Abstract
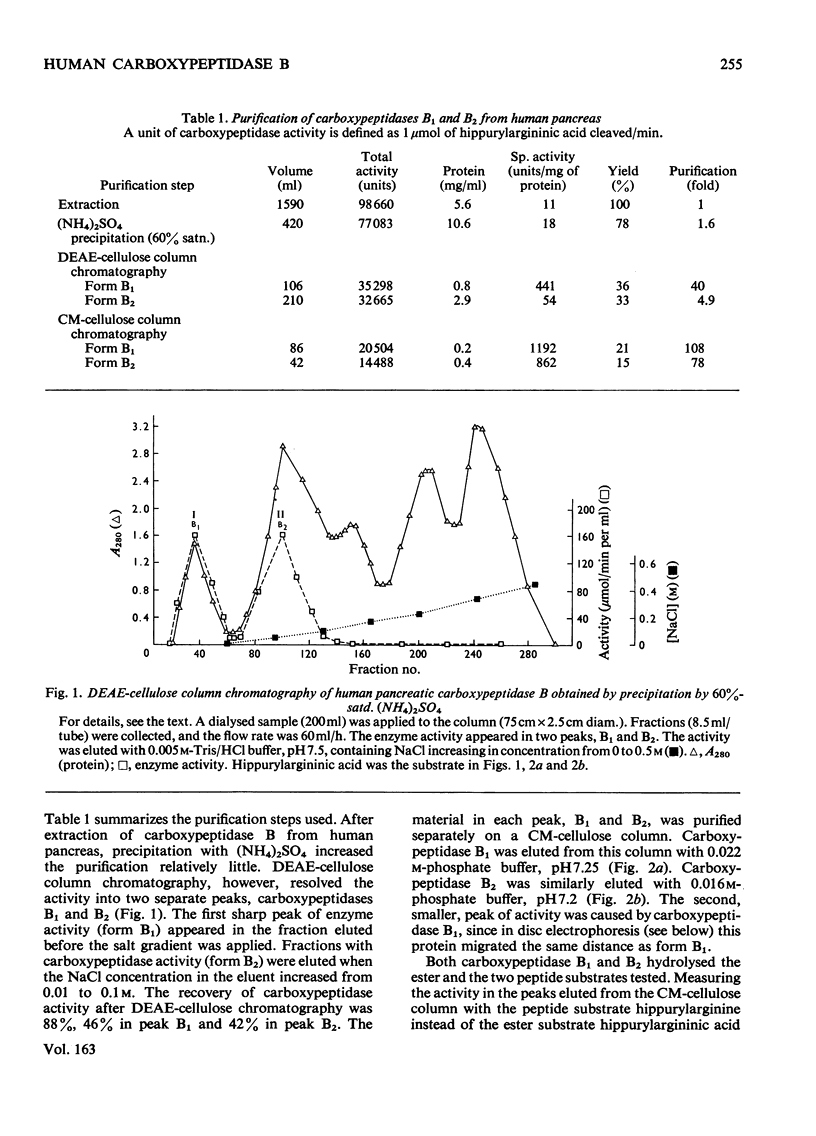
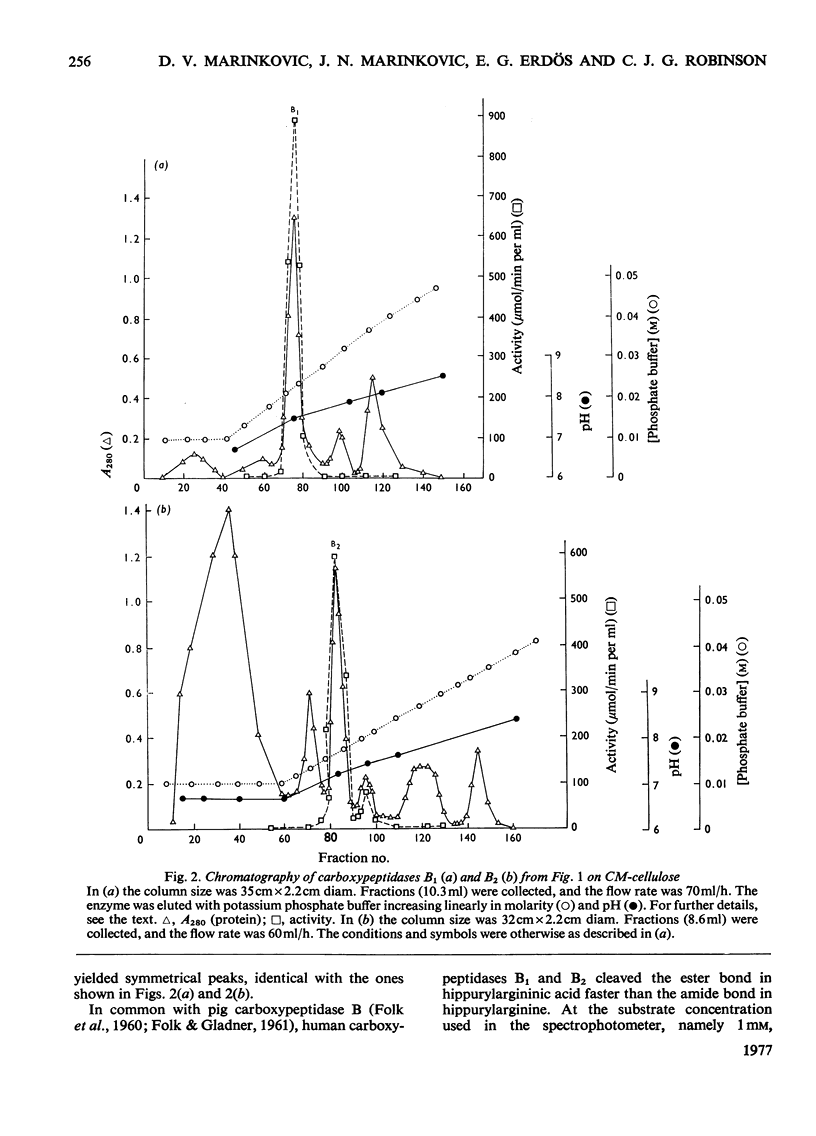
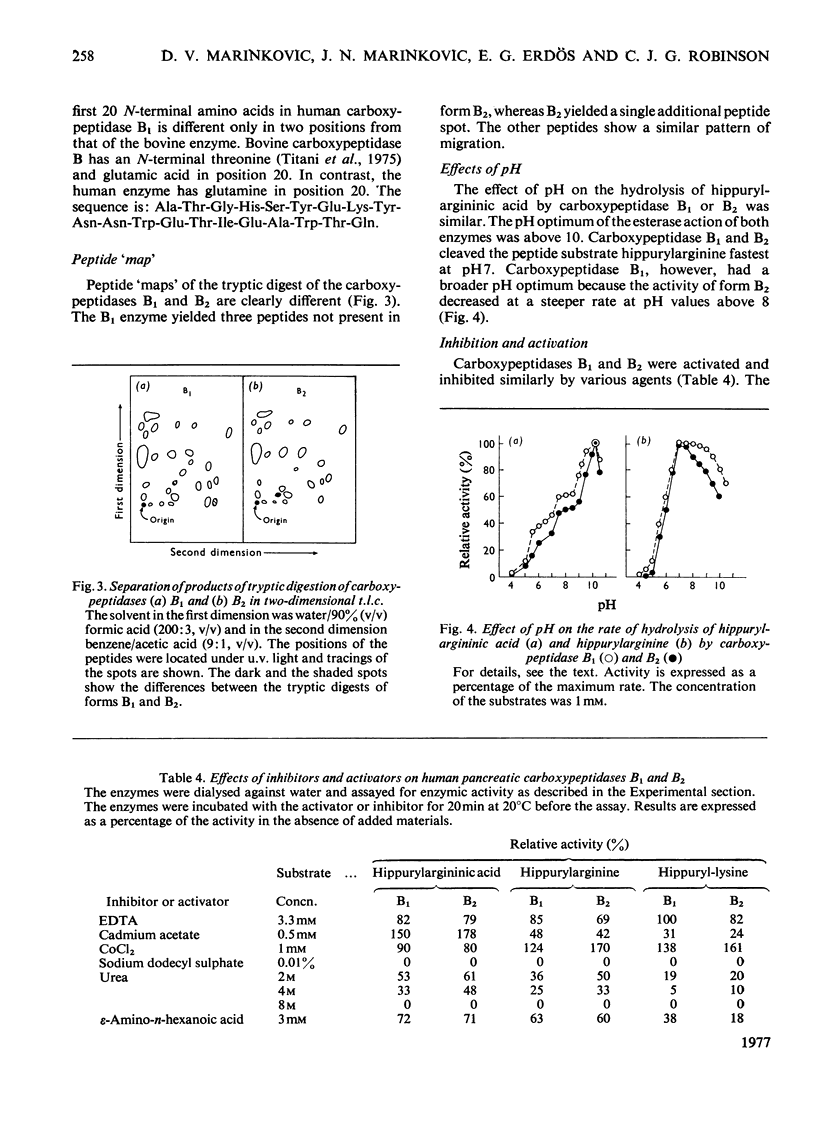
Carboxypeptidase B of the human pancreas was purified by chromatography on DEAE-cellulose and CM-cellulose columns. Two forms of the enzyme, named carboxypeptidase B1 and B2, were separated. They have similar mol.wts. (34250 +/- 590) as established by polyacrylamide-gel disc electrophoresis and by gel filtration. Carboxypeptidase B2 migrates further towards the anode in disc electrophoresis. When the amino acid content of the enzymes was analysed, carboxypeptidase B2 had four more glycine and three more aspartic acid residues than had form B1. The amino acid sequence of the human carboxypeptidase B1 differs from that of the bovine enzyme only in two places in the N-terminal 20-amino-acid sequence. The N-terminal amino acid in carboxypeptidase B1 and B2 is alanine. The peptide 'map' of the tryptic digest of carboxypeptidase B1 contained more peptides than did that of form B2. The Km, the Vmax. and the pH optimum of the cleavage of the peptide substrate hippurylarginine and the ester substrate hippurylargininic acid were similar for both enzymes. CoCl2 accelerated the peptidase activity, and cadmium acetate enhanced the esterase activity, of human carboxypeptidases B1 and B2. Urea and sodium dodecyl sulphate inhibited the enzymes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Clemente F., de Caro A., Figarella C. Composition du suc pancréatique humain. Etude immunoenzymologique. Eur J Biochem. 1972 Nov 21;31(1):186–193. doi: 10.1111/j.1432-1033.1972.tb02518.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- ERDOES E. G., SLOANE E. M., WOHLER I. M. CARBOXYPEPTIDASE IN BLOOD AND OTHER FLUIDS. I. PROPERTIES, DISTRIBUTION, AND PARTIAL PURIFICATION OF THE ENZYME. Biochem Pharmacol. 1964 Jun;13:893–905. doi: 10.1016/0006-2952(64)90033-4. [DOI] [PubMed] [Google Scholar]
