Abstract
Rheumatic heart disease (RHD) and endomyocardial fibrosis (EMF) are major causes of cardiac disease in low-income countries. We present a case of a patient with mitral stenosis and restrictive cardiomyopathy, initially attributed to severe RHD, but with disease progression despite valve replacement, likely secondary to previously undiagnosed EMF.
Key Words: endomyocardial fibrosis, global cardiovascular disease, restrictive cardiomyopathy, rheumatic heart disease
Graphical Abstract
History of Presentation
A 55-year-old female patient who was born in Nigeria and who had a history of rheumatic mitral stenosis (MS) treated with bioprosthetic mitral valve replacement 1 year before presentation, severe pulmonary hypertension, valvular atrial fibrillation, restrictive cardiomyopathy, and sick sinus syndrome seen on Holter monitoring presented to the hospital (Grady Memorial Hospital, Atlanta, Georgia, USA) with fevers, chills, abdominal pain, and increased somnolence over 2 days, in the setting of a recent unsuccessful leadless pacemaker implantation.
Learning Objectives
-
•
To consider endocardial fibrosis as a relatively common although poorly understood cause of restrictive cardiomyopathy in patients from endemic areas.
-
•
To understand the characteristic features of EMF and the role of multimodal imaging in diagnosis.
She was febrile to 39.6 °C and tachycardic. She was ill-appearing and diaphoretic, and her cardiac examination was notable for a systolic murmur at the left sternal border with an open right groin femoral access site that was nontender and without fluctuance or induration. Her initial laboratory results were as follows: white blood cell count, 11.0 × 103/µL; platelets, 95 × 103/μL; creatinine, to 1.8 mg/dL from a baseline of 1.1 mg/dL; C-reactive protein, 140 mg/L; and B-type natriuretic peptide, 237 pg/mL. The electrocardiogram showed sinus rhythm at the time of presentation. Blood culture results were rapidly positive for Streptococcus agalactiae (group B strep), presumed secondary to prosthetic valve endocarditis, and she was started on intravenous antibiotics.
Past Medical History
Rheumatic heart disease (RHD) was identified on an echocardiogram 4 years earlier, during a hospitalization for COVID-19 with an incidentally noted moderate-sized pericardial effusion. She was found to have rheumatic mitral valve changes, with commissural fusion and severe diastolic doming of the mitral leaflets, a mean mitral diastolic gradient 6 to 7 mm Hg, biatrial enlargement, a small left ventricle with a left ventricular ejection fraction (EF) >70%, and moderately to severely reduced right ventricular (RV) systolic function (Figure 1). A transesophageal echocardiogram (TEE) was pursued and revealed a planimetered mitral valve area (MVA) of 2.66 cm2 and a mean diastolic gradient of 6 mm Hg, consistent with moderate MS. Right-sided heart catheterization measured the following: pulmonary artery pressure, 43/15 mm Hg with mean 30 mm Hg; pulmonary capillary wedge pressure, 15 mm Hg; and a Fick cardiac output of 6.31 L/min. Cardiac magnetic resonance (CMR) identified severe biventricular diastolic dysfunction and right systolic dysfunction along with MS consistent with rheumatic valve disease. The patient was monitored with annual echocardiograms, with the TEE 1 year previously showing a diastolic mean gradient of 11 mm Hg and a planimetered MVA of 0.76 cm2, with mild to moderate tricuspid regurgitation and a moderate-sized pericardial effusion (Video 1). Because of her high Wilkins echo score, she was not a candidate for balloon valvuloplasty and instead underwent bioprosthetic mitral valve replacement and tricuspid valve repair. However, she continued to require a maintenance loop diuretic agent and was following a regimen of carvedilol, spironolactone, and dapagliflozin.
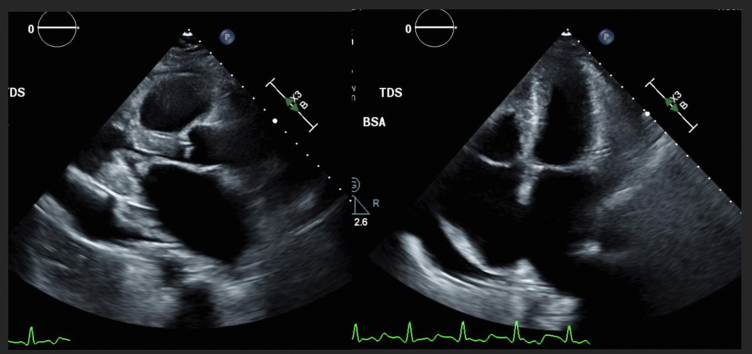
Figure 1.
