Abstract
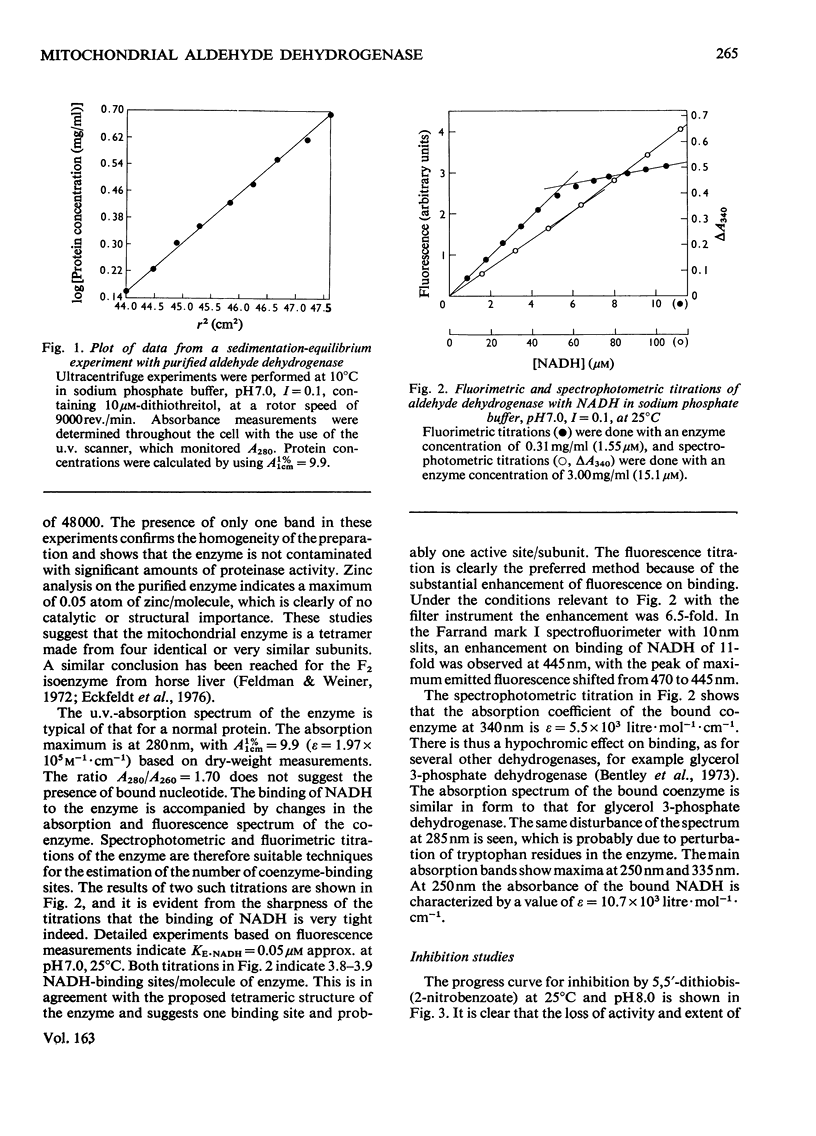
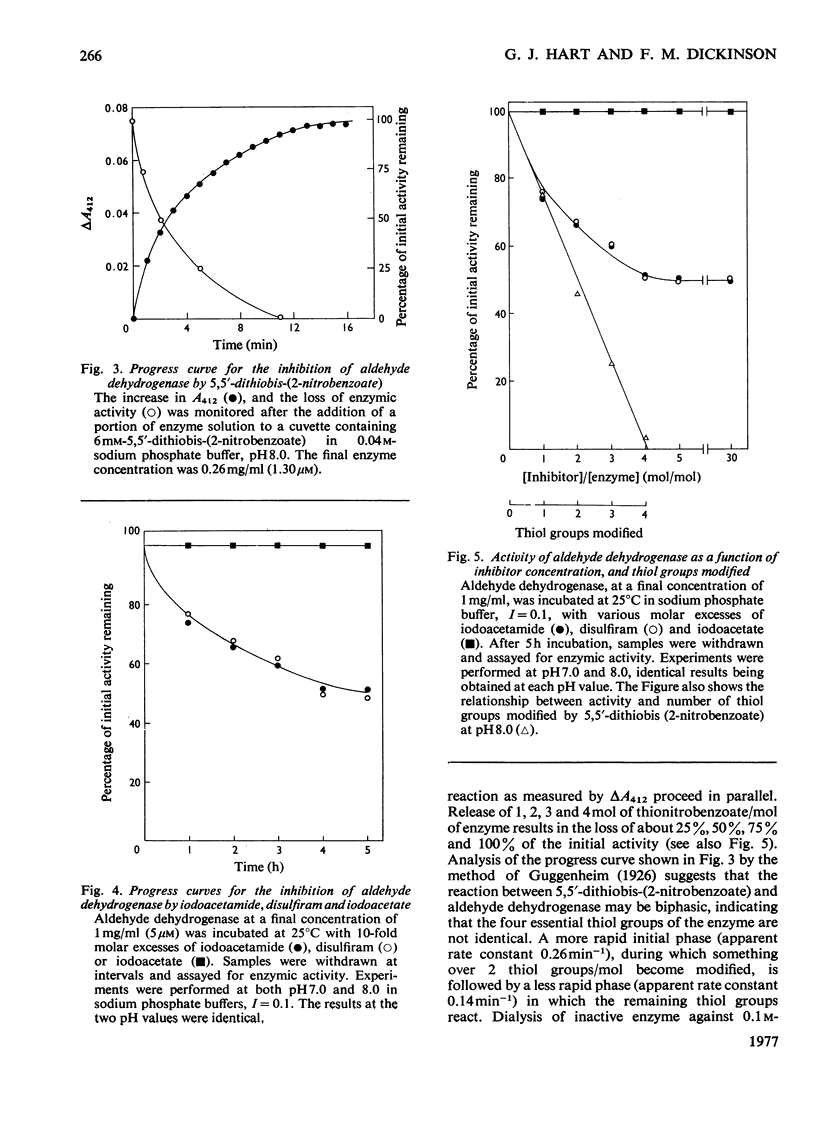
Aldehyde dehydrogenase from sheep liver mitochondria was purified to homogeneity as judged by electrophoresis on polyacrylamide gels, and by sedimentation-equilibrium experiments in the analytical ultracentrifuge. The enzyme has a molecular weight of 198000 and a subunit size of 48000, indicating that the molecule is a tetramer. Fluorescence and spectrophotometric titrations indicate that each subunit can bind 1 molecule of NADH. Enzymic activity is completely blocked by reaction of 4mol of 5,5'-dithiobis-(2-nitrobenzoate)/mol of enzyme. Excess of disulfiram or iodoacetamide decreases activity to only 50% of the control value, and only two thiol groups per molecule are apparently modified by these reagents.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P., Dickinson F. M., Jones I. G. Purification and properties of rabbit muscle L-glycerol 3-phosphate dehydrogenase. Biochem J. 1973 Dec;135(4):853–859. doi: 10.1042/bj1350853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair A. H., Bodley F. H. Human liver aldehyde dehydrogenase: partial purification and properties. Can J Biochem. 1969 Mar;47(3):265–272. doi: 10.1139/o69-041. [DOI] [PubMed] [Google Scholar]
- Bühner M., Sund H. Yeast alcohol dehydrogenase: SH groups, disulfide groups, quaternary structure, and reactivation by reductive cleavage of disulfide groups. Eur J Biochem. 1969 Nov;11(1):73–79. doi: 10.1111/j.1432-1033.1969.tb00741.x. [DOI] [PubMed] [Google Scholar]
- CANFIELD R. E. THE AMINO ACID SEQUENCE OF EGG WHITE LYSOZYME. J Biol Chem. 1963 Aug;238:2698–2707. [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- Castellino F. J., Barker R. Examination of the dissociation of multichain proteins in guanidine hydrochloride by membrane osmometry. Biochemistry. 1968 Jun;7(6):2207–2217. doi: 10.1021/bi00846a025. [DOI] [PubMed] [Google Scholar]
