Abstract
Handfish (Family Brachionichthyidae) is the most threatened marine teleost fish family, however, there is little information on handfish health. We reviewed the results of submissions of mortalities from captive and captive bred spotted handfish (Branchionichthys hirsutus (Lacepède, 1804)) and red handfish (Thymichthys politus (Richardson, 1844)) from a public aquarium from January 2018 to February 2024. Seventeen cases for spotted handfish (comprising 33 individuals) and five cases for red handfish (one individual each) were submitted for mortality investigation. In 2018–2019, six of seven cases were diagnosed with scuticociliatosis. Other conditions included epitheliocystis (1 of 17), infections with metazoan parasites (2 of 17 for spotted handfish and 2 of 5 for red handfish) and hepatic lipidosis (4 of 17). Most submissions were fixed samples for histology with only one fish suitable for microbiology. We recommend development of a coordinated health program for all captive breeders and aquaria, which should include sampling protocols, collection, and preservation in a range of fixatives of all the dying or dead handfish and adapting sublethal sampling, including tank water to assess presence of pathogens and other microorganisms. Risk factors for handfish health in captivity should be assessed. Handfish health database should be established to avoid loss of corporate knowledge in this area.
Keywords: diagnostic sample, epitheliocystis, handfish, histology, public aquarium, scuticociliate
1. INTRODUCTION
Handfish are marine fish from the Family Brachionichthyidae, Order Lophiiformes (anglerfish). Handfish use their modified pectoral fins for moving on the bottom. The family includes 5 genera and 14 species, all of them are endemic to the coastal areas of Southern and Eastern Australia, with 11 species recorded from Tasmania (https://fishesofaustralia.net.au/home/family/169#moreinfo). Conservation status of handfish is a significant issue as three handfish species are critically endangered, four are endangered, six species are data deficient and one is classified as a least concern species, resulting in the Brachionichthyidae identified as the most threatened marine teleost fish family (Edgar et al., 2017, Stuart‐Smith et al., 2020, IUCN https://www.iucnredlist.org/search). Some of the main threats for spotted handfish include climate change and declines in habitat quality: predation on preferred spawning substrate by Northern Pacific seastar, historical scallop dredging, marine moorings, urban development, and heavy metal pollution (Edgar et al., 2017, Stuart‐Smith et al., 2020). Other threats are extreme population fragmentation, degradation of shallow reef habitat quality through increasing urchin densities, possibly as a result of predator release from depletion of Rock lobsters. Remaining handfish colonies are in urban areas, with habitat potentially affected by nutrient runoff, pollution, siltation, and turbidity. Poaching is another potential threat (Stuart‐Smith et al., 2020). This conservation status and recovery efforts for handfish populations resulted first in an assessment of the potential of captive breeding programs (Bruce et al., 1997) and then, in the establishment of captive breeding programs, the first one at Commonwealth Scientific and Industrial Research Organization (CSIRO) in 2017, followed by Seahorse World Tasmania, the Zoo and Aquarium Association, National Environmental Science Program and Sealife Melbourne Aquarium (Hawkins, 2021; Lynch et al., 2017). These programs focused on two species: spotted handfish (Branchionichthys hirsutus (Lacepède, 1804)) and red handfish (Thymichthys politus (Richardson, 1844)), which are both listed as critically endangered on IUCN Red List of threatened species (IUCN https://www.iucnredlist.org/search). The role of these programs is to improve our understanding of the biology of those species and to provide handfish for restocking. Seahorse World has been an active participant in the handfish recovery program and, in addition to running captive breeding, has an important role in raising public awareness of handfish by displaying both species of handfish in the public aquarium (Hawkins, 2021).
To make the conservation efforts even more successful, there is an urgent need for more information about handfish. In particular, little information is available about handfish health (Hawkins, 2021). Despite almost 30 years of research on captive and captive bred handfish, there are no publications summarizing our knowledge of their health and diseases. Here, we review the results of submissions of mortalities from captive and captive bred spotted handfish and red handfish from a public aquarium from January 2018 to February 2024.
2. MATERIALS AND METHODS
2.1. Study population
The first transport of 10 wild adult spotted handfish arrived at Seahorse World Tasmania in 2017, whereas 16 red handfish hatchlings were provided by CSIRO in December 2018 (Hawkins, 2021). From January 2018 to February 2024, spotted handfish contributed 81.4% and red handfish 18.6% to the handfish population held at Seahorse World Tasmania. Of all spotted handfish, 38.1% were wild (captive) and 61.9% were bred in captivity. 5.6% of red handfish were wild and 94.4% were captive bred. All handfish other than those born at Seahorse World were provided to Seahorse World by IMAS Hobart and CSIRO, which have been collecting and breeding those species.
The husbandry of handfish followed the protocols described previously (Hawkins, 2021). Briefly, the fish have been held in different tank systems, each system with its own recirculation. Stocking density was kept low, with 1–10 spotted handfish in each tank and 1–3 red handfish in each tank. The two species were kept separately. Sea water was pumped from the Tamar River and filtered to 0.2 μm (from 2021 new filter to 0.08 μm), then chilled/heated to the required temperature. Some systems had an additional ultra‐violet treatment (Hawkins, 2021). The water quality was checked daily. The water temperature was adjusted to seasonal and ranged from 11 to 17°C (seasonal adjustments to mimic normal range of water temperature at Derwent Estuary). The pH was maintained at 8 and salinity at 33 g/L. Any detectable total ammonia or nitrite resulted in a water change in the tank affected by the increased reading. The adult fish were fed live food to satiation with wild amphipods, Bellorchestia pravidactyla, and sometimes wild mysids. Live hatched enriched Artemia salina was fed to juveniles.
