Abstract
Familial hypercholesterolemia (FH) is an inherited disorder of lipid metabolism that causes marked elevations in low-density lipoprotein cholesterol and is associated with premature atherosclerotic cardiovascular disease. A 71-year-old woman with FH, established atherosclerotic cardiovascular disease, and statin intolerance presents to the cardiology clinic to discuss lipid management. Despite having failed combinations of statins, ezetimibe, and 2 proprotein convertase subtilisin/kexin type 9 inhibitors that use monoclonal antibodies, she was able to achieve low-risk low-density lipoprotein cholesterol levels using a novel way to lower proprotein convertase subtilisin/kexin type 9 levels with inclisiran. Therapeutic persistence is a key trait in the management of FH where multiple changes in treatment are often needed to achieve optimal therapeutic targets.
Key Words: dyslipidemias, hypercholesterolemia, lipid metabolism disorders, primary prevention, secondary prevention
Visual Summary
Visual Summary. Therapeutic Persistence in the Management of Familial Hypercholesterolemia
History of Presentation
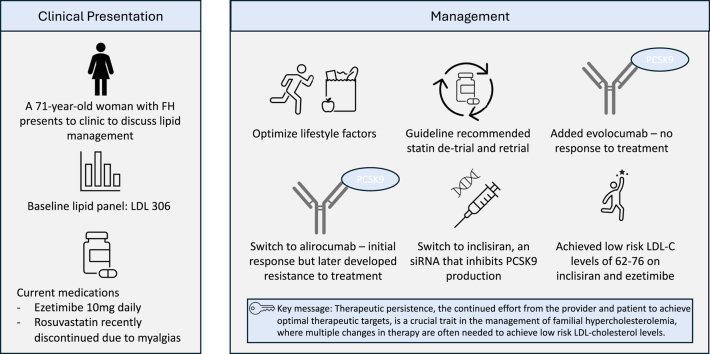
A 71-year-old woman presents to the cardiology clinic to discuss lipid management.
Take-Home Messages
-
•
Familial hypercholesterolemia remains underdiagnosed and undertreated, as recent literature shows many patients still do not achieve low-risk lipid profiles.
-
•
Inclisiran is a novel siRNA targeting PCSK9 with potent lipid-lowering effects and is administered via health care provider injection every 6 months.
Past Medical History
The patient has a known diagnosis of heterozygous familial hypercholesterolemia (FH), established coronary atherosclerosis with 2 prior stents, hypertension, and family history significant for both parents with ischemic heart disease. She is currently taking ezetimibe 10 mg daily after recent discontinuation of rosuvastatin 20 mg due to recurrence of statin-associated muscle symptoms (SAMS) after prior detrial and retrial. She had bilateral leg cramping that improved with statin detrial with return of symptoms after retrial, as well as previous creatine kinase elevation to 1.5 times the upper limit of normal.
Differential Diagnosis
The patient had previously met Dutch Lipid Clinic and Simon Broome criteria for definite FH.
Investigations
Her baseline lipid panel on ezetimibe 10 mg daily showed low-density lipoprotein cholesterol (LDL-C) of 306 mg/dL, which increased from 194 mg/dL when she was also taking rosuvastatin 20 mg daily. The rest of her baseline lipid panel showed total cholesterol 392 mg/dL, triglycerides 137 mg/dL, high-density lipoprotein cholesterol 59 mg/dL. Her lipoprotein(a) was 32 mg/dL.
Management
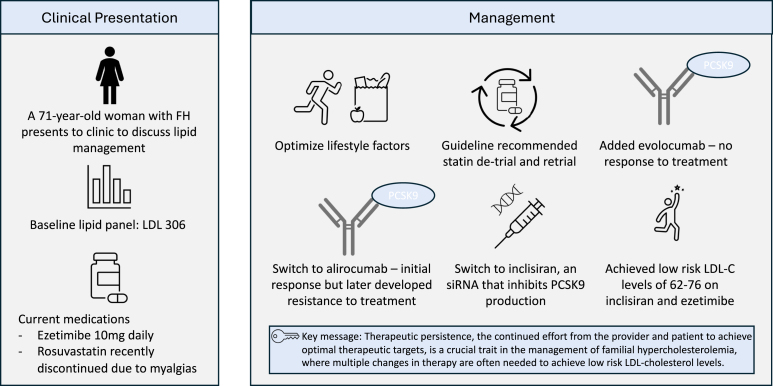
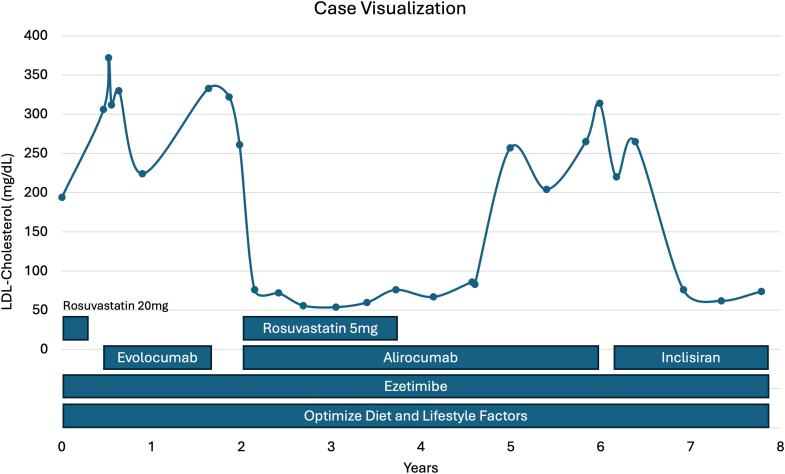
To intensify lipid-lowering therapy, we started by adding evolocumab 140 mg every 2 weeks to her ezetimibe. Unfortunately, her LDL-C did not improve and remained between 312 and 372 mg/dL. We ensured adherence with medications and documented proper injection technique during this period. Thus, we switched from evolocumab to alirocumab 150 mg every 2 weeks and rechallenged with rosuvastatin 5 mg daily. Her repeat LDL-C level fell to 76 mg/dL. Although her statin was later discontinued because of return of SAMS, her LDL-C remained between 56 and 83 mg/dL over 18 months. However, after 2 years of therapy with alirocumab, her LDL-C increased to 257 mg/dL and remained persistently elevated despite adherence to therapy.
