Abstract
Primary carnitine deficiency may mimic hypertrophic cardiomyopathy and be mistakenly attributed to genotype-negative sarcomeric protein dysfunction in hypertrophic cardiomyopathy. Although rare, timely diagnosis may have significant implications on management and should prompt testing of family members.
Key Words: genetics, hypertrophic cardiomyopathy mimics, hypertrophic cardiomyopathy phenocopies, primary carnitine deficiency
Graphical Abstract

The definition of hypertrophic cardiomyopathy (HCM) has evolved over time. The currently accepted definition includes hypertrophy (>15 mm) anywhere within the left ventricular wall that cannot be attributed to another systemic or metabolic disease. HCM is caused by genetic mutations encoding cardiac sarcomeric proteins or sarcomere-related proteins.1 The American College of Medical Genetics and Genomics identifies 14 core genes containing most pathogenic variants of HCM.2 The yield of genetic testing has improved, discovering a pathogenic variant in 30% to 50% of probands.3 Cases without identifiable mutations may be due to undiscovered variants, polygenic etiologies, or HCM phenocopies or mimics. HCM mimics include inborn errors of metabolism, metabolic storage disorders, and neuromuscular disorders. In this report, we review a rare mimic of HCM and explore the challenges and implications in arriving at this diagnosis.
Learning Objectives
-
•
To review the presentation, diagnosis, and management of a rare hypertrophic cardiomyopathy mimic.
-
•
To highlight the challenges in identifying hypertrophic cardiomyopathy mimics.
-
•
To examine contemporary HCM genetic testing and its limitations.
History of Presentation
An 11-year-old boy was referred to pediatric cardiology for evaluation prior to initiating stimulant medication for attention deficit hyperactivity disorder. Although extensive cardiac screening is not routinely recommended prior to stimulant initiation, the patient’s sister had recently been diagnosed with cardiomyopathy which prompted the referral. During the evaluation, the patient reported that he was asymptomatic and had normal exercise tolerance. Physical examination revealed weight 34.6 kg, height 136.7 cm, heart rate 86 beats/min, respiratory rate 24 breaths/min, and blood pressure 119/61 mm Hg. Cardiovascular examination revealed a normal S1 and S2 with a vibratory systolic murmur best heard at the left lower sternal border. No other abnormalities were appreciated.
Past Medical History
The patient was born in Mexico. Aside from newly diagnosed attention deficit hyperactivity disorder, he had no known medical problems. The patient’s sister had been diagnosed with suspected HCM after suffering a dysrhythmia at 9 years of age. Genetic testing of the sister had been unrevealing for the etiology of her cardiomyopathy. The parents and 3 younger siblings had no chronic medical conditions.
Investigations and Differential Diagnosis
Initial electrocardiogram showed sinus rhythm and left ventricular hypertrophy (LVH) with strain pattern (Figure 1A). Echocardiogram showed preserved ejection fraction and concentric LVH with a septal thickness of 13 mm (z score 3.4) and posterior wall thickness of 14 mm (z score 4.6) (Figure 1B). Given these findings and family history, the differential diagnosis most strongly suggested an inherited cardiomyopathy. Potential etiologies included sarcomeric HCM and monogenic systemic disorders such as glycogen storage disease (due to PRKAG2 mutations), Danon disease (LAMP2), Fabry disease (GLA), and the RASopathies.
Figure 1.
Initial Cardiac Investigation
(A) Electrocardiogram consistent with left ventricular hypertrophy (LVH) with strain pattern. (B) Echocardiogram with concentric LVH with a septal thickness 13 mm (z score 3.4) and posterior wall thickness of 14 mm (z score 4.6). (C and D) Cardiac magnetic resonance with concentric LVH without evidence of late gadolinium enhancement.
