1126/science.279.5350.555. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Fangue NA, Bergman JN, Madliger CL, Cech JJ Jr, Eliason EJ, Brauner CJ, Farrell AP (2022) Conservation physiology and the management of wild fish populations in the Anthropocene. In Fish Physiology, Vol. 39. Academic Press, Cambridge MA, pp. 1–31. [Google Scholar]
- Davies-Colley RJ, Nagels JW (2008) Predicting light penetration into river waters. J Geophys Res Biogeo 113: 1–9. 10.1029/2008JG000722. [DOI] [Google Scholar]
- Davies-Colley RJ, Smith DG (2001) Turbidity suspended sediment, and water clarity: a review. Water resources bulletin 37: 1085–1101. 10.1111/j.1752-1688.2001.tb03624.x. [DOI] [Google Scholar]
- Davis BE, Cocherell DE, Sommer T, Baxter RD, Hung TC, Todgham AE, Fangue NA (2019) Sensitivities of an endemic, endangered California smelt and two non-native fishes to serial increases in temperature and salinity: implications for shifting community structure with climate change. Conserv. Physiol. 7: coy076. 10.1093/conphys/coy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis A, Ryer CH, Veloza A, Brodeur RD (2003) Differential effects of turbidity on prey consumption of piscivorous and planktivorous fish. Can J Fish Aquat Sci 60: 1517–1526. 10.1139/f03-123. [DOI] [Google Scholar]
- Dell AI, Pawar S, Savage VM (2011) Systematic variation in the temperature dependence of physiological and ecological traits. PNAS Nexus 108: 10591–10596. 10.1073/pnas.1015178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembiński W (1971) Vertical distribution of vendace Coregonus albula L. and other pelagic fish species in some Polish lakes. J Fish Biol 3: 341–357. 10.1111/j.1095-8649.1971.tb03689.x. [DOI] [Google Scholar]
- Feyrer F, Nobriga ML, Sommer TR (2007) Multidecadal trends for three declining fish species: habitat patterns and mechanisms in the San Francisco Estuary, California, USA. Can J Fish Aquat Sci 64: 723–734. 10.1139/f07-048. [DOI] [Google Scholar]
- Fong S, Louie S, Werner I, Davis J, Connon RE (2016) Contaminant effects on California Bay–Delta species and human health. San. Franc. Estuary Watershed Sci 14: 14. 10.15447/sfews.2016v14iss4/art5. [DOI] [Google Scholar]
- Fortin-Hamel L, Chapman LJ (2024) Interactive effects of sedimentary turbidity and elevated water temperature on the pugnose shiner (Miniellus anogenus), a threatened freshwater fish. Conserv Physiol 12: coae053. 10.1093/conphys/coae053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MB (1981) Effects of turbidity on feeding rates and selectivity of bluegills. Trans Am Fish Soc 110: 446–450. . [DOI] [Google Scholar]
- Garwood RS (2017) Historic and contemporary distribution of longfin smelt (Spirinchus thaleichthys) along the California coast. Calif Fish Game 103: 96–117. [Google Scholar]
- Graham CT, Harrod C (2009) Implications of climate change for the fishes of the British Isles. J Fish Biol 74: 1143–1205. 10.1111/j.1095-8649.2009.02180.x. [DOI] [PubMed] [Google Scholar]
- Gregory RS, Northcote TG (1993) Surface, planktonic, and benthic foraging by juvenile Chinook salmon (Oncorhynchus tshawytscha) in turbid laboratory conditions. Can J Fish Aquat Sci 50: 233–240. 10.1139/f93-026. [DOI] [Google Scholar]
- Grimaldo L, Feyrer F, Burns J, Maniscalco D (2017) Sampling uncharted waters: examining rearing habitat of larval longfin smelt (Spirinchus thaleichthys) in the upper San Francisco Estuary. Estuar Coast 40: 1771–1784. 10.1007/s12237-017-0255-9. [DOI] [Google Scholar]
- Hamel MJ, Brown ML, Chipps SR (2008) Behavioral responses of rainbow smelt to in situ strobe lights. N Am J Fish Manage 28: 394–401. 10.1577/M06-254.1. [DOI] [Google Scholar]