- Erdös E. G., Yang H. Y., Tague L. L., Manning N. Carboxypeptidase in blood and other fluids. 3. The esterase activity of the enzyme. Biochem Pharmacol. 1967 Jul 7;16(7):1287–1297. doi: 10.1016/0006-2952(67)90159-1. [DOI] [PubMed] [Google Scholar]
- FOLK J. E., GLADNER J. A. Influence of cobalt and cadmium on the peptidase and esterase activities of carboxypeptidase B. Biochim Biophys Acta. 1961 Mar 18;48:139–147. doi: 10.1016/0006-3002(61)90524-8. [DOI] [PubMed] [Google Scholar]
- FOLK J. E., PIEZ K. A., CARROLL W. R., GLADNER J. A. Carboxy-peptidase B. 4. Purification and characterization of the porcine enzyme. J Biol Chem. 1960 Aug;235:2272–2277. [PubMed] [Google Scholar]
- FOLK J. E., SCHIRMER E. W. THE PORCINE PANCREATIC CARBOXYPEPTIDASE A SYSTEM. I. THREE FORMS OF THE ACTIVE ENZYME. J Biol Chem. 1963 Dec;238:3884–3894. [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- Geokas M. C., Largman C., Brodrick J. W., Raeburn S., Rinderknecht H. Human pancreatic carboxypeptidase B. I. Isolation, purification, and characterization of fraction II. Biochim Biophys Acta. 1975 Jun 24;391(2):396–402. doi: 10.1016/0005-2744(75)90263-6. [DOI] [PubMed] [Google Scholar]
- Geokas M. C., Wollesen F., Rinderknecht H. Radioimmunoassay for pancreatic carboxypeptidase B in human serum. J Lab Clin Med. 1974 Oct;84(4):574–583. [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneret L., Roth M., Bargetzi J. P. Carboxypeptidase N from pig serum. Hoppe Seylers Z Physiol Chem. 1976 Jun;357(6):867–872. doi: 10.1515/bchm2.1976.357.1.867. [DOI] [PubMed] [Google Scholar]