Given poor treatment response to both anti-proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies and ongoing need for aggressive lipid-lowering therapy, we treated the patient with inclisiran 284 mg every 6 months. Inclisiran is a small interfering RNA (siRNA) that interferes with the hepatic synthesis of PSCK9.
Outcome and Follow-Up
On combination treatment with inclisiran and ezetimibe, our patient achieved low-risk LDL-C levels, ranging from 62 to 76 mg/dL for more than 1 year since the initiation of this therapeutic regimen (Figure 1).
Figure 1.
Case Visualization of Treatment Timeline
LDL = low-density lipoprotein.
Discussion
FH is an autosomal codominant inherited disorder of lipid metabolism that causes marked elevations in LDL-C and is associated with premature atherosclerotic cardiovascular disease (ASCVD). Patients with FH should be treated with aggressive guideline-directed lipid-lowering therapies to minimize risk of premature ASCVD. In this case, we describe multiple therapeutic strategies to treat a patient with FH and established ASCVD, who had elevated lipids despite maximally tolerated statin and ezetimibe.
We addressed SAMS using guideline-recommended attempts to dechallenge and rechallenge, including rechallenging with lower doses or alternative statins.1 However, given marked LDL-C elevation even when previously taking high-intensity statins, the treatment focus was shifted toward alternative lipid-lowering therapies. PCSK9 inhibitors are an important therapeutic option in this patient population, given their potent ability to substantially lower LDL-C levels.
Our patient was treated with evolocumab and had no response to treatment but did initially respond to alirocumab. Proposed mechanisms of poor response to PCSK9 inhibitors include poor adherence or absorption, development of neutralizing antibodies, and pathogenic variants in LDL receptor, apolipoprotein B, and PCSK9.2 Although we did not measure PCSK9 levels, which can help differentiate the mechanism of poor treatment response, her initial improvement with alirocumab suggests delayed formation of neutralizing antibodies as a potential mechanism.
Formation of antidrug antibodies to monoclonal antibodies has been extensively documented with prevalence ranging from <1% up to 70%, with fully human antibodies being the least immunogenic.3 Antidrug antibodies can alter a drug’s bioavailability and pharmacokinetic properties, and are considered neutralizing antibodies when they inhibit binding of the monoclonal antibody to its target. Notably, a humanized murine-derived monoclonal antibody to PCSK9, bococizumab, was withdrawn from development because of high rates of developing antidrug antibodies affecting treatment efficacy.4 In clinical trials of alirocumab and evolocumab, development of antidrug antibodies is rare, with reported rates of 1.3% and 0.16%, respectively,4,5 and did not predict poor treatment response. This suggests development of true neutralizing antibodies is rare, but remains a plausible mechanism for treatment failure.
This case also shows the successful use of inclisiran in our patient who did not respond to either of the available PCSK9 inhibitors that use fully human monoclonal antibodies. Inclisiran is a novel Food and Drug Administration–approved LDL-C lowering drug that uses siRNA to degrade PCSK9 mRNA in the liver, stopping PCSK9 production.6 We chose inclisiran over bempedoic acid because of data showing greater potential degree of LDL-C lowering and our patient’s markedly elevated LDL-C levels. Initial clinical trials show efficacy on a regimen of health care provider injection 3 times in the first year and every 6 months thereafter. Because of the different mechanism of action, inclisiran is not affected by neutralizing antibodies and clinic administration addresses factors such as medication adherence and improper injection technique. The recently published VICTORION-INITIATE (A Randomized Study to Evaluate the Effect of an “Inclisiran First” Implementation Strategy Compared to Usual Care in Patients With Atherosclerotic Cardiovascular Disease and Elevated LDL-C Despite Receiving Maximally Tolerated Statin Therapy) trial7 showed that early inclisiran initiation in patients on maximally tolerated statins achieved significant LDL-C reductions and more patients who achieve low-risk LDL-C levels compared with usual care. However, ongoing randomized clinical trial data on the long-term safety profile and clinical outcomes, as well as cost-effectiveness analysis, are needed to better understand how inclisiran can be best used alongside existing lipid-lowering therapies.