Transthoracic Echocardiogram From 4 Years Earlier
Imaging shows rheumatic changes of the mitral valve with a doming appearance of the leaflets during diastole, biatrial enlargement with small ventricles, and a large pericardial effusion. The mean mitral diastolic gradient was 6 mm Hg. BSA = body surface area.
Differential Diagnosis
Differential diagnoses at the time of presentation included prosthetic valve endocarditis, pulmonary embolism, pericarditis, cardiac tamponade, heart failure exacerbation, and pneumonia.
Investigations
Initial imaging involved computed tomography angiography of the chest, which showed an obliterated RV cavity and severe biatrial dilation (Figure 2). A transthoracic echocardiogram showed an EF of 60% to 65% with an obliterated right ventricle, flattened interventricular septum, biatrial enlargement, and a bioprosthetic mitral valve with mildly thickened leaflets (Videos 2 and 3). A TEE had negative findings for prosthetic valve vegetations, although the findings were concerning for a Gerbode defect, a left ventricular outflow tract (LVOT)–to–right atrial shunt (Video 4). CMR demonstrated smooth myocardium with linear chronic hypoenhancement suggestive of a chronic thrombus or fibrosis with obliteration of the RV apex along with dilation of the RV outflow tract (RVOT) (Figures 3, 4 and 5). Of note, these findings were retroactively identified on previous CMR from 4 years earlier and were consistent with findings commonly seen in endomyocardial fibrosis (EMF). CMR findings were negative for intracardiac shunts but revealed LVOT obstruction secondary to angulation of the prosthetic mitral valve with systolic flow acceleration that was the culprit for the previously suggested Gerbode defect. On the basis of the foregoing multimodal imaging findings, the patient received a formal diagnosis of EMF.
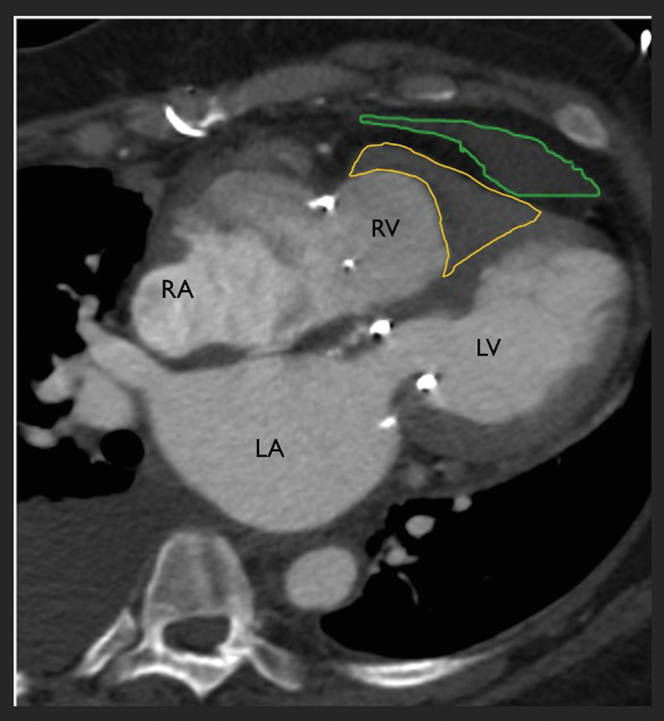
Figure 2.
Computed Tomography Angiography 4-Chamber Long Projection
Imaging following mitral valve replacement and tricuspid valve repair demonstrates obliteration of the right ventricular cavity with the classic V-shaped configuration described in endomyocardial fibrosis (yellow border). The left ventricular myocardium is relatively smooth, and there is a pericardial effusion (green border). LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle.
