Abstract
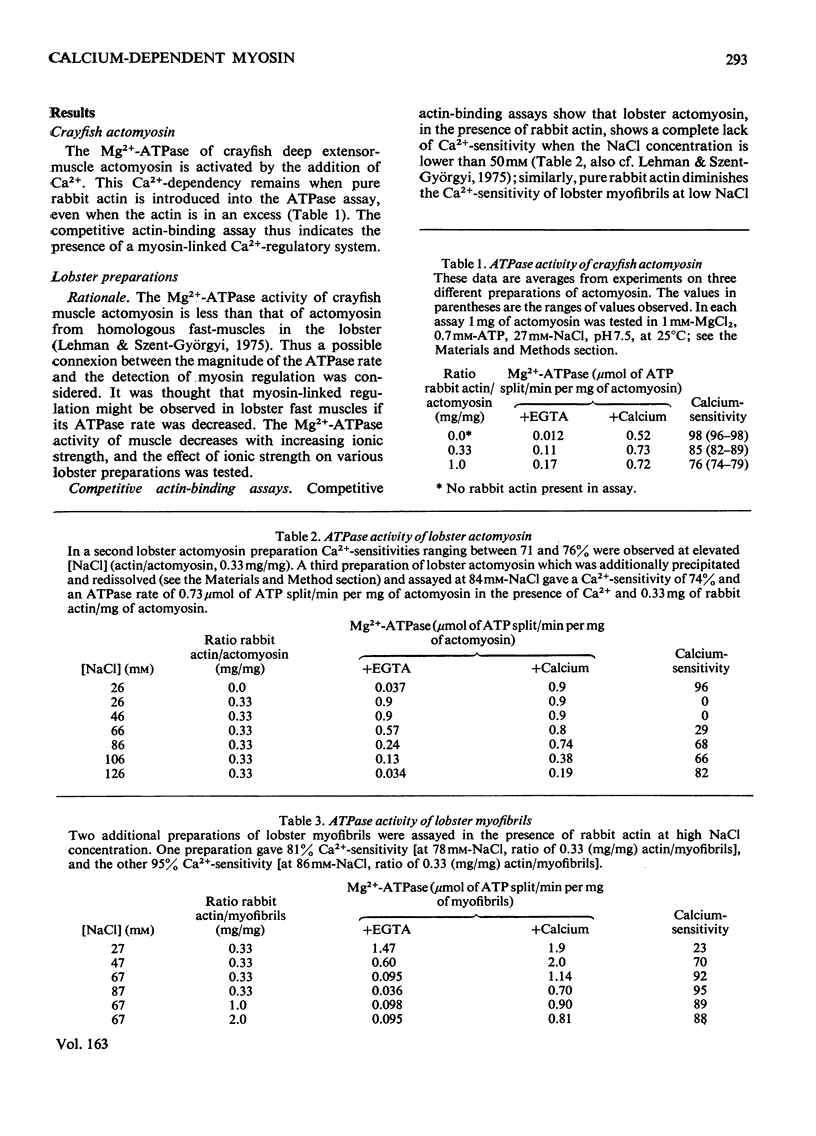
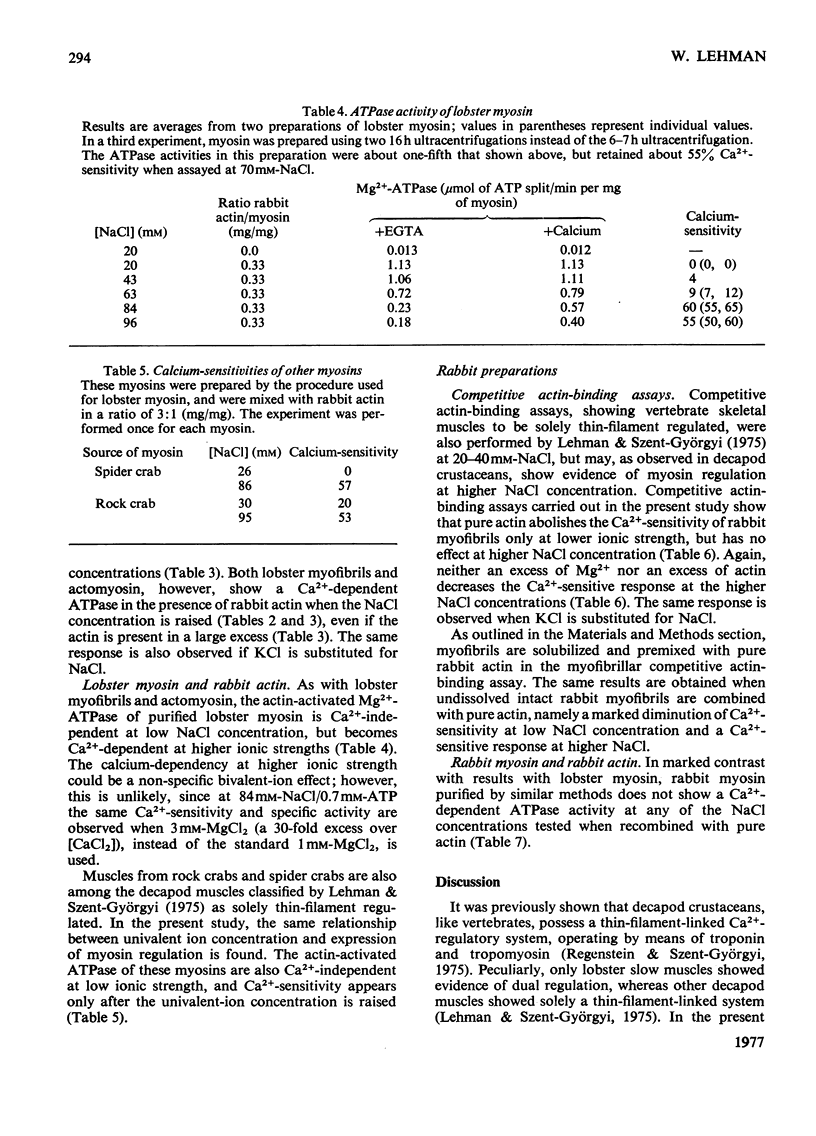
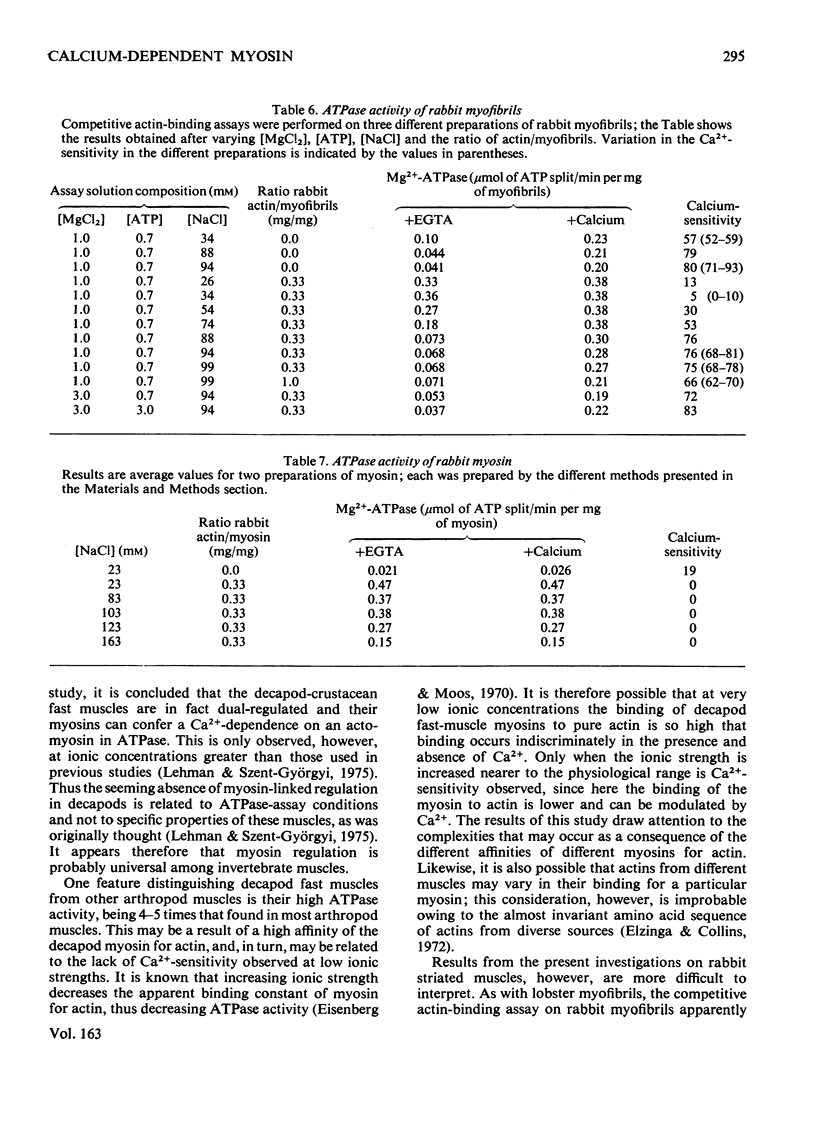
Ca2+ regulation of arthropod actomyosin adenosine triphosphatase is associated with both the thin filaments, as in vertebrates, and with the myosin, as in molluscs. The actomyosin of decapod-crustacean fast muscles was previously considered to be an exception, displaying only a Ca2+-regulatory system linked to the thin filaments and not a myosin-linked regulatory system. In the present study, myosin regulation is demonstrated in a variety of decapod muscles when they are tested under more physiological ionic conditions. Myosin regulation is shown by using mixtures of pure rabbit actin with myofibrils, with actomyosin and with purified myosin, and in each case the adenosine triphosphatase is Ca2+ dependent. Myosin regulation may also occur in vertebrate striated muscle, but seemingly is lost during purification of the myosin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremel R. D. Myosin linked calcium regulation in vertebrate smooth muscle. Nature. 1974 Nov 29;252(5482):405–407. doi: 10.1038/252405a0. [DOI] [PubMed] [Google Scholar]
