Abstract
Porcelain aorta describes circumferential calcification in the ascending aorta that may extend through the aortic arch. This is commonly observed in patients with a history of mediastinal radiation, end-stage renal disease, or chronic vascular inflammation. Mediastinal radiation has been shown to cause intimal and medial calcification of the aorta, as well as diastolic myocardial dysfunction, valvular disease, and coronary artery disease. In recent years, patients with aortic stenosis and concomitant porcelain aorta have been increasingly managed with transcatheter aortic valve replacements rather than surgical replacement to reduce periprocedural risks. We describe the surgical management of a patient with severe mitral valve stenosis, porcelain aorta, and patient prosthesis mismatch from a previously deployed transcatheter aortic valve replacement using right axillary arterial canulation and hypothermic circulatory arrest. To our knowledge, this is the first published case where a porcelain aorta was replaced surgically after failed transcatheter management of severe aortic stenosis.
Key Words: mediastinal radiation, patient prosthesis mismatch, porcelain aorta, transcatheter aortic valve replacement
Graphical Abstract
History of presentation
A 52-year-old female patient with a porcelain aorta given a history of mediastinal radiation for the treatment of Hodgkin’s lymphoma during childhood was referred to us due to severe mitral valve stenosis and significant patient prosthesis mismatch (PPM) following recent transcatheter aortic valve replacement (TAVR). She had a history of severe aortic stenosis with a mean gradient of 39 mm Hg, peak velocity of 4 m/s, dimensionless index of 0.23 cm2/m2, and indexed effective orifice area of 0.79 cm2/m2 for which she received a 23-mm Evolut (Medtronic) valve approximately 18 months before her presentation. Despite an 80-pound weight loss facilitated by a sleeve gastrectomy, the patient remained limited in her activities by dyspnea, chest pain, pre-syncope, and 3-pillow orthopnea, leading to a suspicion of severe mitral valve stenosis with significant PPM due to insufficient expansion of the self-expanding valve due to the calcified aorta. On preoperative echocardiography, the patient was found to have severe mitral stenosis (valve area 0.8 cm2, mean 11 mm Hg) and moderate mitral annular calcification. Additionally, elevated gradients (mean 15.5 mm Hg) and increased velocity (3.2 m/s) were noted across the aortic valve. Extensive calcifications were evident in the ascending aorta extending into zone 3 on computed tomography (CT) angiography (Video 1). The patient had no coronary disease. The primary indication for surgery was her severe mitral valve stenosis. Given the patient’s experienced symptoms, degree of pathology, relatively young age, and concern for poor exposure of the mitral valve, the patient consented to a high-risk complicated surgery involving the aortic root replacement despite a quoted mortality risk of nearly 30%.
Take-Home Messages
-
•
For patients with significant multifaceted heart disease, it is critical to assess the patient holistically to determine the intervention that would result in the greatest clinical benefit.
-
•
For patients with calcified aortas in the setting of mediastinal radiation, it is possible to perform open heart surgery safely to address all contributing pathologies.
Management
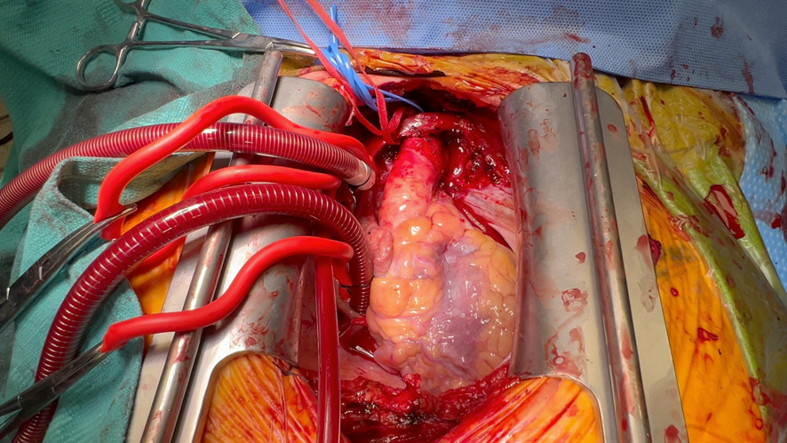
A right axillary cannulation approach was undertaken given her porcelain aorta. The right axillary artery was anastomosed to an 8-mm Dacron graft for initiation of cardiopulmonary bypass. Subsequently the heart was exposed through a standard sternotomy (Figure 1). Bicaval venous return was established, and a left ventricular vent was placed.
Figure 1.
