Abstract
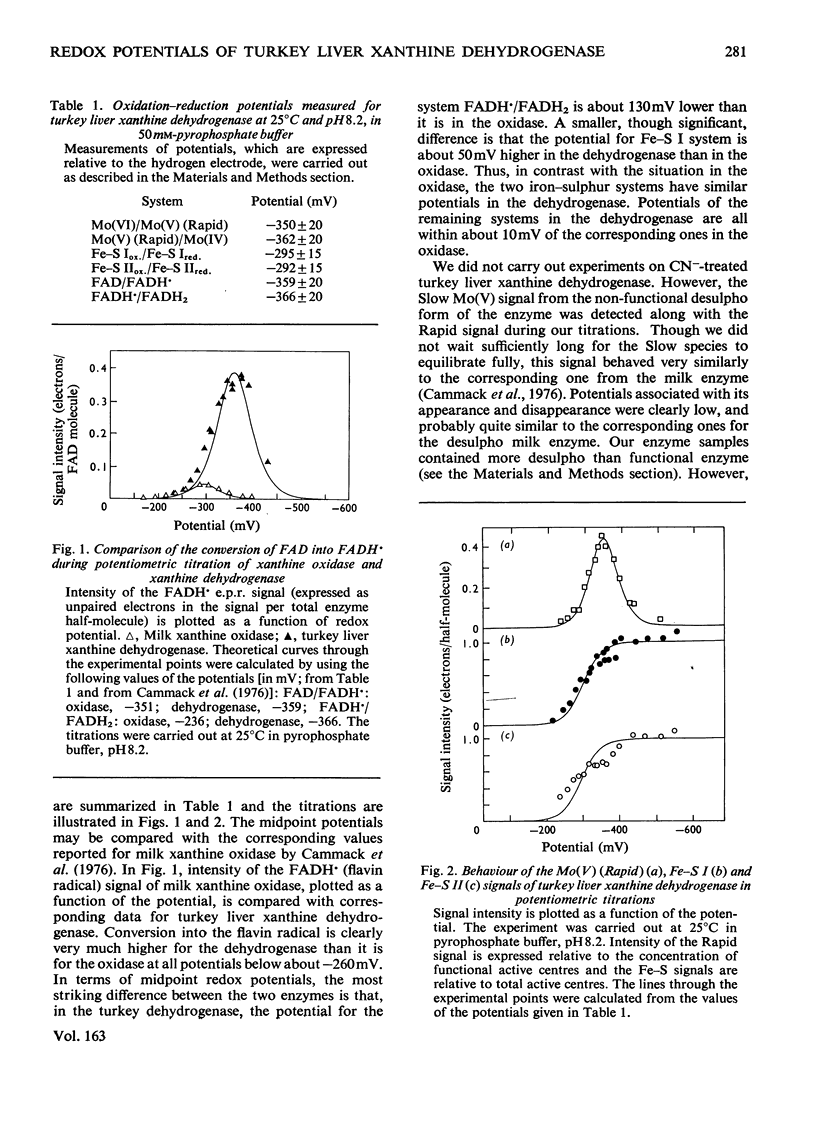
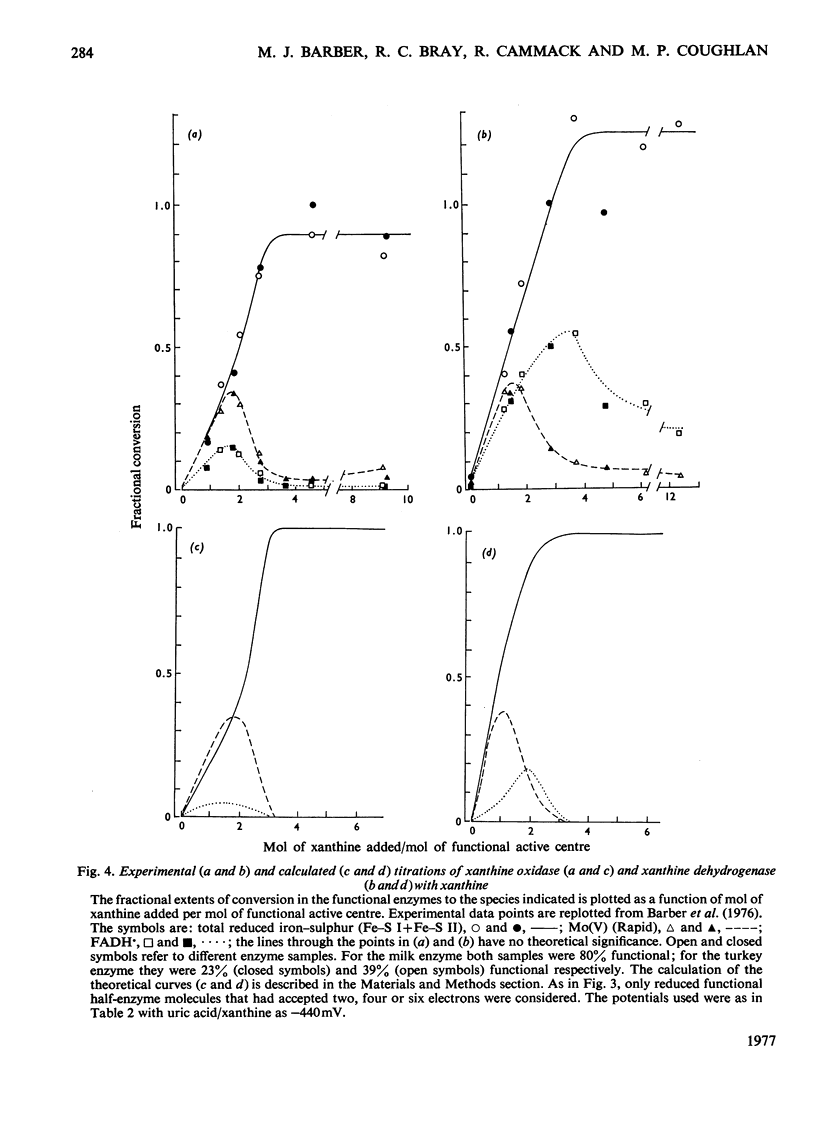
Redox potentials for the various centres in the enzyme xanthine dehydrogenase (EC 1.2.1.37) from turkey liver determined by potentiometric titration in the presence of mediator dyes, with low-temperature electron-paramagnetic-resonance spectroscopy. Values at 25 degrees C in pyrophosphate buffer, pH 8.2, are: Mo(VI)/Mo(V)(Rapid),-350 +/- 20mV; Mo(V) (Rapid)/Mo(IV), -362 +/- 20mV; Fe-S Iox./Fe-S Ired., -295 +/- 15mV; Fe-S IIox./Fe-S IIred., -292 +/- 15mV; FAD/FADH,-359+-20mV; FADH/FADH2, -366 +/- 20mV. This value of the FADH/FADH2 potential, which is 130mV lower than the corresponding one for milk xanthine oxidase [Cammack, Barber & Bray (1976) Biochem. J. 157, 469-478], accounts for many of the differences between the two enzymes. When allowance is made for some interference by desulpho enzyme, then differences in the enzymes' behaviour in titration with xanthine [Barber, Bray, Lowe & Coughlan (1976) Biochem. J. 153, 297-307] are accounted for by the potentials. Increases in the molybdenum potentials of the enzymes caused by the binding of uric acid are discussed. Though the potential of uric acid/xanthine (-440mV) is favourable for full reduction of the dehydrogenase, nevertheless, during turnover, for kinetic reasons, only FADH and very little FADH2 is produced from it. Since only FADH2 is expected to react with O2, lack of oxidase activity by the dehydrogenase is explained. Reactivity of the two enzymes with NAD+ as electron acceptor is discussed in relation to the potentials.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAY R. C., PETTERSSON R., EHRENBERG A. The chemistry of xanthine oxidase. 7. The anaerobic reduction of xanthine oxidase studied by electron-spin resonance and magnetic susceptibility. Biochem J. 1961 Oct;81:178–189. doi: 10.1042/bj0810178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M. J., Bray R. C., Lowe D. J., Coughlan M. P. Studies by electron-paramagnetic-resonance spectroscopy and stopped-flow spectrophotometry on the mechanism of action of turkey liver xanthine