- Bullard B., Dabrowska R., Winkelman L. The contractile and regulatory proteins of insect flight muscle. Biochem J. 1973 Oct;135(2):277–286. doi: 10.1042/bj1350277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBASHI S. THIRD COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF 'NATURAL ACTOMYOSIN'. Nature. 1963 Dec 7;200:1010–1010. doi: 10.1038/2001010a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Moos C. Actin activation of heavy meromyosin adenosine triphosphatase. Dependence on adenosine triphosphate and actin concentrations. J Biol Chem. 1970 May 10;245(9):2451–2456. [PubMed] [Google Scholar]
- HINKE J. A. Glass micro-electrodes for measuring intracellular activities of sodium and potassium. Nature. 1959 Oct 17;184(Suppl 16):1257–1258. doi: 10.1038/1841257a0. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Lehman W., Szent-Györgyi A. G. Regulation in molluscan muscles. J Mol Biol. 1970 Dec 14;54(2):313–326. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehman W., Szent-Györgyi A. G. Regulation of muscular contraction. Distribution of actin control and myosin control in the animal kingdom. J Gen Physiol. 1975 Jul;66(1):1–30. doi: 10.1085/jgp.66.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrwa U., Rüegg J. C. Myosin-linked calcium regulation in vascular smooth muscle. FEBS Lett. 1975 Dec 1;60(1):81–84. doi: 10.1016/0014-5793(75)80423-6. [DOI] [PubMed] [Google Scholar]
- Regenstein J. M., Szent-Gyäorgyi A. G. Regulatory proteins of lobster striated muscle. Biochemistry. 1975 Mar 11;14(5):917–925. doi: 10.1021/bi00676a007. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Myosin-linked calcium regulation in vertebrate smooth muscle. J Mol Biol. 1976 Mar 25;102(1):75–92. doi: 10.1016/0022-2836(76)90074-7. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. G., Cohen C., Kendrick-Jones J. Paramyosin and the filaments of molluscan "catch" muscles. II. Native filaments: isolation and characterization. J Mol Biol. 1971 Mar 14;56(2):239–258. doi: 10.1016/0022-2836(71)90462-1. [DOI] [PubMed] [Google Scholar]