An editorial that reviewed recent data on treatment efficacy in FH indicated that a concerning number of patients with FH, despite their high lifetime risk of atherosclerotic complications, do not attain low-risk levels of LDL-C.8 For example, a recent cohort of patients younger than 50 with FH with myocardial infarction found that a third of patients with FH were not on high-intensity statin at hospital discharge and fewer than a fifth of patients achieved low-risk LDL levels of <70 mg/dL at 1-year follow-up.9 A major barrier to optimal risk factor modification is therapeutic inertia, a failure to initiate or intensify therapy in a timely manner in accordance with evidence-based guidelines.10 Therapeutic inertia has been documented as a major barrier in the management of many chronic conditions such as diabetes and hypertension. Many patient, provider, and system barriers have been identified as contributing to therapeutic inertia.
Despite recurrent hurdles, our case highlights the value of therapeutic persistence, which we would define as a continued effort by the provider to adjust or intensify therapy to achieve optimal therapeutic targets. We feel that therapeutic persistence is a crucial trait in the contemporary era of managing patients with high ASCVD risk, such as patients with FH. The growing number of highly effective lipid-lowering therapeutics makes it possible to achieve low-risk LDL-C levels in many patients who were previously unable to. Therefore, we believe that early and aggressive intensification and adjustment of treatment from the provider is more important than ever. This is exemplified in our patient, who despite having failed combinations of statins and non-statin lipid-lowering therapies such as ezetimibe and 2 PCSK9 inhibitors that use monoclonal antibodies, responded fully to a novel way to lower PCSK9 levels using siRNA with inclisiran.
Conclusions
Treatment of FH is important and urgent, as many patients with FH still do not achieve low-risk LDL-C levels. This case reviews several important concepts in lipid management including statin dechallenge and rechallenge for SAMS, use of ezetimibe and anti-PSCK9 antibodies, and a novel way to lower PSCK9 by using siRNA with inclisiran. Underlying all of these advances in treating hyperlipidemia is the combined effort from the clinician and patient to persist in adjusting and titrating therapies to achieve optimal low-risk targets.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro M.D., Miles J., Tavori H., Fazio S. Diagnosing resistance to a proprotein convertase subtilisin/kexin type 9 inhibitor. Ann Intern Med. 2018;168(5):376–379. doi: 10.7326/M17-2485. [DOI] [PubMed] [Google Scholar]
- 3.Vaisman-Mentesh A., Gutierrez-Gonzalez M., DeKosky B.J., Wine Y. The molecular mechanisms that underlie the immune biology of anti-drug antibody formation following treatment with monoclonal antibodies. Front Immunol. 2020;11:1951. doi: 10.3389/fimmu.2020.01951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth E.M., Goldberg A.C., Catapano A.L., et al. Antidrug antibodies in patients treated with alirocumab. N Engl J Med. 2017;376(16):1589–1590. doi: 10.1056/NEJMc1616623. [DOI] [PubMed] [Google Scholar]
- 5.Koren M.J., Sabatine M.S., Giugliano R.P., et al. Long-term efficacy and safety of evolocumab in patients with hypercholesterolemia. J Am Coll Cardiol. 2019;74(17):2132–2146. doi: 10.1016/j.jacc.2019.08.1024. [DOI] [PubMed] [Google Scholar]
- 6.Raal F.J., Kallend D., Ray K.K., et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–1530. doi: 10.1056/NEJMoa1913805. [DOI] [PubMed] [Google Scholar]
- 7.Koren M.J., Rodriguez F., East C., et al. An “inclisiran first” strategy vs usual care in patients with atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2024;83(20):1939–1952. doi: 10.1016/j.jacc.2024.03.382. [DOI] [PubMed] [Google Scholar]
- 8.Stone N.J., Blum C. If action is delayed, is survival denied? Management of severe hypercholesterolemia is important and urgent. Circulation. 2024;149(6):427–429. doi: 10.1161/CIRCULATIONAHA.123.068039. [DOI] [PubMed] [Google Scholar]
- 9.Singh A., Gupta A., Collins B.L., et al. Familial hypercholesterolemia among young adults with myocardial infarction. J Am Coll Cardiol. 2019;73(19):2439–2450. doi: 10.1016/j.jacc.2019.02.059. [DOI] [PubMed] [Google Scholar]
- 10.Khunti K., Gomes M.B., Pocock S., et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427–437. doi: 10.1111/dom.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]