dehydrogenase. Biochem J. 1976 Feb 1;153(2):297–307. doi: 10.1042/bj1530297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli M. G., Lorenzoni E., Stripe F. Milk xanthine oxidase type D (dehydrogenase) and type O (oxidase). Purification, interconversion and some properties. Biochem J. 1973 Feb;131(2):191–198. doi: 10.1042/bj1310191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack R., Barber M. J., Bray R. C. Oxidation-reduction potentials of molybdenum, flavin and iron-sulphur centres in milk xanthine oxidase. Biochem J. 1976 Aug 1;157(2):469–478. doi: 10.1042/bj1570469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleere W. F., Coughlan M. P. Avian xanthine dehydrogenases. I. Isolation and characterization of the turkey liver enzyme. Comp Biochem Physiol B. 1975 Feb 15;50(2B):311–322. doi: 10.1016/0305-0491(75)90280-1. [DOI] [PubMed] [Google Scholar]
- Cleere W. F., O'Regan C., Coughlan M. P. Turkey liver xanthine dehydrogenase: properties of the enzyme dependent on the content of functional active sites. Biochem J. 1974 Nov;143(2):465–468. doi: 10.1042/bj1430465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton H., Lowe D. J., Pawlik T., Bray R. C. Studies by electron-paramagnetic-resonance spectroscopy on the mechanism of action of xanthine dehydrogenase from Veillonella alcalescens. Biochem J. 1976 Feb 1;153(2):287–295. doi: 10.1042/bj1530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Corte E., Stirpe F. The regulation of xanthine oxidase. Inhibition by reduced nicotinamide-adenine dinucleotide of rat liver xanthine oxidase type D and of chick liver xanthine dehydrogenase. Biochem J. 1970 Mar;117(1):97–100. doi: 10.1042/bj1170097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E. Studies of reversible dehydrogenase systems: The reversibility of the xanthine oxidase system. Biochem J. 1934;28(4):1550–1560. doi: 10.1042/bj0281550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V., Cattau E. L., Jr, Wang P. A comparison of the distribution and electron acceptor specificities of xanthine oxidase and aldehyde oxidase. Comp Biochem Physiol B. 1974 Dec 15;49(4):687–703. doi: 10.1016/0305-0491(74)90256-9. [DOI] [PubMed] [Google Scholar]