2.2. Sample submission and analyses
Handfish samples were received between January 2018 and February 2024 when mortalities occurred. Seventeen cases for spotted handfish (comprising 33 individuals) and five cases for red handfish (one individual each) were submitted for investigation (Table 1). The submitted handfish showed no clinical signs before their sudden death, other than in a few cases lack of appetite or laying on their side. At the time of the handfish submissions, no other fish species held at Seahorse World had any apparent health problems. Most of the submissions consisted of one fish but on a few occasions more individuals were sent. Fish were fixed as soon as possible after they were found dead, but for some fish the fixation was delayed, for example if the fish died at night they were not found until next morning. Not all fish were cut open for fixation, slowing down the penetration of internal organs by the fixative. Most of the samples were sent for histology fixed in 10% phosphate buffered formalin or, on one occasion, the fish was fixed in 70% ethanol. Samples were trimmed, cassetted and processed for routine histology. Briefly, samples were dehydrated using a series of alcohol, cleared by xylene then embedded in paraffin. Fish were serial sectioned at five microns either transversely or longitudinally to include all organs. The fish section were then decalcified in a solution of 10% hydrochloric acid (Decalcifier hydrochloric acid bone decalcifier solution Amber Scientific) then processed for stained with haematoxylin–eosin and examined under a microscope. All organs were included in the examined sections including, liver, kidney, spleen heart, gill, brain, skin, eye, cartilage, bone, and alimentary tract. Images were captured using a digital camera connected to a microscope (Nikon Eclipse Ni‐U). Any parasites present in histological sections were identified based on the published information (Bruno et al., 2006; Dyková & Lom, 2007; Gardiner et al., 1998; Gardiner & Poynton, 1999).
TABLE 1.
Summary of submissions of spotted handfish and red handfish from Seahorse World from January 2018 to February 2024.
| Date (number of fish submitted) | Diagnosis | Case history water quality data |
|---|---|---|
| Spotted handfish | ||
| 27.01.2018 (6) | Hepatic lipidosis, mild multifocal proliferative branchitis | Wild adults (2–3 months in captivity) and a captive bred juvenile, 12–14.1°C, pH 8–1–8.4 34 g/L, ammonia 0–0.1 mg/L, nitrite 0.1–02.mg/L |
| 22.04.2018 (3) | Scuticociliatosis | Small juvenile |
| 7.07.2018 (1) | Scuticociliatosis necrotizing dermatitis | Juvenile, 12.3°C, pH 8 |
| 11.07.2018 (1) | Scuticociliatosis | Juvenile, 12.3°C, pH 8 |
| 30.07.2018 (1) | Scuticociliatosis, nematodes | Wild male, 12.3°C, pH 8 |
| 18.10.2019 (1) | Scuticociliatosis | 17.1°C, pH 8 |
| 28.10.2019 (5) | Scuticociliatosis, mild diffuse lamellar hypertrophy, expansion of the underlying interstitium by oedema, gills autolysed, abundant leucothrix like bacteria over the surface of the lamella (postmortem), moderate autolysis | Adults, sudden mortality, 17.1°C, pH 8 |
| 11.02.2021 (2) | Severe autolysis | Adult male |
| 21.05.2021 (4) |
Hepatic lipidosis (4) Large spleen PMAs (4) Granuloma in liver—trematode metacercaria |
Adults (2 males), first generation bred in captivity |
| 1.02.2022 (1) | Bacteria in autolysed gills (postmortem) | |
| 1.05.2022 (1) | Hepatic lipidosis, Spleen PMAs | |
| 18.06.2022 (1) | Hepatic lipidosis, bacteria in gills | |
| 17.09.2022 (1) | Bacteria in gills, moderate autolysis | Adult female |
| 25.11.2022 (2) | Bacteria in gills, moderate autolysis | |
| 23.01.2023 (1) | Hepatic lipidosis, diffuse, moderate autolysis, detritus between gill lamellae | Adult female, 5 years old, temperature stress event (8–19°C) |
| 29.11.2023 (1) | Trematode metacercaria, larval cestode in the coelomic cavity cross section, autolysis | Wild adult, 5 months in captivity, temperature stress event a few days (8–19°C) |
| 19.02.2024 (1) | Epitheliocystis | Captive bred adult female, 6–7 years old, 17°C pH 8 |
| Red handfish | ||
| 30.12.2020 (1) | Trematode metacercaria | Wild adult female |
| 11.02.2021 (1) | Detritus between gill lamellae, autolysis | Adult female |
| 21.05.2021 (1) | Trematode metacercaria in liver, chronic inflammation heart and gills (PMAs in both) | Adult female |
| 17.01.2022 (1) | Marine invertebrate, possibly phylum Nemertea in oral cavity, moderate autolysis | Adult female |
| 24.03.2022 (1) | Severe autolysis | Adult female |
Note: All submitted fish were mortalities. All fish were submitted for histology and fixed in formalin with the exception of the spotted handfish submitted for PCR (fixed in ethanol) on 22.04.2018, one spotted handfish submitted on ice for microbiology on 28.10.2019 and the spotted handfish submitted for histology (fixed in ethanol) on 19.02.2024.
Abbreviation: PMA, pigmented macrophage aggregates.
2.3. Microbiology methods
Standard microbiology methods were used (Buller, 2014). Briefly, anterior and posterior kidney samples were streaked on Sheep Blood agar and Thiosulfate Citrate Bile Salts Sucrose agar. The agar was commercially formulated Blood Agar Base No 2 (Oxoid Blood Agar Base no.2 CM0271–40.0 g/L) with the addition of defibrinated sheep blood (70 mL/1000 mL based) purchased from Serum Australis, Manila Australia. The plates were incubated at 25°C for 5 days (Buller, 2014).