Surgical Field
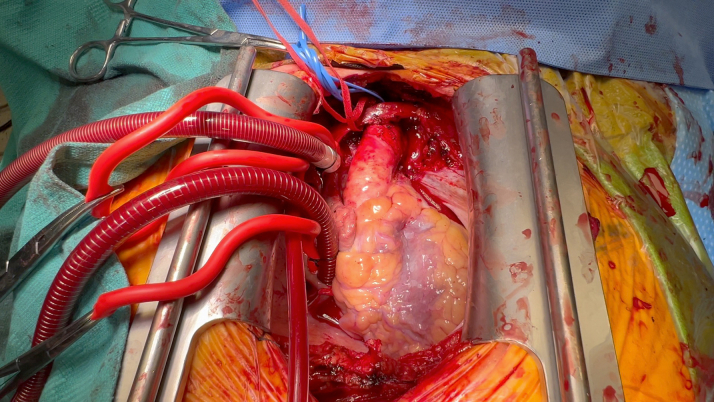
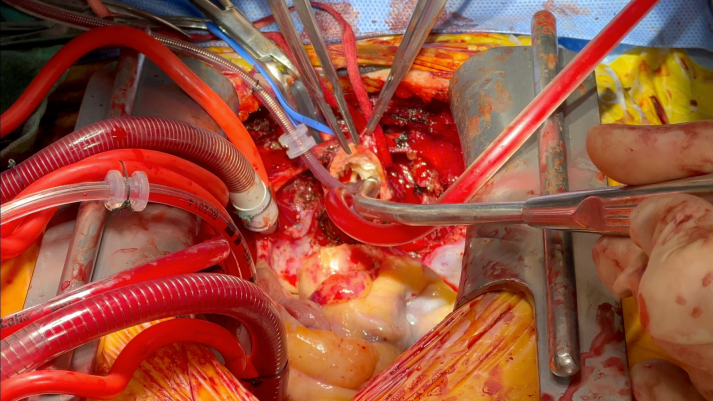
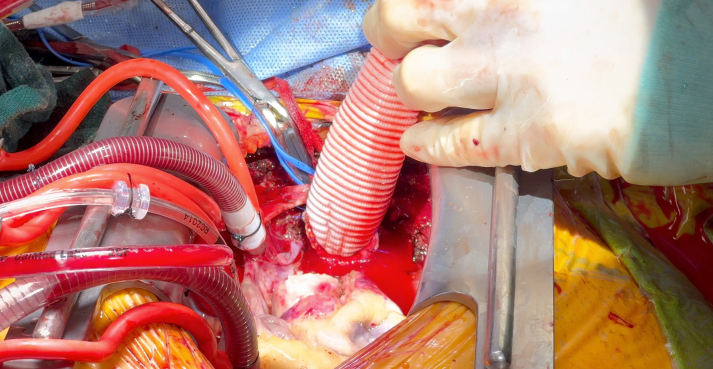
Once the patient was cooled to 24 °C, the innominate artery was snared and antegrade cerebral perfusion was established. While delivering Del Nido cardioplegia in a retrograde fashion, the aorta was carefully opened (Figure 2, Video 2). We then transitioned to direct ostial cardioplegia. The ascending aorta from the sinotubular junction up to the innominate artery takeoff was excised (Video 3). Because of the severe root calcification and previously placed TAVR valve, the mitral valve could not be satisfactorily exposed. The decision was made to replace the aortic root for needed exposure and to address the PPM of the TAVR valve. The Evolut valve was then carefully removed. A 24-mm straight Dacron graft was sewn in hemiarch fashion (Video 4) buttressed with external Teflon felt for hemostasis in the face of circumferential calcium; systemic cardiopulmonary bypass was subsequently restored (Figure 3). The mitral valve was then accessed via a left atriotomy, and the calcified anterior and posterior leaflets were excised. Supra-annular sutures were used to avoid groove disruption and a perivalvular leak while placing a 27-mm St Jude Masters (Abbott) in the mitral position.
Figure 2.
Calcified Aortic Lumen
Figure 3.
Restoration of Cardiopulmonary Bypass After Excision of Porcelain Aorta
Given significant dystrophic calcification in the aortic annulus and its small orifice size, the decision to proceed with an aortic root replacement was made. A 19-mm St Jude Regent valve was sewn into a 24-mm Valsalva graft (Terumo Aortic) to create a mechanical composite root, which was secured to the annulus with circumferential pledgeted stitches in a supra-annular fashion. The indexed effective orifice area following surgical aortic valve replacement was found to be within normal limits at 0.96 cm2/m2 (moderate PPM, 0.65-0.85 cm2/m2; severe PPM, <0.65 cm2/m2). Upon mobilization of the buttons, the right coronary ostium was found to be severely calcified and was therefore bypassed using a saphenous vein graft. The patient was re-warmed, and reperfusion was allowed for 30 minutes at which point the patient was weaned off cardiopulmonary bypass with moderate inotropic support. Antegrade cerebral perfusion duration was 52 minutes, cross-clamp time was 225 minutes, and total bypass time was 304 minutes. Postoperative transesophageal echocardiography revealed no changes in biventricular function, left ventricular ejection fraction of 60%, and well seated aortic and mitral mechanical valves with mean gradients of 5 mm Hg and 2 mm Hg, respectively.
Outcome and follow-up
The patient was extubated on postoperative day (POD) 1 and was neurologically intact. Her postoperative course was complicated by complete heart block. Because the arrhythmia was thought to be temporary, a leadless pacemaker was implanted on POD 4. Serial transthoracic echocardiography showed vigorous biventricular function with normal gradients across both mechanical valves. After optimizing volume and establishing therapeutic international normalized ratio, the patient was successfully discharged home on POD 16.
