Abstract
Giant cell myocarditis (GCM) and cardiac sarcoidosis share clinical and histologic features, but whether they represent separate processes or lie on an inflammatory cardiomyopathy spectrum is unclear. We present a case of cardiogenic shock thought to be secondary to biopsy-proven GCM with a subsequent post-transplant diagnosis of sarcoidosis through 18-fluorodeoxyglucose positron emission tomography and biopsy.
Key Words: cardiac sarcoidosis, giant cell myocarditis, inflammatory cardiomyopathy
Visual Summary
Visual Summary. Clinical Timeline
Longitudinal view of all relevant clinical events and timing of diagnoses are summarized. Bx = biopsy; GCM = giant cell myocarditis; HF = heart failure; PET = positron emission tomography; SCC = squamous cell carcinoma.
History of Presentation
A 55-year-old man with nonischemic cardiomyopathy (NICM) presented to our center (MedStar Washington Hospital Center, Washington, DC) with cardiogenic shock associated with ventricular arrhythmias. He was not known to our team on arrival to our institution. The patient reported a diagnosis of heart failure (HF) 5 years previously that was managed by an outside cardiologist and was complicated by complete heart block necessitating dual-chamber pacemaker placement 1 year after diagnosis. Results of an ischemic evaluation were negative; however, no additional investigation of high-grade heart block was conducted. His ejection fraction had remained stable at 45% until 6 months before hospitalization, when his ejection fraction worsened to <35% despite guideline-directed medical therapy.
Take-Home Messages
-
•
This case highlights the differential diagnosis of high-grade atrioventricular block and inflammatory cardiomyopathy, including CS, GCM, and arrhythmogenic cardiomyopathy.
-
•
FDG PET and genetic testing play important roles in the initial evaluation of inflammatory cardiomyopathy.
-
•
Although differences exist between CS and GCM, several similarities make the diagnosis challenging.
-
•
Additional studies are needed to determine whether the 2 entities exist on a spectrum of disease or represent distinct disorders.
Right heart catheterization on arrival confirmed a low cardiac output state. Antiarrhythmic agents and inotropic therapy were initiated with concomitant intra-aortic balloon pump placement, resulting in hemodynamic stabilization. Given his acute shock presentation with ventricular arrhythmia, an endomyocardial biopsy was pursued to investigate an underlying disease of suspected inflammatory cardiomyopathy.
Past Medical History
The patient had no other medical disease or family history of cardiac disease or unexplained sudden death. Minimal alcohol use and lifelong tobacco and drug abstinence were noted.
Differential Diagnosis
Our initial differential diagnosis was focused on the acute presentation of an inflammatory or genetic cardiomyopathy, including viral myocarditis, giant cell myocarditis (GCM), cardiac sarcoidosis (CS), lamin A/C disease, or arrhythmogenic cardiomyopathy.
Investigations
Results of laboratory testing were notable for normal kidney and liver function, with an N-terminal pro–B-type natriuretic peptide level of 1,749 pg/mL and a high-sensitivity troponin level of 388 ng/L. Chest imaging showed no hilar or mediastinal lymphadenopathy. T-Spot (Quest Diagnostics) tuberculosis test results and Lyme titers were normal, although testing for atypical mycobacterial infection was not performed. Results of pulmonary function testing were normal, as were levels of angiotensin-converting enzyme, calcium,1,25-dihydroxy-vitamin D3, and immunoglobulin. Genetic testing for familial or arrhythmogenic cardiomyopathy would have been important diagnostic data, but it was not conducted because of logistical limitations and inpatient status.