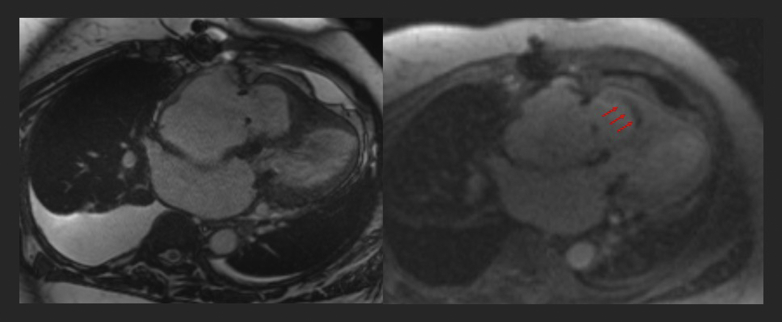
Figure 3.
Cardiac Magnetic Resonance 4-Chamber Long View Post-Mitral Valve Repair
Imaging reveals obliteration of the right ventricular cavity with smooth left ventricular myocardium and moderately to severely dilated atria. Perfusion scan demonstrates thin linear chronic hypoenhancement (arrows), suggestive of chronic linear thrombus or scar in the setting of endomyocardial fibrosis. This was also retroactively noted on previous cardiac magnetic resonance 4 years earlier and was the likely culprit for failure to capture a leadless pacemaker.
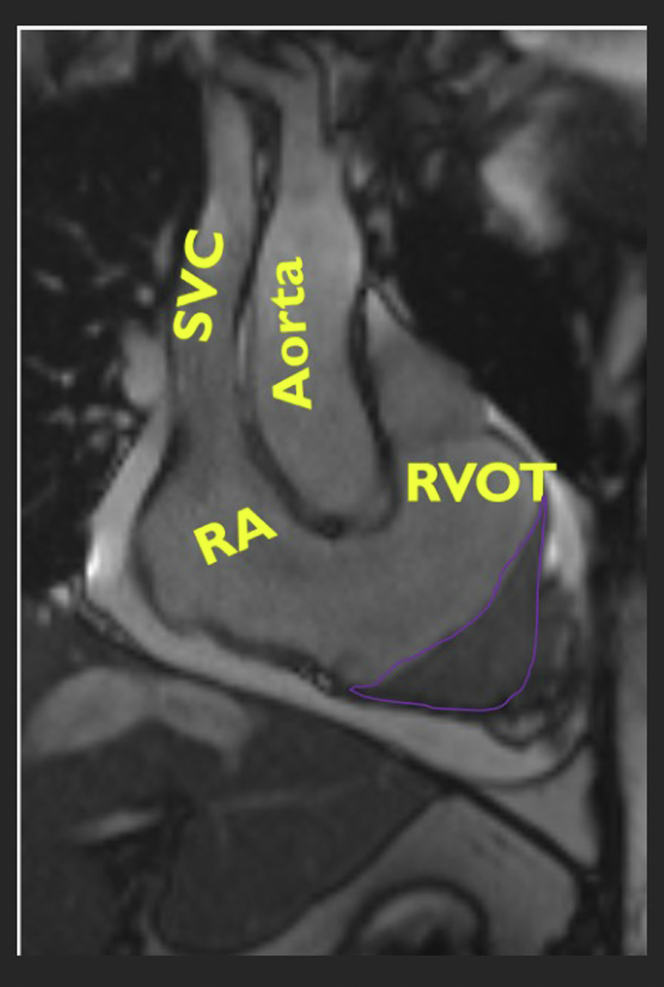
Figure 4.
Cardiac Magnetic Resonance Right Ventricular Inflow Tract View
Imaging shows a dilated right ventricular outflow tract (RVOT) with a severely obliterated and scarred right ventricular cavity (outlined in purple). RA = right atrium; SVC = superior vena cava.
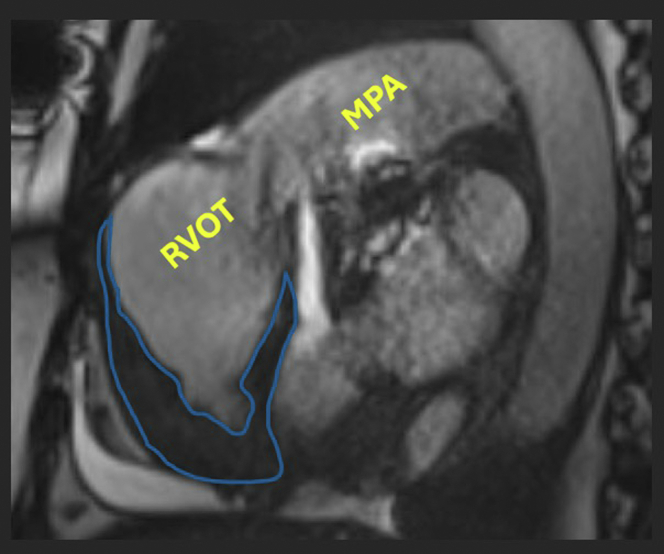
Figure 5.
