Abstract
This study presents an elderly man with sequential hemodynamic obstructions caused by hypertrophic cardiomyopathy and aortic stenosis. Septal reduction therapy was performed to avoid outflow tract obstruction associated with potential future transcatheter aortic valve replacement. This case highlights the importance of resolving outflow tract obstruction during assessment of aortic valve disease.
Key Words: aortic stenosis, obstructive hypertrophic cardiomyopathy
Graphical Abstract
History of Presentation
An 82-year-old man presented to hypertrophic cardiomyopathy (HCM) clinic for second opinion regarding previously advised transcatheter aortic valve replacement (TAVR) in the setting of HCM. The patient reported progressive exertional dyspnea over several years with minimal activity, including showering, and exertional intolerance to <1 flight of stairs (NYHA functional class III). Physical examination was notable for a grade 3/6 ejection systolic murmur over the aortic area that radiated to the carotids and a late systolic murmur grade 2/6 at the left sternal border.
Learning Objectives
-
•
To recognize obstructive hypertrophic cardiomyopathy on echocardiography and/or cardiac MRI.
-
•
To understand the importance of treating dynamic outflow tract obstruction prior to assessing aortic valve disease for accurate hemodynamic assessment in patients with sequential obstruction.
-
•
To recognize that undiagnosed HCM can be a complicating diagnosis in patients undergoing TAVR and has been associated with worse clinical outcomes, including cardiogenic shock, renal failure, and death.
Past Medical History
The patient had a history of hypertension, hyperlipidemia, HCM, aortic stenosis (AS), ascending aortic aneurysm, benign prostatic hyperplasia, and acid reflux. His medication list was notable for metoprolol tartrate 75 mg twice a day, losartan 100 mg daily, nifedipine 30 mg daily, tamsulosin 0.4 mg daily, aspirin 81 mg daily, and atorvastatin 40 mg daily.
Differential Diagnosis
The differential diagnosis included AS progression, new valvular heart disease (eg, aortic regurgitation in the setting of aortic dilatation), obstructive HCM, obstructive coronary artery disease, and/or arrhythmia.
Investigations
Electrocardiogram demonstrated sinus bradycardia (heart rate 57 beats/min) with inferior infarct pattern and non-specific T-wave changes through the lateral limb and precordial leads (Figure 1). Transthoracic echocardiography showed normal left ventricular (LV) size and systolic function, focal basal hypertrophy (2.0 cm), a small LV cavity, and systolic anterior motion of the anterior mitral valve leaflet (Figures 2A and 2B). The aortic valve (AV) leaflets appeared thickened and calcified with severely restricted motion (Figure 2A) and mild regurgitation. Continuous wave Doppler across the left ventricular outflow tract (LVOT) and AV identified a 4 m/s mid-systolic peak velocity and 34 mm Hg mean gradient, with a 0.45 cm2/m2 indexed aortic valve area (AVAi), 0.33 dimensionless valve index (DVI), and 39.7 mL/m2 stroke volume index (Figure 3A). In alternate examinations, continuous wave Doppler detected a 5.3 m/s late-peaking velocity (112 mm Hg peak gradient) through the LVOT and AV, and pulse wave Doppler identified an ∼3 m/s late-peaking velocity at the LVOT (Figures 3B and 3C). Calculation of the peak gradient across the fixed AV obstruction using the Bernoulli equation yielded 64 mm Hg. Cardiac magnetic resonance demonstrated moderate basal septal hypertrophy, with 1.8-cm maximum wall thickness (Figure 4A), along with severely restricted movement of the AV leaflets with proton-dephasing through the LVOT and across the AV, suggestive of hemodynamic obstruction (Figure 4B). Computed tomography for TAVR planning demonstrated a severely calcified AV with a calcium score of 2,863. Cardiac catheterization revealed a peak-to-peak gradient of 131 mm Hg across the LVOT and AV and 63 mm Hg across the AV (308 mm Hg peak LV pressure, 177 mm Hg peak aortic pressure) (Figure 5). Coronary angiogram identified no significant obstructive disease.
Figure 1.
Baseline Electrocardiogram
Electrocardiogram demonstrated sinus bradycardia.
Figure 2.
Diagnosis of Hypertrophic Cardiomyopathy by Echocardiography
(A) Parasternal long-axis view demonstrated 2.0-cm basal septal hypertrophy (red arrow), along with calcification and restricted motion of the aortic valve leaflets (yellow arrow). (B) M-mode across the mitral valve demonstrated systolic anterior motion, with systolic displacement of the anterior mitral valve leaflet (red arrow) toward the left ventricular outflow tract.