- Hammock BG, Moose SP, Solis SS, Goharian E, Teh SJ (2019) Hydrodynamic modeling coupled with long-term field data provide evidence for suppression of phytoplankton by invasive clams and freshwater exports in the San Francisco Estuary. Environ Manag 63: 703–717. 10.1007/s00267-019-01159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenbein M, Fangue NA, Geist J, Komoroske LM, Truong J, McPherson R, Connon RE (2016) Assessments at multiple levels of biological organization allow for an integrative determination of physiological tolerances to turbidity in an endangered fish species. Conserv Physiol 4: cow004. 10.1093/conphys/cow004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenbein M, Komoroske LM, Connon RE, Geist J, Fangue NA (2013) Turbidity and salinity affect feeding performance and physiological stress in the endangered delta smelt. Integr Comp Biol 53: 620–634. 10.1093/icb/ict082. [DOI] [PubMed] [Google Scholar]
- Healey M, Goodwin P, Dettinger M, Norgaard R (2016) The state of Bay–Delta science 2016: an introduction. San. Franc. Estuary Watershed Sci 14. 10.15447/sfews.2016v14iss2art5. [DOI] [Google Scholar]
- Hecht T, Van der Lingen CD (1992) Turbidity-induced changes in feeding strategies of fish in estuaries. Afr Zool 27: 95–107. 10.1080/02541858.1992.11448269. [DOI] [Google Scholar]
- Heist BG, Swenson WA (1983) Distribution and abundance of rainbow smelt in western Lake Superior as determined from acoustic sampling. J Great Lakes Res 9: 343–353. 10.1016/S0380-1330(83)71905-2. [DOI] [Google Scholar]
- Henley WF, Patterson MA, Neves RJ, Lemly AD (2000) Effects of sedimentation and turbidity on lotic food webs: a concise review for natural resource managers. Rev Fish Sci 8: 125–139. 10.1080/10641260091129198. [DOI] [Google Scholar]
- Hobbs J, Moyle PB, Fangue N, Connon RE (2017) Is extinction inevitable for delta smelt and longfin smelt? An opinion and recommendations for recovery. San Franc Estuary Watershed Sci 15. 10.15447/sfews.2017v15iss2art2. [DOI] [Google Scholar]
- Honey K, Baxter R, Hymanson Z, Sommer T, Gingras M, Cadrett P (2004) IEP long-term fish monitoring program element review. Interagency Ecological Program cow004. [Google Scholar]
- Horppila J, Liljendahl-Nurminen A, Malinen T (2004) Effects of clay turbidity and light on the predator prey interaction between smelts and chaoborids. Can J Fish Aquat Sci 61: 1862–1870. 10.1139/f04-123. [DOI] [Google Scholar]
- Hung TC, Ellison L, Stevenson T, Sandford M, Schultz AA, Eads AR (2022) Early weaning in endangered delta smelt: effect of weaning time on growth and survival. N Am J Aquac 84: 249–260. 10.1002/naaq.10230. [DOI] [Google Scholar]
- Hung TC, Rahman MM, Lewis LS, Yang YC, Stevenson TA Jr, Menard KL, Connon RE, Bell H, Fangue NA (2024) Laboratory-bred longfin smelt produced offspring in the first year in captivity. N Am J Aquac 86: 228–233. 10.1002/naaq.10327. [DOI] [Google Scholar]
- Iwama GK, Pickering AD, Sumpter JP, Schreck CB (eds) (1997) Fish stress and health in aquaculture. In Soc. Exp. Biol. Sem. Ser. 62. Cambridge Univ. Press, Cambridge, U.K. [Google Scholar]
- Jackson JB, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JAet al. (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293: 629–637. 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- Jeffries KM, Connon RE, Davis BE, Komoroske LM, Britton MT, Sommer T, Todgham AE, Fangue NA (2016) Effects of high temperatures on threatened estuarine fishes during periods of extreme drought. J Exp Biol 219: 1705–1716. 10.1242/jeb.134528. [DOI] [PubMed] [Google Scholar]
- Kang Y, Song X, Liu Z (2013) Sediment resuspension dampens the effect of nutrient inputs on the phytoplankton community: a mesocosm experiment study. Hydrobiologia 710: 117–127. 10.1007/s10750-012-1221-y. [DOI] [Google Scholar]
- Kimmerer W (2004) Open water processes of the San Francisco Estuary: from physical forcing to biological responses. San Franc Estuary Watershed Sci 2: 1–142. 10.15447/sfews.2004v2iss1art1. [DOI] [Google Scholar]