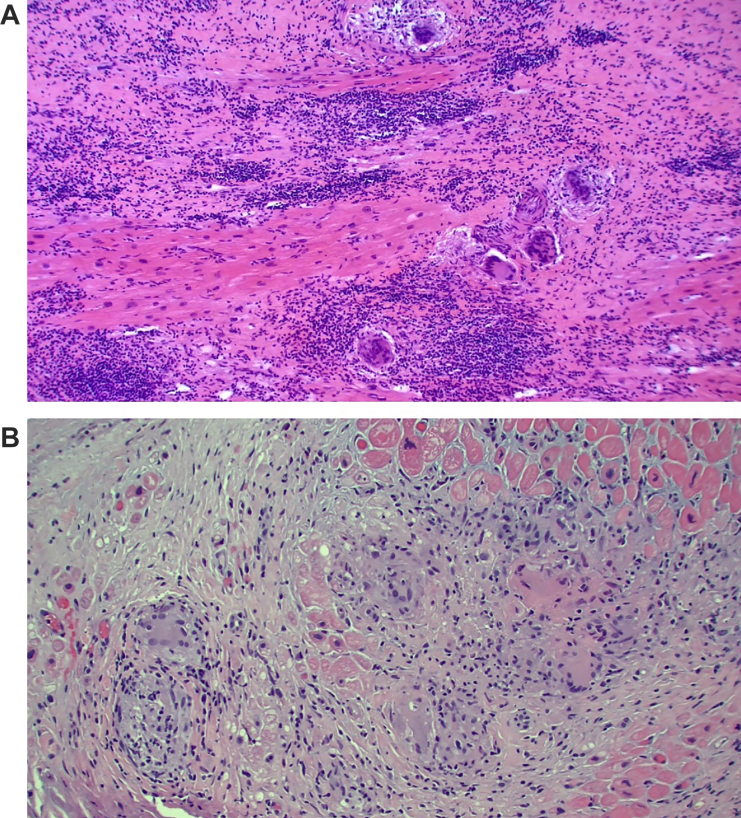
An echocardiogram demonstrated a mildly dilated left ventricle with concentric left ventricular hypertrophy and an ejection fraction of 20% to 25% with aneurysmal and akinetic basal inferior and inferoseptal walls with biatrial dilation. Left heart catheterization showed no coronary artery disease. Fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) would have been another crucial investigation to identify the presence of cardiac and extracardiac inflammation; however, this was not attainable because of the patient’s hemodynamic instability and the presence of temporary mechanical circulatory support. Endomyocardial biopsy demonstrated free multinucleated giant cells on a background of chronic inflammatory cells and myocardial fibrosis without granuloma or myocardial necrosis, a picture thought by our pathology and clinical team to be consistent with GCM given the absence of granuloma (Figure 1A).
Figure 1.
Initial Endomyocardial Biopsy and Native Heart Pathology Findings
(A) Endomyocardial biopsy with evidence of giant cells. (B) Pathologic features of the explanted heart with evidence of giant cells.
Management
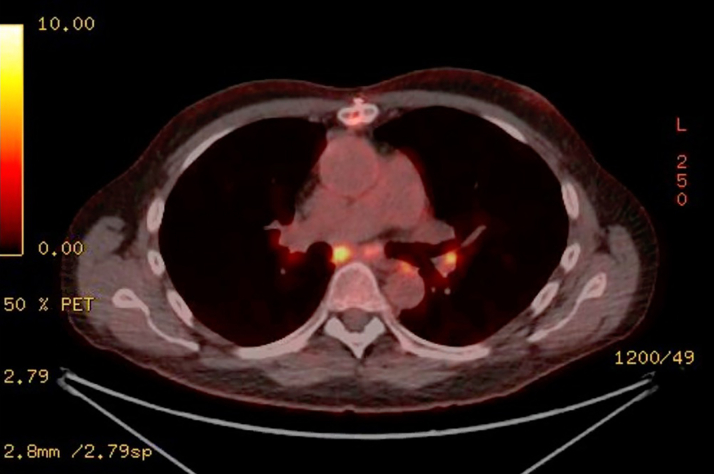
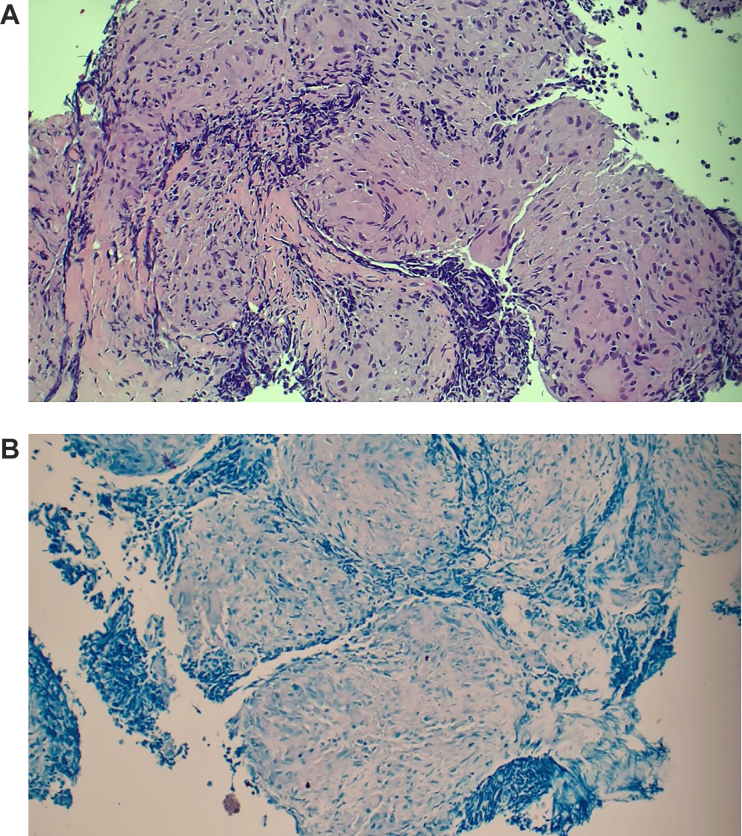
The patient was started on cyclosporine, mycophenolate mofetil, and an intravenous solumedrol pulse with subsequent taper. His cardiogenic shock was refractory to these treatments, necessitating heart transplant (HT) listing. A suitable donor was identified, and the patient underwent HT with an unremarkable post-HT course. On pathologic examination, the explanted heart demonstrated myocyte hypertrophy with multifocal interstitial fibrosis, lymphoplasmacytic cell infiltrates, and numerous giant cells believed to be consistent with GCM on top of chronic cardiomyopathy (Figure 1B). Five months post-HT, a tonsillar mass was identified that was biopsied and revealed to be squamous cell carcinoma. The patient underwent full-body FDG PET for cancer staging and was noted to have FDG-avid hilar lymphadenopathy (Figure 2). A transbronchial lymph node biopsy was subsequently performed, revealing noncaseating granulomas consistent with pulmonary sarcoidosis (Figures 3A and 3B), thus undermining the initial pre-HT diagnosis of GCM. No other post-HT clinical events were associated with the discovery of this lymphadenopathy.