2.4. DNA extraction
Three handfish (H1, H2, and H3) from the scuticociliate outbreak in 2018 were preserved in 70% ethanol for further identification of which species of scuticociliate was involved. DNA extraction was carried out using the ISOLATE II Genomic DNA kit (Meridian Bioscience) as per the manufacturer's protocol with the following modifications. The pre‐lysis step took 5 h, binding DNA required a 5‐min centrifugation due to the column retaining liquid, and the washing of the silica membrane required a 3‐min centrifugation with the GW1 wash and a 2‐min centrifugation with the GW2 wash. Additionally, both the dry silica membrane step and the elution steps both required a 2‐min centrifugation. As the fish were between 41 mg and 54 mg in weight, the whole fish was used for DNA extraction. DNA concentration of the extractions was assessed using NanoDrop (ThermoFisher Scientific).
2.5. Polymerase chain reaction amplification of the extracted DNA
PCRs were performed to determine the presence of SSU rRNA of scuticociliates using the 2/4 primer combination (Table 2) (Jung et al., 2005). PCRs (50 μL) were set up with 25 μL of AmpliTaq® Gold 360 master mix (Agilent Technologies), 17 μL of DNA free water, 1 μL of each primer (10 μM), 1 μL of GC Enhancer and 5 μL of sample DNA. PCRs included a negative control (no template control) to test for contamination and a positive control (Miamensis avidus, isolate U02 from Southern bluefin tuna Port Lincoln South Australia, GenBank accession: KX842462.1) to determine PCR efficacy.
TABLE 2.
Oligonucleotide sequences used for polymerase chain reactions in this study.
Each PCR was vortexed and centrifuged before being placed in the T100™ Thermal cycler (Bio‐Rad). PCR samples were initially denatured at 95°C for 10 min before undergoing 35 cycles of denaturation (95°C, 30 s), annealing (50°C, 30 s) and elongation (72°C, 60 s). A final extension of 72°C for 7 min was followed by an infinite hold at 4°C. PCR products were visualized on gel electrophoresis with a 1.5% (w/v) agarose gel containing 5 μL of SYBR Safe (Invitrogen) for 1 h at 80 V. Lanes were loaded up with 5 μL of sample and 2 μL of 5× loading dye (Meridian Bioscience).
2.6. Amplicon sequencing
PCR products were sent to the Ramaciotti Centre for Genomics at the University of NSW for an Illumina indexing PCR and MiSeq v3 2 × 300 bp sequencing run generating paired end reads. The Miseq Reporter carried out demultiplexing and created both forward and reverse reads for each sample.
2.7. Taxonomic assignment of molecular operational taxonomic units
A lower limit of 2000 sequence reads for each sample was assigned and sequence identification analysis was performed using the GHAP Amplicon pipeline (Greenfield, 2017). Sequences that originate from genomic DNA of individual organisms are known as molecular operational taxonomic units (MOTUs) (Floyd et al., 2002). This pipeline uses both USEARCH and RDP classifier to analyse the data generated by Illumina platforms for metabarcoding purposes. Reads were merged and trimmed before assigning MOTUs. The list of MOTUs was assessed against the SILVA database (https://www.arb‐silva.de/) to apply taxonomic information to the sequences (Chariton et al., 2015).
2.8. Quality control for sequencing data
MOTUs which had a sequence match lower than 97% to the identification databases and less than 10 reads per sample were discarded from the analysis. Sequences were blasted against the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome) to check for consistency with the SILVA database.
3. RESULTS
Spotted handfish was the main species submitted. Between 2018 and March 2024 there were 17 submissions of spotted handfish (77.3%) and 5 submissions of red handfish (22.3%) (Table 1), roughly representing the proportion of how many fish of each species were held at Seahorse World Tasmania during that period. All submissions included only mortalities. There were no gross external lesions in any of the submitted fish.
For spotted handfish, scuticociliatosis was the most common diagnosis, followed by hepatic lipidosis and the presence of bacteria on the gills. Three spotted handfish were infected by metazoan parasites (one also had scuticociliatosis and another hepatic lipidosis) and one fish was affected by epitheliocystis (Table 1, Figure 1). Three red handfish submitted for examination were infected by metazoan parasites (Table 1). All submitted red handfish were adult females.
FIGURE 1.
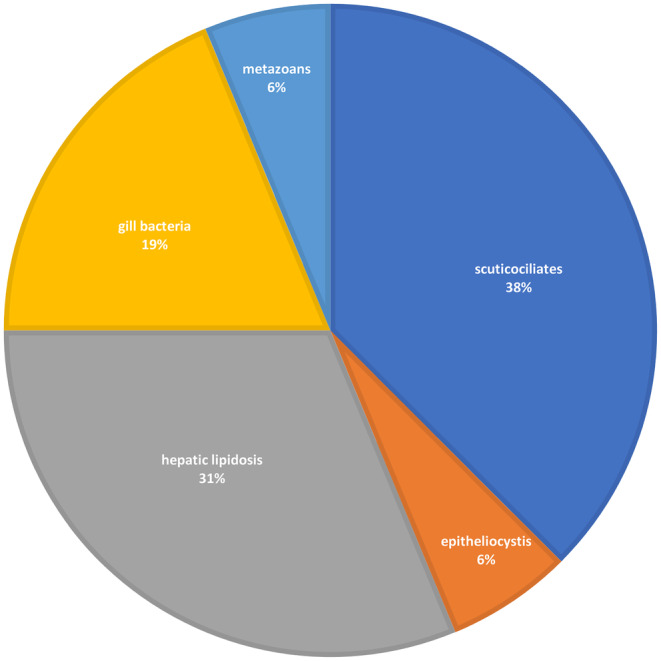
Diagnostic outcomes for spotted handfish histology submissions from 2018 to 2024. If more than one conditions were present in the submission, the one more likely contributing to the mortality was included in this graph. Severe autolysis cases (1 case for spotted handfish and 1 case for red handfish) were excluded from the dataset.