- Kimmerer WJ (2002) Effects of freshwater flow on abundance of estuarine organisms: physical effects or trophic linkages? Mar Ecol Prog Ser 243: 39–55. 10.3354/meps243039. [DOI] [Google Scholar]
- Kirk JT (1985) Effects of suspensoids (turbidity) on penetration of solar radiation in aquatic ecosystems. Hydrobiologia 125: 195–208. 10.1007/BF00045935. [DOI] [Google Scholar]
- Komoroske LM, Connon RE, Lindberg J, Cheng BS, Castillo G, Hasenbein M, Fangue NA (2014) Ontogeny influences sensitivity to climate change stressors in an endangered fish. Conserv Physiol 2: cou008. 10.1093/conphys/cou008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe D, Cooperman MS, Chapman LJ, Clark TD, Val AL, Ferreira MS, Balirwa JS, Mbabazi D, Mwanja M, Chhom Let al. (2018) Predicted impacts of climate warming on aerobic performance and upper thermal tolerance of six tropical freshwater fishes spanning three continents. Conserv. Physiol. 6: coy056. 10.1093/conphys/coy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LS, Willmes M, Barros A, Crain PK, Hobbs JA (2020) Newly discovered spawning and recruitment of threatened longfin smelt in restored and underexplored tidal wetlands. Ecology 101: e02868. 10.1002/ecy.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg JC, Tigan G, Ellison L, Rettinghouse T, Nagel MM, Fisch KM (2013) Aquaculture methods for a genetically managed population of endangered delta smelt. N American J Aquac 75: 186–196. 10.1080/15222055.2012.751942. [DOI] [Google Scholar]
- Mahardja B, Young MJ, Schreier B, Sommer T (2017) Understanding imperfect detection in a San Francisco Estuary long-term larval and juvenile fish monitoring programme. Fish Manag Ecol 24: 488–503. 10.1111/fme.12257. [DOI] [Google Scholar]
- Moyle PB (2002) Inland Fishes of California. Univ of California Press, Berkeley, California [Google Scholar]
- Moyle PB, Lund JR, Bennett WA, Fleenor WE (2010) Habitat variability and complexity in the upper San Francisco Estuary. San Franc Estuary Watershed Sci 8. 10.15447/sfews.2010v8iss3art1. [DOI] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403: 853–858. 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Nichols FH, Cloern JE, Luoma SN, Peterson DH (1986) The modification of an estuary. Science 231: 567–573. 10.1126/science.231.4738.567. [DOI] [PubMed] [Google Scholar]
- Nobriga ML, Rosenfield JA (2016) Population dynamics of an estuarine forage fish: disaggregating forces driving long-term decline of longfin smelt in California's San Francisco Estuary. T Am Fish Soc 145: 44–58. 10.1080/00028487.2015.1100136. [DOI] [Google Scholar]
- Ortega JC, Figueiredo BR, Graca WJ, Agostinho AA, Bini LM (2020) Negative effect of turbidity on prey capture for both visual and non-visual aquatic predators. J Anim Ecol 89: 2427–2439. 10.1111/1365-2656.13329. [DOI] [PubMed] [Google Scholar]
- Pasparakis C, Lohroff T, Biefel F, Cocherell DE, Carson EW, Hung TC, Connon RE, Fangue NA, Todgham AE (2023) Effects of turbidity, temperature and predation cue on the stress response of juvenile delta smelt. Conserv. Physiol. 11: coad036. 10.1093/conphys/coad036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis C, Wampler AN, Lohroff T, DeCastro F, Cocherell DE, Carson EW, Hung TC, Connon RE, Fangue NA, Todgham AE (2022) Characterizing the stress response in juvenile delta smelt exposed to multiple stressors. Comp Biochem Phys A 274: 111303. 10.1016/j.cbpa.2022.111303. [DOI] [PubMed] [Google Scholar]
- Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308: 1912–1915. 10.1126/science.1111322. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, R Core Team (2023). Nlme: linear and nonlinear mixed effects models. R package version 3.1–163, https://CRAN.R-project.org/package=nlme.