- Land E. J., Swallow A. J. One-electron reactions in biochemical systems as studied by pulse radiolysis. I. Nicotinamide-adenine dinucleotide and related compounds. Biochim Biophys Acta. 1968 Oct 1;162(3):327–337. doi: 10.1016/0005-2728(68)90119-9. [DOI] [PubMed] [Google Scholar]
- Massey V., Müller F., Feldberg R., Schuman M., Sullivan P. A., Howell L. G., Mayhew S. G., Matthews R. G., Foust G. P. The reactivity of flavoproteins with sulfite. Possible relevance to the problem of oxygen reactivity. J Biol Chem. 1969 Aug 10;244(15):3999–4006. [PubMed] [Google Scholar]
- Mayhew S. G., Foust G. P., Massey V. Oxidation-reduction properties of flavodoxin from Peptostreptococcus elsdenii. J Biol Chem. 1969 Feb 10;244(3):803–810. [PubMed] [Google Scholar]
- Muir Wood P. The redox potential of the system oxygen--superoxide. FEBS Lett. 1974 Aug 15;44(1):22–24. doi: 10.1016/0014-5793(74)80297-8. [DOI] [PubMed] [Google Scholar]
- Olson J. S., Ballou D. P., Palmer G., Massey V. The mechanism of action of xanthine oxidase. J Biol Chem. 1974 Jul 25;249(14):4363–4382. [PubMed] [Google Scholar]
- Pick F. M., Bray R. C. Complex-formation between reduced xanthine oxidase and purine substrates demonstrated by electron paramagnetic resonance. Biochem J. 1969 Oct;114(4):735–742. doi: 10.1042/bj1140735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. T., Rajagopalan K. V., Handler P. Purification and properties of xanthine dehydroganase from Micrococcus lactilyticus. J Biol Chem. 1967 Sep 25;242(18):4108–4117. [PubMed] [Google Scholar]
- Stirpe F., Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem. 1969 Jul 25;244(14):3855–3863. [PubMed] [Google Scholar]
- Swann J. C., Bray R. C. Multiple phases in the reduction of xanthine oxidase by substrates. Eur J Biochem. 1972 Apr 11;26(3):407–415. doi: 10.1111/j.1432-1033.1972.tb01781.x. [DOI] [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (type D) to an O2-dependent form (type O). Arch Biochem Biophys. 1976 Feb;172(2):365–379. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]