During the first 2 years, the most common finding was the presence of moderate numbers of ellipsoid, holotrichous, ciliated protozoa, 20–30 μm in diameter within the epidermis, dermis and subcutis, coelomic cavity, skeletal muscle and adhered to the olfactory orifice. These scuticociliates were present in six submissions of spotted handfish in 2018 and 2019, which were diagnosed as scuticociliatosis (Table 1, Figures 1 and 2ab). Multifocal mixed inflammation and necrosis were associated with the infection by scuticociliates. Necrosis of the epidermis and dermis and presence of bacteria suggested that the scuticocilliate infiltration was secondary (Figure 2b). However, necrotizing dermatitis was detected only in one of the six cases of scuticociliatosis (Table 1). All the handfish samples from the outbreak in 2018 showed positive bands in PCR amplification for the primers 2,4 (Figure 3). Subsequent sequencing indicated the presence of three MOTUs. All three of these MOTUs were present in H3, only one was present in H2 and no MOTUs were present in H1. One MOTU has been identified as Porpostoma notata, another MOTU was a species from the genus Uronema, and the last MOTU was inconclusive. No scuticociliates were detected in the histological sections from handfish submitted after 2019.
FIGURE 2.
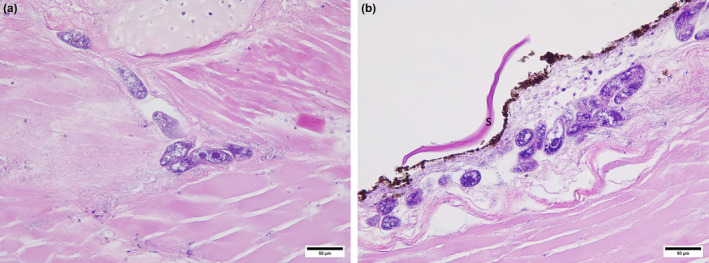
Scuticociliatosis in spotted handfish. (a) Scuticociliates colonizing skeletal muscles. (b) Scuticociliates in dermis, note completely eroded epidermis, necrotic dermis and bacteria suggesting secondary infection, possibly postmortem. S, scale.
FIGURE 3.
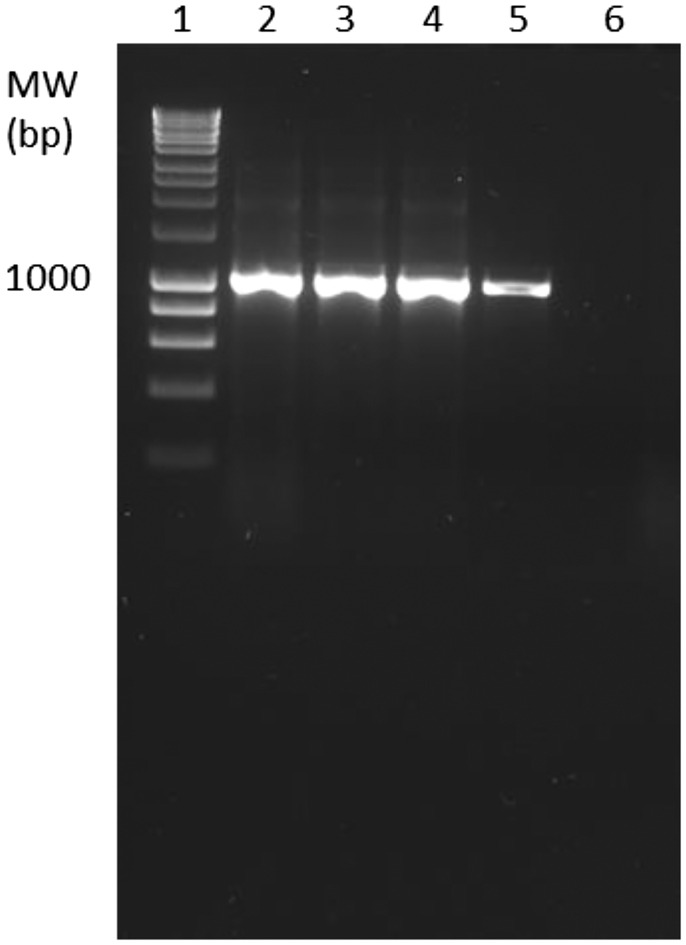
PCR amplification of samples visualized on a 1.5% (w/v) agarose gel. Gel lanes: 1. 1 kb Hyperladder, 2. Handfish 1 (H1), 3. Handfish 2 (H2), 4. Handfish 3 (H3), 5. Positive Control (U02), 6. Negative Control (no template control).
One spotted handfish submitted in 2024 was affected by epitheliocystis associated with multifocal branchitis and mild epithelial hyperplasia resulting in multifocal fusion of lamellae (Figure 4a). Multiple cysts were present in single lamellae (Figure 4b). The cysts were 100 to 200 μm in diameter and had slightly granular content. While the quality of the histological sections was poor due to the initial fixation in ethanol, there was some evidence of hyperplastic host response leading to lamellar fusion and oedema at the tip of gill filament (Figure 4a).
FIGURE 4.