Apical-Predominant Obliteration of the Right Ventricular Cavity
Scarring is outlined in blue. Of note, the right ventricular outflow tract (RVOT) and main pulmonary artery (MPA) have increased in dilatation from 4 years earlier, suggesting worsening pulmonary hypertension secondary to endomyocardial fibrosis.
Management
The rest of her course was otherwise unremarkable because her bacteremia, likely from a culture-negative genitourinary vs cutaneous source, resolved with intravenous antibiotics. She was discharged with outpatient follow-up in the cardiology clinic.
Discussion
This is a complex case of a patient with diastolic heart failure initially attributed to RHD with worsening heart failure despite successful valve repair. During the 3 years of observation, she continued to have persistent symptoms, attributed to progression of her rheumatic MS, but she had unexplained restrictive filling patterns, pulmonary hypertension out of proportion to previous moderate MS, and poorly understood biventricular apical obliteration. In retrospect, she was likely received a misdiagnosis of severe MS, which explains her lack of clinical improvement after a mitral valve replacement. It was not until 1 year previously that EMF was formally diagnosed and thought to be the potentially larger contributing factor to her heart failure.
This case demonstrates the difficulty of recognizing 2 concomitant causes of cardiomyopathy that can manifest in similar populations from tropical and lower-resourced regions. Her heart failure was initially attributed to only a single cause, RHD, and EMF was not considered at the time of initial presentation because of the lack of widespread familiarity with the condition and its diagnostic features. Additionally, although EMF can cause fibrosis that extends to the valve, echocardiography demonstrated the typical “hockey-stick” appearance caused by restricted motion of the anterior mitral leaflet that was strongly suggestive of RHD.1 On retrospective review, CMR 4 years earlier showed similar findings of a 3-layered delayed enhancement pattern in the RV apex (double V sign), suggestive of chronic EMF and thrombosis with associated shrunken ventricles and biatrial enlargement. Our patient had also developed dilatation of her RVOT to accommodate for obliteration of her RV apex. Most recently, she experienced device failure with leadless pacemaker implantation because of the inability to implant the device in smoothened endocardium and noncapture of pacing stimuli from extensive myocardial fibrosis. It was not until 3 years later that a cardiothoracic radiologist who was familiar with EMF provided the formal diagnosis, thus demonstrating the importance of awareness and maintaining an index of suspicion in patients presenting from endemic regions.
EMF is a major contributor to heart failure in low-income countries and is estimated to affect up to 10 million people globally.2,3 It is most highly prevalent in tropical and subtropical areas of Africa, Asia, and South America.4 It is characterized by deposition of fibrous tissue in the endocardium resulting in restrictive cardiomyopathy.2 Unfortunately, the diagnosis is often late in the disease course, and this delay contributes to the high EMF mortality of approximately 75% within 2 years.5
The pathogenesis and origin of EMF are unclear but likely multifactorial from the combined effects of factors that induce an inflammatory state that causes EMF. These factors include hypereosinophilia, given the burden of parasitic diseases, dietary, environmental, and toxic factors. However, data on each of these causes are mixed,5, 6, 7 and it is hypothesized that the cause of EMF involves many coexisting triggers that lead to the hyperinflammatory state.