Management and Outcome
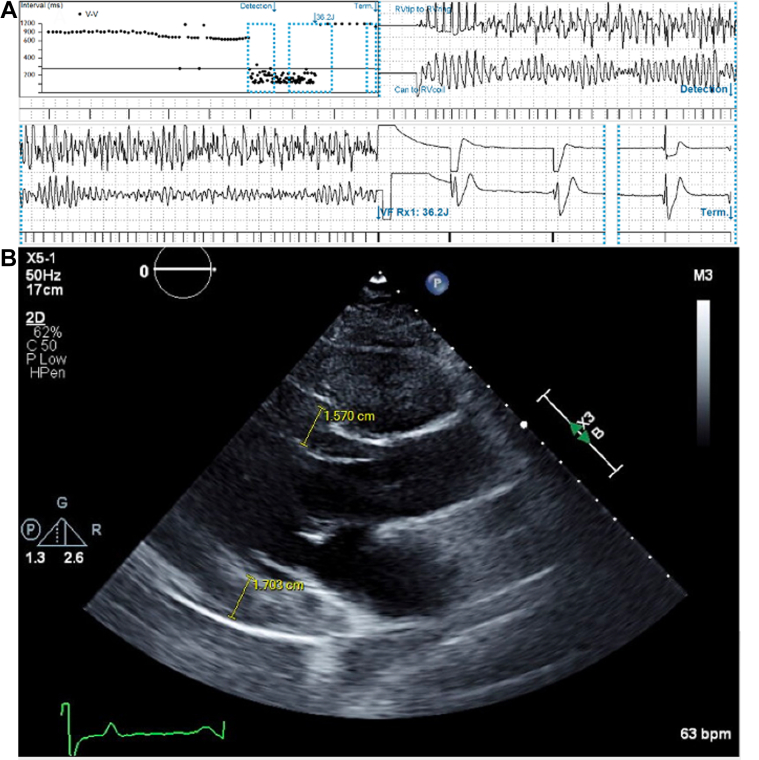
He continued to follow with pediatric cardiology for surveillance echocardiograms. Genetic testing was not performed initially given that it was negative in his sister. Unfortunately, the patient experienced ventricular fibrillation at 13 years of age. He was appropriately resuscitated by emergency medical services and survived without neurologic sequelae. During the postarrest hospital admission, a cardiac magnetic resonance was obtained and showed HCM with relative septal sparing and no evidence of obstruction or interstitial fibrosis (Figures 1C and 1D). He received a single chamber implantable cardiac defibrillator for secondary prevention. A GeneDx Hypertrophic Cardiomyopathy Panel was obtained and did not identify a pathogenic variant. This panel sequenced the following genes: ACTC (ACTC1), CAV3, GLA, LAMP2, MTTG, MTTI, MTTK, MTTQ, MYBPC3, MYH7, MYL2, MYL3, PRKAG2, TNNC1, TNNI3, TNNT2, TPM1, and TTR. His sister was later discovered to have low carnitine levels and underwent whole exome sequencing which revealed a pathogenic variant in SLC22A5, indicative of autosomal recessive systemic primary carnitine deficiency (CDSP). Carnitine levels were obtained in the presented patient and revealed total carnitine of 3 nmol/mL (reference, 25-69 nmol/mL), free carnitine 3 (reference, 16-60 nmol/mL), and acylcarnitine short chain <1 nmol/mL (reference, 0-19 nmol/mL). He subsequently underwent directed genetic testing revealing homozygosity for the same pathogenic variant as his sister. He was placed on carnitine supplementation; however, he had multiple subsequent device rescues for ventricular arrhythmias and progression of LVH in the setting of nonadherence to supplementation (Figures 2A and 2B). During his last follow-up at 21 years of age, he reported doing well and was compliant with carnitine supplementation and sotalol.
Figure 2.
Subsequent Cardiac Outcomes
(A) Ventricular fibrillation successfully terminated by a single 36-J defibrillation. (B) Progression of concentric left ventricular hypertrophy with interventricular septum thickness 15.7 mm and left ventricular posterior wall thickness 17 mm.
Discussion
CDSP has an estimated prevalence of 1 per 50,000 in the United States4 and results from dysfunction of the cationic transporter (OCTN2) which is encoded by the SLC22A5 gene. Mutations in SLC22A5 impair the ability of OCTN2 to import carnitine into cells, leading to low intracellular carnitine levels and urinary carnitine wasting. Low intracellular carnitine levels impair fatty acid oxidation because carnitine is essential for the transport of fatty acids into the mitochondria through the carnitine shuttle. CDSP is therefore characterized by hypoketotic hypoglycemia, hyperammonemia, liver dysfunction, cardiomyopathy, and skeletal hypotonia.5 Phenotypic presentation can range from metabolic decompensation in infancy, childhood myopathy, to fatiguability in adults or complete absence of symptoms. Cardiac arrest may precede diagnosis.5 Despite the broad range of phenotypic presentations associated with CDSP, cardiac dysfunction appears to be the most prevalent, particularly in those presenting at an older age.6 This is most likely due to cardiomyocytes reliance on fatty acids as the primary substrate for oxidative metabolism. Although cardiomyocytes can use other substrates for metabolism, the heart is particularly vulnerable to the reduced capacity to oxidize fatty acids because it has the highest adenosine triphosphate requirement of any organ.7
Typically, the disorder is discovered through routine newborn screening; however, false-negative results may occur because carnitine is transported transplacentally and will reflect the mother’s level for several days after birth. Once CDSP is suspected, diagnosis is established by testing for pathogenic variants in the SLC22A5 gene and demonstrating low plasma carnitine levels.