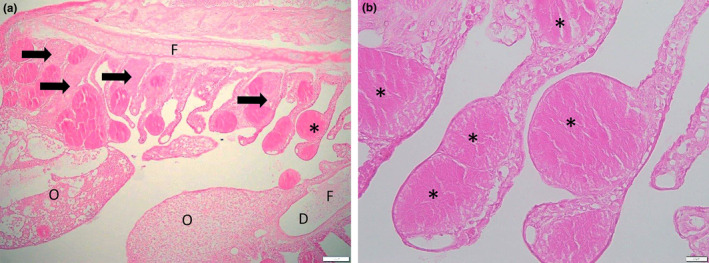
Epitheliocystis, * ‐ asterisks showing hypertrophied cells containing bacteria. (a) Host hyperplastic response resulting in lamellar fusion shown by arrows, F—filament, D—dilation of the vascular channel of the filament, O—oedema (expansion of the adjacent interstitium by eosinophilic proteinaceous material). (b) Multiple cysts in one gill lamella. Sample fixed in ethanol resulting in poor fixation and staining.
Metazoan parasites were present in some handfish, for example nematodes (Figure 5ab) were present in wild spotted handfish submitted in 2018 (diagnosed with scuticociliatosis), digenean metacercariae in wild red handfish in 2020 and another wild in 2021, digenean metacercariae in spotted handfish in 2021 and digenean metacercariae and a larval cestode in a spotted handfish in 2023. In some cases, pigmented macrophage aggregates were present in an organ to which the parasites were adjacent (Figure 5a) and slight pressure atrophy could be observed (Figure 5ab). Some host response was present, with granuloma forming around the nematodes (Figure 5ab). The digenean infection was often severe and affected multiple organs including liver, spleen, kidney, and intestine causing focal necrosis and pressure atrophy (Figure 6ab). Pigmented macrophage aggregates were prominent in a number of individuals, sometimes present in heart and gills and appeared to occupy most of the volume of spleen. These pigmented macrophage aggregates were not always associated with a parasitic infection.
FIGURE 5.
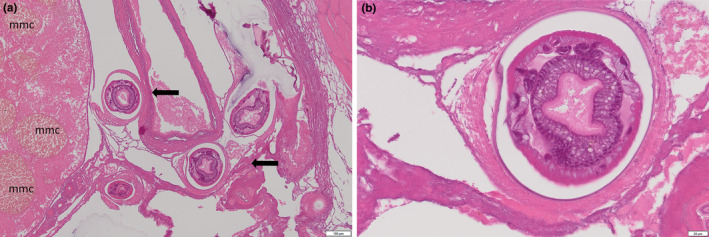
Nematode infection in spotted handfish. (a) Cross sections of nematodes showing round body and external acellular cuticle. Note granuloma forming around the parasites. Some pressure atrophy present (arrows). Pigmented macrophage aggregate (mmc) in the adjacent organ were more numerous than in other unaffected handfish. (b) Eggs present in a cross‐section of a female nematode surrounded by a granuloma.
FIGURE 6.
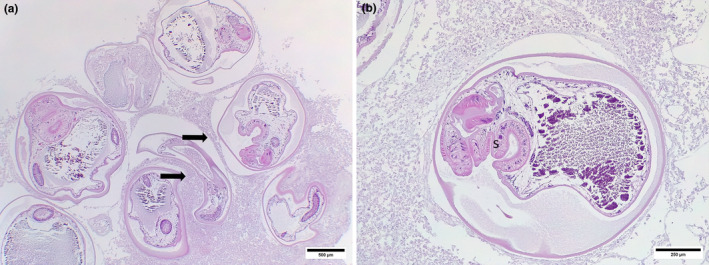
Digenean infection in red handfish. (a) Severe digenean infection as shown by the number of digenean metacercariae causing pressure atrophy (arrows). (b) Encysted digenean metacercariae showing sucker with radially arranged muscles (s). Liver autolysis post‐mortem.
Various stages of increased lipid like vacuolation ranging to multifocal hepatic lipidosis were diagnosed in five cases of spotted handfish (including all four individuals in one case) submitted between 2018 and 2023 (Table 1, Figure 7ab). Hepatocyte membranes appeared to disintegrate leading to the fusion of the fat globules (Figure 7ab). In some individuals, fat deposits replaced large volume of liver and were associated with an inflammatory response (Figure 7ab). Two of those fish were females, two males and sex could not be determined for the other individual.
FIGURE 7.
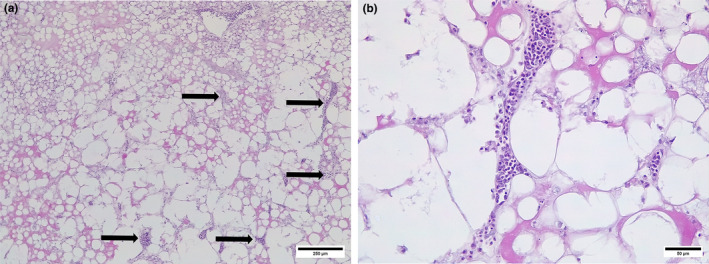
Hepatic lipidosis. (a) Hepatic lipidosis showing focal inflammation (arrows). (b) Hepatic lipidosis, note coalescing lipid vacuoles, inflammatory response and a few normal hepatocytes.
Presence of leucothrix‐like bacteria in the gills was noted in all submissions in 2022 (Table 1). However, it was likely that the colonization occurred post‐mortem based on autolytic changes to the gills. Five of the 17 submissions of spotted handfish and three of the 5 submissions of red handfish showed signs of autolysis affecting diagnosis (Table 1). This included one spotted handfish (of the 17 submissions) and one red handfish (of the five submissions), which were severely autolysed to the extent that no histological observations could be made.
Only one sample, which was from the kidney, was provided for microbiology on 28.10.2019. There was a mixed bacterial growth from kidney on sheep blood agar, which was not clinically significant.