Patients in the early stage of the disease present with an acute febrile illness characterized by pancarditis and acute eosinophilia.8 Patients have recurrent flare-ups of disease characterized as worsening heart failure as it progresses into the chronic phase. The chronic phase generally manifests with biventricular failure and severe restrictive disease caused by myocardial fibrosis, often with severe cardiomegaly, arrhythmias, and volume overload.9
Echocardiography has emerged as a cost-effective gold standard diagnostic tool for both EMF and RHD. A scoring system composed of major and minor criteria has been created to diagnose and assess the severity of EMF,10 with the diagnosis made on the basis of 2 major criteria or 1 major criterion and 2 minor criteria, although this scoring system has not been validated. Endocardial biopsy is no longer frequently used given the patchy nature of EMF and a 50% yield. CMR provides excellent morphologic and functional analysis of the myocardium but is limited by cost and access.11
Treatment is often limited given that patients are often at the end stage on presentation. Medical management involves diuretic agents to treat symptoms of volume overload, and angiotensin-converting enzyme inhibitors are theorized to slow the progression of fibrosis.12 There is growing evidence that early endocardial decortication and valve repair or replacement improve survival,13,14 but many patients are not suitable for surgery given the severity of disease. There are case reports of cardiac transplantation in these patients, but the data is limited.15
Follow-Up
The patient was taken off carvedilol and continues to require maintenance diuretic agents but is otherwise doing well and has not had any recurrent heart failure hospitalizations.
Conclusions
RHD and EMF are two common causes of cardiomyopathy in low-income countries, with significant geographic overlap. Although there is appropriate global awareness of RHD, EMF is a poorly understood and underrecognized condition that can lead to severe restrictive cardiomyopathy. Our patient had moderate rheumatic MS, and she continued to experience worsening heart failure after valve replacement, at which point multimodal imaging was suggestive of a concomitant diagnosis of advanced EMF. Additional research and improved awareness are needed to provide insight into how to treat this complex cardiac condition and to better understand the intersection of RHD and EMF.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
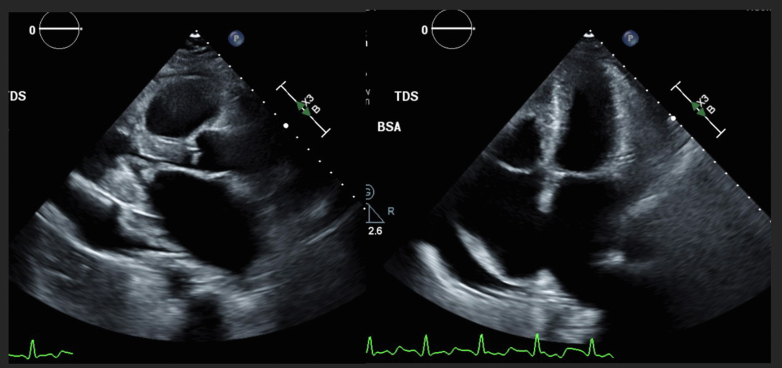
TEE From Early 1 Year Previously
Imaging shows severe rheumatic changes in the mitral valve with leaflet thickening and diastolic doming, small left ventricular cavity size, and a small right ventricle with apical infiltration. There is also a large pericardial effusion predominantly along the lateral wall, biapical enlargement (right more than left), and patchy endocardial scarring.
TTE From 6 Months Previously
Imaging reveals an ejection fraction of 60% to 65% with obliteration of the right ventricular apex and a shrunken right ventricle, a flattened septum, and a bioprosthetic mitral valve with mildly thickened leaflets but only mild mitral regurgitation.
Patchy Endocardial Scars Present on the Apical 2-Chamber View
TEE From 6 Months Previously Revealing Trace Mitral Regurgitation Without Evidence of Bioprosthetic Valve Endocarditis
There is a suggestion of a right-to-left shunt predominantly during late systole, initially concerning for Gerbode defect. Cardiac magnetic resonance was pursued for definitive diagnosis.