- Pörtner HO, Peck MA (2010) Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol 77: 1745–1779. 10.1111/j.1095-8649.2010.02783.x. [DOI] [PubMed] [Google Scholar]
- R Core Team (2023). R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria. <https://www.R-project.org/> 111303. [Google Scholar]
- Rieger PW, Summerfelt RC (1997) The influence of turbidity on larval walleye, Stizostedion vitreum, behavior and development in tank culture. Aquaculture 159: 19–32. 10.1016/S0044-8486(97)00187-7. [DOI] [Google Scholar]
- Robinson RA, Crick HQ, Learmonth JA, Maclean IM, Thomas CD, Bairlein F, Forchhammer MC, Francis CM, Gill JA, Godley BJet al. (2009) Travelling through a warming world: climate change and migratory species. Endanger Species Res 7: 87–99. 10.3354/esr00095. [DOI] [Google Scholar]
- Rodrigues JN, Ortega JC, Petsch DK, Padial AA, Moi DA, Figueiredo BR (2023) A meta-analytical review of turbidity effects on fish mobility. Rev Fish Biol Fish 33: 1113–1127. 10.1007/s11160-023-09785-4. [DOI] [Google Scholar]
- Rohan SK, Beauchamp DA, Essington TE, Hansen AG (2021) Merging empirical and mechanistic approaches to modeling aquatic visual foraging using a generalizable visual reaction distance model. Ecol Model 457: 109688. 10.1016/j.ecolmodel.2021.109688. [DOI] [Google Scholar]
- Rosenfield JA, Baxter RD (2007) Population dynamics and distribution patterns of longfin smelt in the San Francisco Estuary. T Am Fish Soc 136: 1577–1592. 10.1577/T06-148.1. [DOI] [Google Scholar]
- Sağlam İK, Hobbs J, Baxter R, Lewis LS, Benjamin A, Finger AJ (2021) Genome-wide analysis reveals regional patterns of drift, structure, and gene flow in longfin smelt (Spirinchus thaleichthys) in the northeastern Pacific. Can J Fish Aquat Sci 78: 1793–1804. 10.1139/cjfas-2021-0005. [DOI] [Google Scholar]
- Sandblom E, Gräns A, Axelsson M, Seth H (2014) Temperature acclimation rate of aerobic scope and feeding metabolism in fishes: implications in a thermally extreme future. P R Soc B- Biol Sci 281: 20141490. 10.1098/rspb.2014.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoellhamer DH (2011) Sudden clearing of estuarine waters upon crossing the threshold from transport to supply regulation of sediment transport as an erodible sediment pool is depleted: San Francisco Bay, 1999. Estuar Coast 34: 885–899. 10.1007/s12237-011-9382-x. [DOI] [Google Scholar]
- Sirois P, Dodson JJ (2000) Influence of turbidity, food density and parasites on the ingestion and growth of larval rainbow smelt Osmerus mordax in an estuarine turbidity maximum. Mar Ecol Prog Ser 193: 167–179. 10.3354/meps193167. [DOI] [Google Scholar]
- Skinner JE (1962) An historical review of the fish and wildlife resources of the San Francisco Bay area (No. 1). Resources Agency of California, Department of Fish and Game, Water Projects Branch. 1–226. [Google Scholar]
- Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA (2012) Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar Environ Res 79: 1–15. 10.1016/j.marenvres.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Somero GN (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 213: 912–920. 10.1242/jeb.037473. [DOI] [PubMed] [Google Scholar]