4. DISCUSSION
Systemic scuticociliatosis affected juvenile spotted handfish in 2018 and 2019. Scuticociliates are free living detritivores which can become opportunistic histophagous parasites and can cause fish mortalities (Iglesias et al., 2001). Scuticociliatosis has been reported in farmed fish and in captive or captive reared fish from public aquaria, for example systemic scuticociliatosis caused by Philasterides dicentrachi resulted in mortalities of three species of sharks in a European aquarium and one of the same species of sharks in American display aquarium (Stidworthy et al., 2014). The same species caused mortalities in multispecies public aquarium (Jalenques et al., 2021), in seadragons Phycodurus eques and Phyllopteryx taeniolatus (see Rossteuscher et al., 2008) and in seahorses, including potbellied seahorse, Hippocampus abdominalis, in Vancouver Marine Science Center (Di Cicco et al., 2013). The scuticociliate Porpostoma notata was isolated from a batch of moribund Hippocampus hippocampus juveniles, which were showing high mortality and were reared in captivity in the Institute of Marine Investigations in Vigo Spain (Ofelio et al., 2014). While a range of species of scuticociliates have been reported from fish infections, they were not host specific, which means that an infection can spread between different fish species within a facility. However, this was not the case here, as other fish in the aquarium were not infected, suggesting that the system separation provided enough biosecurity to reduce risks of spread of infections between tanks.
One group of handfish was infected by scuticociliates despite being treated with acriflavine, which is considered an effective treatment against a range of external infections affecting fish (Lieke et al., 2020). In other fish species, systemic scuticociliatosis was successfully treated with antimicrobials, for example using metronidozole in seahorse, H. abdominalis (see Di Cicco et al., 2013), or trimethoprim in squarespot anthias, Pseudanthias pluerotaenia, infected by Uronema nigricans (see Kubiski et al., 2011).
Some handfish infections by scuticociliates appeared to be secondary, related to skin necrosis, however most infected handfish had no skin damage suggesting the infections were primary. This is consistent with reports from other hosts. For example, skin abrasion was necessary for a successful experimental infection of turbot Scophthalmus maximus, by P. dicentrachi (see Paramá et al., 2004), but skin damage was not necessary to induce experimental scuticociliatosis in Japanese flounder, Paralichthys olivaceus, using M. avidus (see Moustafa et al., 2010). Infection by scuticociliates can cause skin damage, as described in seadragons P. eques and P. taeniolatus infected by P. dicentrachi (see Rossteuscher et al., 2008).
Scuticociliate DNA suggested a co‐infection with at least two species. Co‐infections of scuticociliates have been reported in farmed fish previously, for example three different species of scuticociliates, including M. avidus, Uronema marinum, and Parauronema virginianum were associated with mortality events in New Zealand groper, Polyprion oxygeneios, farmed in sea cages (Salinas et al., 2011, 2012). Similarly, both U. nigricans and M. avidus were reported from Southern Bluefin Tuna, Thunnus maccoyii, affected by swimmer syndrome during ranching in sea pens (Balli Garza et al., 2017; Munday et al., 1997; Power et al., 2019).
To the best of our knowledge this is the first report of epitheliocystis in a handfish or anglerfish. Epitheliocystis was present only in one individual of spotted handfish. This condition is often benign and was associated with mortalities only if the infection was severe (Nowak & LaPatra, 2006). Based on the severity of the infection and host response observed in the handfish, it is possible that it contributed to death of this fish. Multiple cysts in an individual lamella, as present in the handfish, were reported before only in a few cases, including striped trumpeter, Latris lineata (see Lai et al., 2013), and largemouth bass, Micropterus salmoides (see Goodwin et al., 2005), and suggest a hyperinfection. In handfish, the presence of proliferative host response indicated chronic infection and the variable size of the hypertrophic cells may be due to the spread of the infection over time. Epitheliocystis has been reported from many marine fish species, both wild and farmed, but the pathogenic bacteria causing this condition appear to be host‐specific (Blandford et al., 2018; Stride et al., 2014). If this is the case then epitheliocystis would not spread between different fish species in an aquarium facility.
A few wild handfish were infected by metazoan parasites, including trematodes, nematodes, and cestodes. Some of the parasites caused localized host response, including granulomas. While some infections were quite severe, it was unclear how much they contributed to the mortality as the fish appeared to be in a good condition. Captive reared fish are usually at lower risk of infection by parasites with complex life cycles if the intermediate hosts and infective stages are not present in the tank. However, not all fish infected by metazoan parasites were wild. The risk of introducing parasites or other pathogens would increase when live food is used, as it is the case with handfish. Both mysids and amphipods were reported to be intermediate hosts of fish parasites, for example digenean Coitocaecum parvum was recovered from common bully, Gobiomorphus cotidianus, 4 weeks after feeding with experimentally infected amphipod, Paracalliope fluviatilis, and naturally infected mysid, Tenagomysis chiltoni (see Holton, 1984). Larval nematodes Pseudoterranova decipiens, Hysterothylacium aduncum, and Paracuaria adunca, and the digenean fish parasite Hemiurus levinseni were found in mysids sampled in North West Atlantic (Jackson et al., 1997). Larval nematodes P. adunca and Tetrameres sp., and Ascarophis sp., were present in amphipods from North West Atlantic (Jackson et al., 1997). Furthermore, amphipods and mysids can carry other pathogens, including pathogenic bacteria or protozoans. It is essential that the amphipods and mysids used to feed handfish are screened for presence of fish parasites and other organisms pathogenic to fish.