Figure 2.
Post-Transplant Fluorine-18 Fluorodeoxyglucose PET Scan
Fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging demonstrates hilar FDG avidity.
Figure 3.
Post-Transplant Lymph Node Biopsy
(A) Biopsy pathology findings of fluorine-18 fluorodeoxyglucose (FDG)-avid lymph node, revealing noncaseating granuloma. (B) Biopsy pathology findings of FDG-avid lymph node with negative acid-fast bacilli stain.
Discussion
Giant cell myocarditis is a rapidly progressive cause of HF caused by T-cell lymphocyte-mediated myocardial inflammation.1 It affects younger men aged 30 to 45 years and, less commonly, women of the same age; both men and women may present with high-degree atrioventricular block, ventricular arrythmia, and myocardial injury.1 The diagnosis is made through histopathologic findings alongside clinical suspicion, and treatment consists of cyclosporine-based immunosuppression. Without treatment, the disease has 90% mortality at 1 year and a median diagnosis to time of death of 6 months. Patients may be evaluated for advanced HF therapies such as HT when there are no signs of myocardial recovery, and these patients are frequently stabilized with temporary mechanical circulatory support to allow evaluation for destination therapies. Therefore, a high index of suspicion and timely diagnostic testing with endomyocardial biopsy are crucial.2
Cardiac sarcoidosis can manifest insidiously and can be discovered as an advanced form of disease, including late-stage HF. The disease hallmark is the presence of noncaseating granuloma formation. The pathogenesis remains unknown, but CS is often chronic and slowly developing, with end-stage forms requiring advanced HF therapies.3 Cardiac magnetic resonance (CMR) and FDG PET are the best modalities to use when evaluating for CS because the patchy nature of disease in the myocardium can lead to false negative results on endomyocardial biopsy. If discovered early, CS can be monitored with FDG PET or CMR and treated with immunosuppression, with appropriate risk stratification for ventricular arrhythmia.