- Sommer T, Armor C, Baxter R, Breuer R, Brown L, Chotkowski M, Culberson S, Feyrer F, Gingras M, Herbold Bet al. (2007) The collapse of pelagic fishes in the upper San Francisco Estuary. Fisheries 32: 270–277. 10.1577/1548-8446(2007)32[270:TCOPFI]2.0.CO;2. [DOI] [Google Scholar]
- Sutherland AB, Meyer JL (2007) Effects of increased suspended sediment on growth rate and gill condition of two southern Appalachian minnows. Environ Biol Fishes 80: 389–403. 10.1007/s10641-006-9139-8. [DOI] [Google Scholar]
- Taylor SG (2008) Climate warming causes phenological shift in pink salmon, Oncorhynchus gorbuscha, behavior at Auke Creek, Alaska. Glob Change Biol Bioenergy 14: 229–235. 10.1111/j.1365-2486.2007.01494.x. [DOI] [Google Scholar]
- Tigan G, Mulvaney W, Ellison L, Schultz A, Hung TC (2020) Effects of light and turbidity on feeding, growth, and survival of larval delta smelt (Hypomesus transpacificus, Actinopterygii, Osmeridae). Hydrobiologia 847: 2883–2894. 10.1007/s10750-020-04280-4. [DOI] [Google Scholar]
- Tort L (2011) Stress and immune modulation in fish. Dev Comp Immunol 35: 1366–1375. 10.1016/j.dci.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Utne-Palm AC (2002) Visual feeding of fish in a turbid environment: physical and behavioural aspects. Mar Freshw Behav Physiol 35: 111–128. 10.1080/10236240290025644. [DOI] [Google Scholar]
- Vroom J, Van der Wegen M, Martyr-Koller RC, Lucas LV (2017) What determines water temperature dynamics in the San Francisco Bay-Delta system? Water Resour Res 53: 9901–9921. 10.1002/2016WR020062. [DOI] [Google Scholar]
- Wendelaar Bonga SW (1997) The stress response in fish. Physiol Rev 77: 591–625. 10.1152/physrev.1997.77.3.591. [DOI] [PubMed] [Google Scholar]
- Werner I, Deanovic LA, Markiewicz D, Khamphanh M, Reece CK, Stillway M, Reece C (2010) Monitoring acute and chronic water column toxicity in the northern Sacramento–San Joaquin Estuary, California, USA, using the euryhaline amphipod, Hyalella azteca: 2006 to 2007. Environ Toxicol Chem 29: 2190–2199. 10.1002/etc.281. [DOI] [PubMed] [Google Scholar]
- Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, Jackson JB, Lotze HK, Micheli F, Palumbi SRet al. (2006) Impacts of biodiversity loss on ocean ecosystem services. Science 314: 787–790. 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- Wright SA, Schoellhamer DH (2004) Trends in the sediment yield of the Sacramento River, California, 1957–2001. San Franc Estuary Watershed Sci 2: 1–14. 10.15447/sfews.2004v2iss2art2. [DOI] [Google Scholar]
- Yanagitsuru YR, Main MA, Lewis LS, Hobbs JA, Hung TC, Connon RE, Fangue NA (2021) Effects of temperature on hatching and growth performance of embryos and yolk-sac larvae of a threatened estuarine fish: longfin smelt (Spirinchus thaleichthys). Aquaculture 537: 736502. 10.1016/j.aquaculture.2021.736502. [DOI] [Google Scholar]
- Yanagitsuru YR, Mauduit F, Lundquist AJ, Lewis LS, Hobbs JA, Hung TC, Connon RE, Fangue NA (2024) Effects of incubation temperature on the upper thermal tolerance of the imperiled longfin smelt (Spirinchus thaleichthys). Conserv Physiol 12: coae004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the online supplementary material.