Hepatic lipidosis is often overdiagnosed in fish and it should be called “increased lipid‐like vacuolation” in most cases (Wolf et al., 2015). However, coalescencing of lipid vacuoles resulting from cell membrane rupture as shown in the handfish is indicative of adverse effects on liver and therefore the diagnosis of hepatic lipidosis is justified (Wolf et al., 2015). Hepatic lipidosis could be due to nutritional factors, such as type of food or amount of food, or as a result of exposure to pollutants. Overfeeding on energy‐rich diet or lipid peroxidation as a result of feeding diets high in polyunsaturated fats and/or low vitamin E or induced by an exposure to toxicants (Wolf & Wolfe, 2005). Marine fish species appear to have a higher risk of hepatic lipidosis, because they have a reduced potential for hepatocyte peroxisome proliferation and their diets may contain high proportion of mono‐unsaturated fatty acids (Spisni et al., 1998). In females, hepatic lipidosis may also be a result of fat re‐absorption from eggs due to the fish producing eggs but not spawning. It is unclear if hepatic lipidosis is irreversible and what effect it has on health of fish (Wolf & Wolfe, 2005), but it was considered a cause of death in a captive African stonefish, Synanceja verrucosa (see Penrith et al., 1994).
In the current study, only one handfish was in a suitable condition for kidney collection for microbiology and culture, because the period from death to submission to the laboratory was less than 24 h and the animal had been refrigerated. This time period and storage method were within the guidelines recommended for microbiological samples from fish (Elliott, 2020). For future disease investigations of captive handfish collecting, where practical, suitable fresh samples (e.g. kidney or any gross lesion) from recently deceased captive handfish for microbiological culture will be of benefit. Samples for molecular detection of pathogens should be included in the investigation of handfish mortalities. Molecular detection and identification of pathogens has been increasingly important in fish diseases (Cunningham, 2002). Microbial pathogen identification will contribute to future biosecurity management improvements for captive handfish. Monitoring of aquatic systems, for example aquaria, can be done using environmental DNA (eDNA) which means that samples could be collected without sampling the fish (Peters et al., 2018). This is important due to handfish conservation status.
All submitted fish appeared to be in a good condition. As some submitted fish were older, senescence was suggested as a cause of death. This has been suggested for other handfish mortalities as well (Lynch, Green et al., 2022). Spotted handfish showed ~90% mortality by 5 years and the oldest fish in the wild was estimated to be 10 years old (Bessell, 2018; Lynch, Soo et al., 2022). In captivity, one individual spotted handfish received as an adult in 2017 is still alive in 2024 and captive bred spotted handfish hatched in 2017 are alive in 2024 (Hawkins, 2021). Some of the submitted spotted handfish were close to the maximum age reported, so we cannot rule out that old age was a major factor in mortality of those fish. The presence of large and numerous pigmented macrophage aggregates further suggests that the fish were old, as at least in some species the relationship between fish age and increase in pigmented macrophage aggregates size or number of volume (for review see Steinel & Bolnick, 2017).
Most handfish diagnostic testing did not identify the cause of mortality. This could be because histology was often the only analysis performed, most fish were sampled after their death (up to a few hours) and sometimes autolytic changes were present. Opening coelomic cavity, removing operculum, rapid fixation, and euthanasia when moribund are recommended to avoid autolysis. It would be beneficial to run a full range of analyses to investigate handfish mortalities as suggested for other fish species (Blazer et al., 2018). For example, molecular detection of pathogens should be done, in particular if histology suggests an infection. If nonlethal sampling was required due to handfish conservation status, eDNA could be used to investigate presence of pathogens in the fish tank.
Due to the high conservation status of handfish and our current lack of knowledge of handfish health, it is important to sample as many individuals as possible and optimize the use of those samples. It is a missed opportunity for handfish research if not all mortalities are submitted for analysis or at least preserved for diagnosis. Our understanding of handfish health could be significantly increased if a coordinated health program was adopted by all captive breeders and aquaria, including sampling protocols, collection, and preservation in a range of fixatives of all the dying or dead handfish and adapting sublethal sampling, including tank water to assess presence of pathogens and other microorganisms. If a pathogen is detected in a handfish population, follow‐up research should identify the pathogen and effective treatments should be developed. The risk of introduction of pathogens with live food should be assessed. Other risk factors, including environmental, should be determined. Handfish health database and health management protocols should be created to summarize all information available.
AUTHOR CONTRIBUTIONS
Barbara F. Nowak: Conceptualization; investigation; writing – original draft; writing – review and editing; project administration; resources; supervision; methodology. Graeme Knowles: Investigation; writing – original draft; writing – review and editing; methodology; conceptualization. Judith Handlinger: Investigation; writing – review and editing. Rachelle Hawkins: Investigation; writing – review and editing; resources. Khattapan Jantawongsri: Investigation; writing – review and editing; visualization. Mai Dang: Investigation; visualization; writing – review and editing. Andrew Thompson: Investigation; writing – review and editing; writing – original draft. Rebecca van Gelderen: Investigation; methodology; writing – original draft; writing – review and editing; formal analysis. Nathan J. Bott: Resources; project administration; supervision; conceptualization; investigation; writing – original draft; writing – review and editing.
FUNDING INFORMATION
No external funding.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
We would like to thank Professor Ian Beveridge for help with the identification of metazoan parasites in histological sections and Jon Bryan for professional preparation of the final images.