Figure 3.
Baseline Hemodynamics by Echocardiography
(A) Continuous-wave Doppler across the left ventricular outflow tract (LVOT) and aortic valve (AV) identified a 4 m/s mid-systolic peak velocity and 34 mm Hg mean gradient. In an alternate examination, (B) continuous-wave Doppler detected a 5.3 m/s late-peaking velocity across the LVOT and AV and (C) pulse wave Doppler identified a 3 m/s late-peaking velocity at the LVOT.
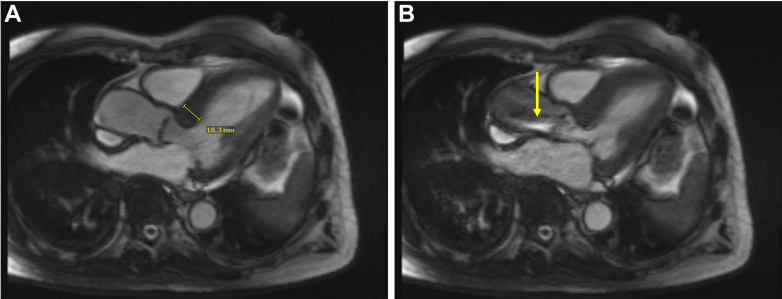
Figure 4.
Baseline Measurements by Cardiac Magnetic Resonance
Baseline cardiac magnetic resonance demonstrated (A) 1.8-cm basal septal hypertrophy and (B) severely restricted movement of the aortic valve leaflets with proton-dephasing across the left ventricular outflow tract and aortic valve (yellow arrow).
Figure 5.
Baseline Hemodynamics by Cardiac Catheterization
Representative tracing demonstrating peak-to-peak pressure differences between the left ventricle (yellow) and aorta (teal) before and after withdrawal of catheter from the left ventricle (LV) toward the cranial aspect of the left ventricular outflow tract (LVOT). Electrocardiogram leads II, aVL, and V1 are shown in black. Plethysmography tracing is shown in blue.
Management
The clinical data were consistent with HCM associated with dynamic outflow tract obstruction and moderate-to-severe AS. A late-peaking outflow tract obstruction >50 mm Hg present at rest or with exertion, accompanied by symptoms, warrants consideration of septal reduction therapy (SRT) when medical therapy options have been exhausted.1 The patient’s previously prescribed nifedipine was discontinued to avoid exacerbating outflow tract obstruction with an arterial vasodilator in a normotensive patient. The patient was already on maximally tolerated beta-blocker dosing, limited by resting bradycardia. Disopyramide was deferred because of a history of urinary retention and benign prostatic hyperplasia. Additionally, the patient was not suitable for myosin-inhibitor therapy due to concomitant AS, an exclusion criterion in the evaluation of myosin-inhibitor efficacy.2
Sequential dynamic and fixed obstructions of the LVOT pose a high risk for hemodynamic collapse as a function of hyperdynamic left ventricle if the distal fixed obstruction is relieved ahead of the proximal dynamic outflow obstruction.3,4 Accordingly, SRT was prioritized to adequately address LVOT obstruction prior to potential valve replacement. Invasive therapy involving septal myectomy and surgical aortic valve replacement was considered. However, given his age, frailty, high risk for surgery, and patient preference, the patient was referred for SRT with minimally invasive catheter-based alcohol septal ablation (ASA) and future discussion of TAVR.
The patient underwent successful ASA to the first septal perforator of the left anterior descending artery. Four-month post-procedural transthoracic echocardiography demonstrated moderate basal septal thickening of 2.0 cm without LV dysfunction (Figure 6A). AV leaflet movement remained restricted, and peak velocity across the LVOT and AV remained elevated at 4.1 m/s, with a 43 mm Hg mean gradient, 0.72 cm2/m2 AVAi, 0.43 DVI, and 75 mL/m2 stroke volume index, consistent with moderate AS in the setting of high cardiac output (Figures 6B and 6C). A late-peaking velocity >30 mm Hg could not be detected across the LVOT. The patient denied shortness of breath with ordinary activities and was able to climb at least 2 flights of stairs without exertional limitation.
Figure 6.