Discussion
Mediastinal radiation, used throughout the 20th century, results in a significant insult to the cardiovascular system.1, 2, 3 The aggregate incidence of cardiac and aortic diseases has previously been reported to be as high as 30% at 10 years following radiation.2 Given the large field of radiation, patients, as the one described, frequently suffer from multifactorial disease involving valves and vasculature; treatment of isolated sequalae—such as aortic stenosis with TAVRs—may therefore be insufficient.4
Providing definitive surgical treatment comes with substantial risks. In the setting of cardiac surgery, a porcelain aorta may complicate or preclude the ability to cannulate or cross-clamp the aorta due to significantly higher risks of embolic strokes, dissection, and death.1,5 Surgeons have avoided disrupting the aorta by relying on off-pump technique, fibrillating heart, and peripheral arterial cannulation with hypothermic circulatory arrest.1,6 Although the use of occlusive aortic endo-balloon for achieving cross-clamp is possible in the presence of a porcelain aorta, in the face of a balloon expandable valve, the high post of the valve prevents adequate balloon-to-wall apposition. In addition, surgical intervention following TAVR is known to have higher rates of mortality compared to isolated surgical aortic valve replacement.7 TAVR implantation may damage native anatomy requiring an aortic root replacement.8
In the setting of a porcelain aorta, preoperative cross-sectional imaging is essential to assess the extent of calcification and develop an appropriate operative plan. Spiral CT is the most effective, noninvasive technique used in the preoperative setting to detect and quantify the calcific burden in the vasculature. Assessment of calcific burden is necessary to determine sites of cannulation, snaring, and ultimately cross-clamp. Therefore, there should be a low threshold for surgeons to use CT imaging in patients in whom calcific aortas are anticipated.
Conclusions
In this case, we have discussed a technically challenging case involving a TAVR explant in the setting of porcelain aorta, with concomitant mechanical Bentall, mechanical mitral valve replacement, ascending aorta and hemiarch replacement, and coronary artery bypass to the right coronary artery. In the setting of multifaceted disease, it was essential to prepare for an operative approach that addressed all concerns, especially given the young age of this otherwise healthy woman. We demonstrate a safe surgical approach and highlight the necessity to consider mediastinal radiation as a global insult to the cardiovascular system. We also discuss operative technique as well as drawbacks of pursuing TAVR in patients with cardiovascular disease secondary to mediastinal radiation. This case emphasizes the importance of cross-sectional imaging in the setting of aortic surgery. In conclusion, we report a successful surgical intervention on a porcelain aorta in a 52-year-old patient without major bleeding or neurological injury who is now recovering at home.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Preoperative Computed Tomography Scan of Porcelain Aorta
Aortotomy
Excision of Porcelain Aorta
Sewing of Dacron Graft in Hemiarch Fashion
References
- 1.Abu Rmilah A.A., Yandrapalli S., Boudi F.B. Porcelain Aorta. StatPearls [Internet] 2023 StatPearls Publishing; PMID: 33085311. [PubMed] [Google Scholar]
- 2.Salz T., Zabor E.C., Brown P.N., et al. Cardiovascular risk factors, radiation therapy, and myocardial infarction among lymphoma survivors. Acta Oncol. 2022;61(9):1064–1068. doi: 10.1080/0284186X.2022.2107402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patil S., Pingle S.R., Shalaby K., Kim A.S. Mediastinal irradiation and valvular heart disease. CardioOncology. 2022;8(1):7. doi: 10.1186/s40959-022-00133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer B., Vekstein A.M., Bishop P.D., et al. Choosing transcatheter aortic valve replacement in porcelain aorta: outcomes versus surgical replacement. Eur J Cardiothorac Surg. 2023;63(5) doi: 10.1093/ejcts/ezad057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitoulis F., Pamias-Lopez B., Fraser C., et al. Transaortic TAVR and mitral repair under deep hypothermic circulatory arrest in a porcelain aorta patient. JACC Case Rep. 2023;29(1) doi: 10.1016/j.jaccas.2023.102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrel T., Vogt P.R. Porcelain aorta does not mean inoperability but needs special strategies. Interact Cardiovasc Thorac Surg. 2022;35(4) doi: 10.1093/icvts/ivac222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins R.B., Deeb G.M., Sukul D., et al. Redo surgical aortic valve replacement after prior transcatheter versus surgical aortic valve replacement. JACC Cardiovasc Interv. 2023;16(8):942–953. doi: 10.1016/j.jcin.2023.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Vitanova K., Zaid S., Tang G.H.L., et al. Aortic valve versus root surgery after failed transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2023;166(5):1418–1430.e4. doi: 10.1016/j.jtcvs.2021.12.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preoperative Computed Tomography Scan of Porcelain Aorta
Aortotomy
Excision of Porcelain Aorta
Sewing of Dacron Graft in Hemiarch Fashion