American College of Cardiology/American Heart Association guidelines recommend genetic testing for all patients with HCM. The HCM genetic panel initially sent for this patient did not include SLC22A5 variants, which remains the case on contemporary expanded genetic cardiomyopathy panels. Other limitations to identifying CDSP and other HCM mimics include variable penetrance and expressivity of HCM variants, racial disparities in identifying pathogenic variants, and complex underlying genetics such as the cumulative role of multiple variants.8 American College of Cardiology/American Heart Association guidelines also stress the importance of a 3-generation family history and genetic counseling, when available, for all patients with HCM. The importance of this recommendation is exemplified by the subsequent discovery that in addition to his sister being diagnosed with HCM, consanguinity existed between the parents, and there was a distant relative who had died in her 30s while awaiting heart transplant.
The primary treatment of CDSP is carnitine supplementation and avoidance of fasting to reduce disease progression. An important difference between HCM and HCM mimics like CDSP are treatment options. Whereas pathogenic variant identification typically does not change management in HCM, pathogenic variant identification of HCM mimics almost always alters management. When initiated before irreversible organ damage occurs, carnitine supplementation in CDSP has been shown to slow disease progression and even improve cardiac muscle function.5 Nonadherence to supplementation can be devastating as evidenced by this patient’s multiple device rescues and progression of LVH.
Conclusions
HCM mimics (eg, primary carnitine deficiency) are rare and often nonspecific in presentation, and therefore challenging to identify. HCM phenocopies may be correctable with enzyme or protein replacement/stabilization, leading to improved cardiac outcomes and systemic symptoms. Clinicians must maintain a high clinical suspicion for HCM phenocopies when evaluating patients with apparent HCM to facilitate prompt diagnosis and proper disease-modifying treatment.
Funding Support and Author Disclosures
Dr Jensen has received research funding from grants R01 HL140067 and R01 HL165294. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Gersh B.J., Maron B.J., Bonow R.O., et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58(25):2703–2738. doi: 10.1016/j.jacc.2011.10.825. [DOI] [PubMed] [Google Scholar]
- 2.Biddinger K.J., Jurgens S.J., Maamari D., et al. Rare and common genetic variation underlying the risk of hypertrophic cardiomyopathy in a national biobank. JAMA Cardiol. 2022;7(7):715–722. doi: 10.1001/jamacardio.2022.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butters A., Bagnall R.D., Ingles J. Revisiting the diagnostic yield of hypertrophic cardiomyopathy genetic testing. Circ Genom Precis Med. 2020;13(2) doi: 10.1161/CIRCGEN.120.002930. [DOI] [PubMed] [Google Scholar]
- 4.Magoulas P.L., El-Hattab A.W. Systemic primary carnitine deficiency: an overview of clinical manifestations, diagnosis, and management. Orphanet J Rare Dis. 2012;7:68. doi: 10.1186/1750-1172-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Hattab AW. Systemic Primary Carnitine Deficiency. 2012 Mar 15 [Updated 2016 Nov 3]. In: Adam MP, Feldman J, Mirzaa GM, et al, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024. https://www.ncbi.nlm.nih.gov/books/NBK84551/ [PubMed]
- 6.Shibbani K., Fahed A.C., Al-Shaar L., et al. Primary carnitine deficiency: novel mutations and insights into the cardiac phenotype. Clin Genet. 2014;85(2):127–137. doi: 10.1111/cge.12112. [DOI] [PubMed] [Google Scholar]
- 7.Lopaschuk G.D., Ussher J.R., Folmes C.D., Jaswal J.S., Stanley W.C. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90(1):207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 8.Hoss S., Habib M., Silver J., et al. Genetic testing for diagnosis of hypertrophic cardiomyopathy mimics: yield and clinical significance. Circ Genom Precis Med. 2020;13(2) doi: 10.1161/CIRCGEN.119.002748. [DOI] [PubMed] [Google Scholar]