The diagnosis of GCM vs CS is often challenging, and the spectrum of disease can overlap. On the basis of these features, our patient’s initial diagnosis of GCM could have been supported by his age, cardiogenic shock presentation, ventricular arrhythmia, and multinucleated giant cells on endomyocardial biopsy. Conversely, his history of NICM with wall thinning noted on an echocardiogram and the absence of severe myocardial injury argued for chronicity and against GCM.4 Although immunosuppression of some form would have been warranted for either process, the diagnosis was challenging and would have implications for future surveillance and management. Even though endomyocardial biopsy and heart explant pathology suggested GCM as the diagnosis, we could not fully exclude CS as a cause, in retrospect. FDG PET imaging pre-HT would have been essential to screen for extracardiac sarcoid; however, this could not be done. Given the high mortality associated with GCM, his age at presentation, and the ventricular arrhythmia presence, therapy targeted to GCM was pursued to stabilize our patient to HT.
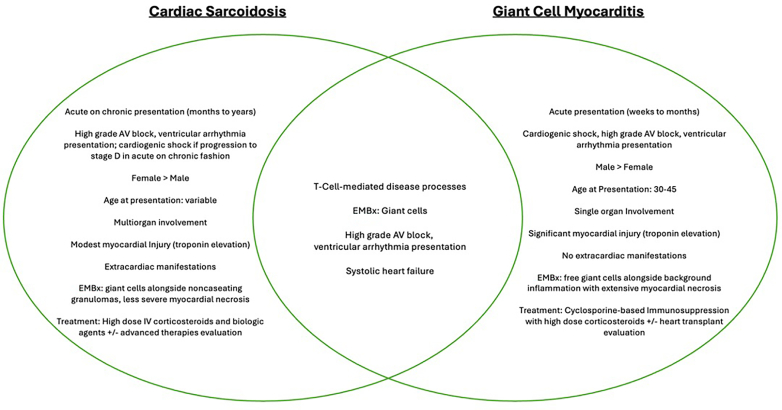
It is well established that GCM and CS share clinical and pathologic features. Both diseases are T-lymphocyte mediated and manifest with high-grade atrioventricular block alongside ventricular arrythmia.5 However, they differ in natural history, the presence of extracardiac manifestations, the urgency of immunosuppression, and their overall trajectory and prognosis. GCM manifests with rapid evolution and acute deterioration, affecting only the myocardium, whereas CS manifests either chronically or in an acute on chronic manner, often with multiorgan involvement of granulomatous disease. Giant cells may be found on pathology examination in both diseases but are unfortunately not specific for either entity.5,6 Salient differences and “watershed” similarities between the 2 entities are summarized in Figure 4.
Figure 4.
Clinical Differences and Overlap Between Giant Cell Myocarditis and Cardiac Sarcoidosis
Salient pathologic and clinical differences between the 2 inflammatory processes. AV = atrioventricular; EMBx = endomyocardial biopsy; IV = intravenous.
A study by Nordenswan et al4 described the histopathologic features of 351 cases of CS and 28 of GCM and posited that the 2 entities exist on a spectrum of what is ultimately the same disease. These investigators found that patients with GCM presented with more advanced HF, less ventricular arrhythmia, and higher biomarkers of myocardial injury compared with patients with CS. However, despite a histopathologic diagnosis, a patient’s degree of myocardial injury, rather than histologic findings, was the strongest indicator of prognosis and the need for HT. In the study by Nordenswan et al,4 on readjudication of histopathologic findings, 62% of GCM cases were reclassified as CS, thereby suggesting that GCM is a form of severe and aggressive CS rather than its own entity. An editorial by Birnie et al7 suggested otherwise—that CS and GCM remain 2 separate treatable entities. Birnie et al7 re-reviewed the data, which, after adjustment for left ventricular function and clinical status at presentation, revealed a significantly worse mortality, HT-free survival, and prognosis in GCM compared with CS. These investigators argued that because sarcoidosis is a systemic disease and GCM solely affects the myocardium, the 2 must be distinct entities.7
At first glance, our case could support either theory, that the initial diagnosis was misclassified (and the disease was CS all along), or that this was indeed histologic GCM with an acute presentation, existing on a spectrum of inflammatory cardiomyopathy with CS. However, the key to diagnosis in this case was the patient’s post-HT full-body FDG PET. In hindsight, the patient’s minimal myocardial injury, long-standing systolic HF, and nonspecific nature of giant cells on pathology examination support an initial diagnosis of CS, later confirmed by biopsy-proven extracardiac sarcoidosis. FDG PET pre-HT may have allowed us to cinch the diagnosis of CS from the outset and superseded the conclusion derived from the endomyocardial and heart explant pathology findings.