References
- 1.Pandian N.G., Kim J.K., Arias-Godinez J.A., et al. Recommendations for the use of echocardiography in the evaluation of rheumatic heart disease: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2023;36(1):3–28. doi: 10.1016/j.echo.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Bhatti K., Bandlamudi M., Lopez-Mattei J. StatPearls. StatPearls Publishing; 2024. Endomyocardial fibrosis [Updated 2022]https://www.ncbi.nlm.nih.gov/books/NBK513293/ [PubMed] [Google Scholar]
- 3.Mocumbi A.O., Stothard J.R., Correia-de-Sá P., Yacoub M. Endomyocardial fibrosis: an update after 70 years. Curr Cardiol Rep. 2019;21(11):148. doi: 10.1007/s11886-019-1244-3. [DOI] [PubMed] [Google Scholar]
- 4.Duraes A.R., de Souza Lima Bitar Y., Roever L., Neto M.G. Endomyocardial fibrosis: past, present, and future. Heart Fail Rev. 2020;25(5):725–730. doi: 10.1007/s10741-019-09848-4. [DOI] [PubMed] [Google Scholar]
- 5.Grimaldi A., Mocumbi A.O., Freers J., et al. Tropical endomyocardial fibrosis: natural history, challenges, and perspectives. Circulation. 2016;133(24):2503–2515. doi: 10.1161/CIRCULATIONAHA.115.021178. [DOI] [PubMed] [Google Scholar]
- 6.Patel A.K., Ziegler J.L., D'Arbela P.G., Somers K. Familial cases of endomyocardial fibrosis in Uganda. Br Med J. 1971;4(5783):331–334. doi: 10.1136/bmj.4.5783.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oke O.L. The role of hydrocyanic acid in nutrition. World Rev Nutr Diet. 1969;11:170–198. doi: 10.1159/000387578. [DOI] [PubMed] [Google Scholar]
- 8.Andy J.J., Ogunowo P.O., Akpan N.A., et al. Helminth associated hypereosinophilia and tropical endomyocardial fibrosis (EMF) in Nigeria. Acta Trop. 1998;69(2):127–140. doi: 10.1016/s0001-706x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- 9.Mocumbi A.O., Ferreira M.B. Neglected cardiovascular diseases in Africa: challenges and opportunities. J Am Coll Cardiol. 2010;55(7):680–687. doi: 10.1016/j.jacc.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 10.Mocumbi A.O., Ferreira M.B., Sidi D., Yacoub M.H. A population study of endomyocardial fibrosis in a rural area of Mozambique. N Engl J Med. 2008;359(1):43–49. doi: 10.1056/NEJMoa0708629. [DOI] [PubMed] [Google Scholar]
- 11.León D., Martín M., Corros C., Santamarta E., Costilla S., Lambert J.L. Usefulness of cardiac MRI in the early diagnosis of endomyocardial fibrosis. Rev Port Cardiol. 2012;31(5):401–402. doi: 10.1016/j.repc.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Mocumbi A.O., Yacoub S., Yacoub M.H. Neglected tropical cardiomyopathies: II. Endomyocardial fibrosis: myocardial disease. Heart. 2008;94(3):384–390. doi: 10.1136/hrt.2007.136101. [DOI] [PubMed] [Google Scholar]
- 13.Joshi R., Abraham S., Kumar A.S. New approach for complete endocardiectomy in left ventricular endomyocardial fibrosis. J Thorac Cardiovasc Surg. 2003;125(1):40–42. doi: 10.1067/mtc.2003.70. [DOI] [PubMed] [Google Scholar]
- 14.Schneider U., Jenni R., Turina J., et al. Long-term follow up of patients with endomyocardial fibrosis: effects of surgery. Heart. 1998;79(4):362–367. doi: 10.1136/hrt.79.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Freitas H.F., de Castro P.P., Chizzola P.R., Bocchi E.A. Transplante cardíaco em portadora de endomiocardiofibrose [Heart transplantation in a patient with endomyocardial fibrosis] Arq Bras Cardiol. 2005;84(1):49–50. doi: 10.1590/s0066-782x2005000100011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TEE From Early 1 Year Previously
Imaging shows severe rheumatic changes in the mitral valve with leaflet thickening and diastolic doming, small left ventricular cavity size, and a small right ventricle with apical infiltration. There is also a large pericardial effusion predominantly along the lateral wall, biapical enlargement (right more than left), and patchy endocardial scarring.
TTE From 6 Months Previously
Imaging reveals an ejection fraction of 60% to 65% with obliteration of the right ventricular apex and a shrunken right ventricle, a flattened septum, and a bioprosthetic mitral valve with mildly thickened leaflets but only mild mitral regurgitation.
Patchy Endocardial Scars Present on the Apical 2-Chamber View
TEE From 6 Months Previously Revealing Trace Mitral Regurgitation Without Evidence of Bioprosthetic Valve Endocarditis
There is a suggestion of a right-to-left shunt predominantly during late systole, initially concerning for Gerbode defect. Cardiac magnetic resonance was pursued for definitive diagnosis.