Follow-Up Echocardiography
Four-month follow-up echocardiography demonstrated (A) 2.0-cm basal septal thickness and (B and C) moderate aortic stenosis with high flow.
Discussion
HCM is a genetic cardiomyopathy inherited in autosomal dominant fashion and defined by pathologic hypertrophy of the ventricular myocardium (>1.5 cm) and structurally aberrant mitral valve and/or subvalvular apparatus.5,6 Structural features of HCM can provoke LVOT obstruction to blood flow during the cardiac cycle, leading to symptoms such as dyspnea and syncope.1 Studies estimate the prevalence of HCM to be 1:500 to 1:200 in the general population, affecting more than 20 million individuals worldwide.1 However, HCM is often underdiagnosed, especially among patients presenting with heart failure and valve disease.1
Herein we describe the clinical evaluation of an 82-year-old man with progressive exertional intolerance associated with obstructive HCM and moderate-to-severe AS. The patient was referred for ASA ahead of TAVR given risk of rapid hemodynamic collapse associated with sudden afterload reduction after TAVR in patients with marked LV hypertrophy and/or HCM with dynamic outflow tract obstruction.3,4 ASA resulted in resolution of heart failure symptoms (from NYHA functional class III to I) and improvement in AV hemodynamics (from DVI of 0.33 to 0.43 and AVAi of 0.45 to 0.72 cm2/m2), reclassifying the AS grade as moderate. The patient no longer met criteria for urgent or semiurgent valve replacement and could be monitored in the outpatient setting.7
Follow-Up
The patient continued to remain asymptomatic at 4 months of follow-up. No further intervention was indicated.
Conclusions
The presence of sequential outflow tract obstructions complicates the hemodynamic and clinical assessment of AV disease.8 A clinician may be led to believe that AV disease is the primary cause of symptoms if underlying obstructive HCM is not considered. However, HCM is associated with worse in-hospital outcomes, including cardiogenic shock, renal failure, and death, among patients undergoing TAVR.3,4,9,10 We thus advise careful assessment of underlying structural heart disease, particularly obstructive HCM, in patients being evaluated for AV disease.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Ommen S.R., Mital S., Burke M.A., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2020;76(25):e159–e240. doi: 10.1016/j.jacc.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 2.Olivotto I., Oreziak A., Barriales-Villa R., et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;396(10253):759–769. doi: 10.1016/s0140-6736(20)31792-x. [DOI] [PubMed] [Google Scholar]
- 3.Maron M.S., Olivotto I., Harrigan C., et al. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation. 2011;124(1):40–47. doi: 10.1161/circulationaha.110.985812. [DOI] [PubMed] [Google Scholar]
- 4.Kwon D.H., Setser R.M., Thamilarasan M., et al. Abnormal papillary muscle morphology is independently associated with increased left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Heart. 2008;94(10):1295–1301. doi: 10.1136/hrt.2007.118018. [DOI] [PubMed] [Google Scholar]
- 5.Koliastasis L., Drakopoulou M., Latsios G., et al. Overcoming the obstacle of suicide left ventricle after transcatheter aortic valve replacement phenomenon. JACC Case Rep. 2023;26 doi: 10.1016/j.jaccas.2023.102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lioufas P.A., Kelly D.N., Brooks K.S., Marasco S.F. Unexpected suicide left ventricle post-surgical aortic valve replacement requiring veno-arterial extracorporeal membrane oxygenation support despite gold-standard therapy: a case report. Eur Heart J Case Rep. 2022;6(2) doi: 10.1093/ehjcr/ytac020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):450–500. doi: 10.1016/j.jacc.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Geske J.B., Cullen M.W., Sorajja P., Ommen S.R., Nishimura R.A. Assessment of left ventricular outflow gradient: hypertrophic cardiomyopathy versus aortic valvular stenosis. JACC Cardiovasc Interv. 2012;5(6):675–681. doi: 10.1016/j.jcin.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Bandyopadhyay D., Chakraborty S., Amgai B., et al. Association of hypertrophic obstructive cardiomyopathy with outcomes following transcatheter aortic valve replacement. JAMA Netw Open. 2020;3(2) doi: 10.1001/jamanetworkopen.2019.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasahira Y., Yamada R., Doi N., Uemura S. Urgent percutaneous transluminal septal myocardial ablation for left ventricular outflow tract obstruction exacerbated after surgical aortic valve replacement. Clin Case Rep. 2021;9(9) doi: 10.1002/ccr3.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]