- Corrall R. J., Havre P., Margolis J., Kong M., Landau B. R. Subcellular site of acetaldehyde oxidation in rat liver. Biochem Pharmacol. 1976 Jan;25(1):17–20. doi: 10.1016/0006-2952(76)90166-0. [DOI] [PubMed] [Google Scholar]
- Crow K. E., Kitson T. M., MacGibbon A. K., Batt R. D. Intracellular localisation and properties of aldehyde dehydrogenases from sheep liver. Biochim Biophys Acta. 1974 May 20;350(1):121–128. doi: 10.1016/0005-2744(74)90209-5. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Eckfeldt J. H., Yonetani T. Kinetics and mechanism of the F1 isozyme of horse liver aldehyde dehydrogenase. Arch Biochem Biophys. 1976 Mar;173(1):273–281. doi: 10.1016/0003-9861(76)90260-5. [DOI] [PubMed] [Google Scholar]
- Eckfeldt J., Mope L., Takio K., Yonetani T. Horse liver aldehyde dehydrogenase. Purification and characterization of two isozymes. J Biol Chem. 1976 Jan 10;251(1):236–240. [PubMed] [Google Scholar]
- Feldman R. I., Weiner H. Horse liver aldehyde dehydrogenase. I. Purification and characterization. J Biol Chem. 1972 Jan 10;247(1):260–266. [PubMed] [Google Scholar]
- Kraemer R. J., Deitrich R. A. Isolation and characterization of human liver aldehyde dehydrogenase. J Biol Chem. 1968 Dec 25;243(24):6402–6408. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marjanen L. Intracellular localization of aldehyde dehydrogenase in rat liver. Biochem J. 1972 May;127(4):633–639. doi: 10.1042/bj1270633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrilla R., Okawa K., Lindros K. O., Zimmerman U. J., Kobayashi K., Williamson J. R. Functional compartmentation of acetaldehyde oxidation in rat liver. J Biol Chem. 1974 Aug 10;249(15):4926–4933. [PubMed] [Google Scholar]
- REITHEL F. J., ROBBINS J. E., GORIN G. A STRUCTURAL SUBUNIT MOLECULAR WEIGHT OF UREASE. Arch Biochem Biophys. 1964 Dec;108:409–413. doi: 10.1016/0003-9861(64)90421-7. [DOI] [PubMed] [Google Scholar]
- Sund H., Weber K., Mölbert E. Dissoziation der Rinderleber-Katalase in ihre Untereinheiten. Eur J Biochem. 1967 Jun;1(4):400–410. doi: 10.1111/j.1432-1033.1967.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