Finally, this case highlights the utility of whole body FDG-PET imaging after HT to aid in or confirm a diagnosis of multisystem sarcoidosis and screen for extracardiac disease. Although CMR and FDG PET remain the mainstay of management of CS in chronic HF, no guidelines exist to inform surveillance for recurrent cardiac inflammation or detection of extracardiac disease after advanced HF therapies for inflammatory cardiomyopathies such as GCM or CS despite known evidence that both diseases can recur post-HT.8,9 Prospective studies are needed to determine the role of surveillance imaging after HT and its ability, as in this case, to elucidate an underlying cardiomyopathy diagnosis.
Follow-Up
After treatment of the primary malignant disease with carboplatin monotherapy, the patient has been cancer free. He remains on an immunosuppression regimen of tacrolimus and sirolimus with normal allograft function and has been maintained on prednisone, 5 mg daily, for sarcoidosis.
Conclusions
This case calls attention to the controversy surrounding the diagnosis of GCM and CS and offers insight into the spectrum of clinical manifestations of inflammatory cardiomyopathy, as well as diagnostic pitfalls and challenges in work-up. Early FDG PET imaging is key for evaluating CS in patients with suspected inflammatory cardiomyopathy. Distinguishing between GCM and CS remains challenging given the overlap in pathologic and clinical features, and ongoing controversy exists about whether the diseases lie along the same spectrum or are 2 separate processes.
Funding Support and Author Disclosures
Dr Sheikh has received institutional research support from Abbott, Alnylam, and Akcea; has received honoraria for educational presentations from Abbott; and has served as a consultant for Alnylam. Dr Gupta has served as a consultant for and has received honoraria for educational presentations from CVRx. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Bang V., Ganatra S., Shah S.P., et al. Management of patients with giant cell myocarditis. J Am Coll Cardiol. 2021;77:1122–1134. doi: 10.1016/j.jacc.2020.11.074. [DOI] [PubMed] [Google Scholar]
- 2.Kociol R.D., Cooper L.T., Fang J.C., et al. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141(6):e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 3.Kouranos V., Sharma R. Cardiac sarcoidosis: state-of-the-art review. Heart. 2021;107:1591–1599. doi: 10.1136/heartjnl-2019-316442. [DOI] [PubMed] [Google Scholar]
- 4.Nordenswan H., Lehtonen J., Ekström K., et al. Manifestations and outcome of cardiac sarcoidosis and idiopathic giant cell myocarditis by 25-year nationwide cohorts. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okura Y., Dec G.W., Hare J.M., et al. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003;41:322–329. doi: 10.1016/s0735-1097(02)02715-8. [DOI] [PubMed] [Google Scholar]
- 6.Cooper L.T., Berry G.J., Shabetai R. Idiopathic giant-cell myocarditis — natural history and treatment. N Engl J Med. 1997;336(26):1860–1866. doi: 10.1056/NEJM199706263362603. [DOI] [PubMed] [Google Scholar]
- 7.Birnie D.H., Nair V., Veinot J.P. Cardiac sarcoidosis and giant cell myocarditis: actually, 2 ends of the same disease? J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frankel E.S., Hajduczok A.G., Rajapreyar I.N., Brailovsky Y. Recurrent giant cell myocarditis after heart transplant: a case report. Eur Heart J Case Rep. 2022;6(9) doi: 10.1093/ehjcr/ytac362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandya K., Vaidya A., Cheng R., Baran D., Depasquale E. Management of cardiac sarcoidosis post heart transplantation: survey of transplant centers. J Heart Lung Transplant. 2020;39(4):S261. doi: 10.1016/j.healun.2020.01.567. [DOI] [Google Scholar]