- Kemmler W., Peterson J. D., Steiner D. F. Studies on the conversion of proinsulin to insulin. I. Conversion in vitro with trypsin and carboxypeptidase B. J Biol Chem. 1971 Nov 25;246(22):6786–6791. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marinkovic D. V., Marinkovic J. N. Studies of human carboxypeptidase A purification and properties from human pancreas. Biochem Med. 1975 Sep;14(1):125–134. doi: 10.1016/0006-2944(75)90027-7. [DOI] [PubMed] [Google Scholar]
- Mole L. E., Goodfriend L., Lapkoff C. B., Kehoe J. M., Capra J. D. The amino acid sequence of ragweed pollen allergen Ra5. Biochemistry. 1975 Mar 25;14(6):1216–1220. doi: 10.1021/bi00677a019. [DOI] [PubMed] [Google Scholar]
- Neurath H., Bradshaw R. A., Pétra P. H., Walsh K. A. 3. Carboxypeptidase. Bovine carboxypeptidase A--activation, chemical structure and molecular heterogeneity. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):159–176. doi: 10.1098/rstb.1970.0019. [DOI] [PubMed] [Google Scholar]
- Oshima G., Kato J., Erdös E. G. Plasma carboxypeptidase N, subunits and characteristics. Arch Biochem Biophys. 1975 Sep;170(1):132–138. doi: 10.1016/0003-9861(75)90104-6. [DOI] [PubMed] [Google Scholar]
- Reeck G. R., Walsh K. A., Neurath H. Isolation and characterization of carboxypeptidases A and B from activated pancreatic juice. Biochemistry. 1971 Dec 7;10(25):4690–4698. doi: 10.1021/bi00801a015. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Titani K., Ericsson L. H., Walsh K. A., Neurath H. Amino-acid sequence of bovine carboxypeptidase B. Proc Natl Acad Sci U S A. 1975 May;72(5):1666–1670. doi: 10.1073/pnas.72.5.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]