Nowak, B. F. , Knowles, G. , Handlinger, J. , Hawkins, R. , Jantawongsri, K. , Dang, M. , Thompson, A. , van Gelderen, R. , & Bott, N. J. (2025). Submissions of diagnostic samples of two critically endangered species of handfish (Branchionichthys hirsutus and Thymichthys politus) from a public aquarium from 2018 to 2024. Journal of Fish Diseases, 48, e14024. 10.1111/jfd.14024
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Balli Garza, J. , Bott, N. J. , Hammond, M. D. , Shepherd, N. , & Nowak, B. F. (2017). Molecular characterisation of Miamiensis avidus (Ciliophora: Scuticociliata) from ranched southern bluefin tuna, Thunnus Maccoyii off Port Lincoln, South Australia. Aquaculture, 469, 44–49. 10.1016/j.aquaculture.2016.11.040 [DOI] [Google Scholar]
- Bessell, T. J. (2018). Using autonomous photo‐identification systems and otoliths to estimate age, growth and movement of the spotted handfish. Institute for Marine and Antarctic Studies (Honours Thesis). University of Tasmania.
- Blandford, M. I. , Taylor‐Brown, A. , Schlacher, T. A. , Nowak, B. , & Polkinghorne, A. (2018). Epitheliocystis in fish: An emerging aquaculture disease with a global impact. Transboundary and Emerging Diseases, 65(6), 1436–1446. 10.1111/tbed.12908 [DOI] [PubMed] [Google Scholar]
- Blazer, V. S. , Walsh, H. L. , Braham, R. P. , & Smith, C. (2018). Necropsy‐based wild fish health assessment. Journal of Visualized Experiments, 139, 57946. 10.3791/57946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce, B. D. , Green, M. A. , & Last, P. R. (1997). Developing captive husbandry techniques for spotted Handfish, (Brachionichthys hirsutus) and monitoring the 1996 spawning season. Final report to the endangered species unit, environment Australia (p. 29). CSIRO Division of Marine Research. [Google Scholar]
- Bruno, D. W. , Nowak, B. , & Elliott, D. G. (2006). Guide to the identification of fish protozoan and metazoan parasites in stained tissue sections. Diseases of Aquatic Organisms, 70(1–2), 1–36. 10.3354/dao070001 [DOI] [PubMed] [Google Scholar]
- Buller, N. B. (2014). Chapter 2 bacteriological culture techniques: Microscopy, culture and identification. In Buller N. B. (Ed.), Bacteria and fungi from fish and other aquatic animals: A practical identification manual (pp. 425–450). CABI. [Google Scholar]
- Chariton, A. A. , Stephenson, S. , Morgan, M. J. , Steven, A. D. L. , Colloff, M. J. , Court, L. N. , & Hardy, C. M. (2015). Metabarcoding of benthic eukaryote communities predicts the ecological condition of estuaries. Environmental Pollution, 203, 165–174. 10.1016/j.envpol.2015.03.047 [DOI] [PubMed] [Google Scholar]
- Cunningham, C. O. (2002). Molecular diagnosis of fish and shellfish diseases: Present status and potential use in disease control. Aquaculture, 206, 19–55. 10.1016/S0044-8486(01)00864-X [DOI] [Google Scholar]
- Di Cicco, E. , Paradis, E. , Stephen, C. , Turba, M. E. , & Rossi, G. (2013). Scuticociliatid ciliate outbreak in Australian pot‐bellied seahorse, Hippocampus abdominalis (lesson, 1827): Clinical signs, histopathologic findings and treatment with metronidazole. Journal of Zoo and Wildlife Medicine, 44(2), 435–440. http://www.jstor.org/stable/24550020 [DOI] [PubMed] [Google Scholar]
- Dyková, I. , & Lom, J. (2007). Histopathology of protistan and myxozoan infections in fishes.
- Edgar, G. J. , Stuart‐Smith, R. D. , Cooper, A. , Jacques, M. , & Valentine, J. (2017). New opportunities for conservation of handfishes (family Brachionichthyidae) and other inconspicuous and threatened marine species through citizen science. Biological Conservation, 208, 174–182. 10.1016/j.biocon.2016.07.028 [DOI] [Google Scholar]
- Elliott, D. (2020). General procedures for bacteriology In: AFS‐FHS (American Fisheries Society‐Fish Health Section). FHS blue book: suggested procedures for the detection and identification of certain finfish and shellfish pathogens, 2020 edition. Accessible at: https://virtuallearn.wpengine.com/fhs/wp‐content/uploads/sites/30/2017/08/1.1.1Gen_Proc_BacT_2014.pdf
- Floyd, R. , Abebe, E. , Papert, A. , & Blaxter, M. (2002). Molecular barcodes for soil Nematode identification. Molecular Ecology, 11, 839–850. 10.1046/j.1365-294X.2002.01485.x [DOI] [PubMed] [Google Scholar]
- Gardiner, C. H. , Fayer, R. , & Dubey, J. P. (1998). An atlas of protozoan parasites in animal tissues. Registry of Veterinary Pathology, Armed Forces Institute of Pathology. [Google Scholar]
- Gardiner, C. H. , & Poynton, S. L. (1999). An atlas of metazoan parasites in animal tissues. Registry of Veterinary Pathology, Armed Forces Institute of Pathology. [Google Scholar]
- Goodwin, A. E. , Park, E. , & Nowak, B. F. (2005). Successful treatment of largemouth bass, Micropterus salmoides (L.) with epitheliocystis hyperinfection. Journal of Fish Diseases, 28, 623–625. [DOI] [PubMed] [Google Scholar]
- Greenfield, P. (2017). Greenfield hybrid analysis pipeline (GHAP). v1. CSIRO. Software collection. 10.4225/08/59f98560eba25 [DOI]
- Hawkins, R. (2021). Aquarium industry offers hope for Tasmania's critically endangered handfish. Papers and Proceedings of the Royal Society of Tasmania, 155, 71–77. 10.26749/rstpp.155.1.71 [DOI] [Google Scholar]
- Holton, A. L. (1984). A redescription of Coitocaecum parvum Crowcroft, 1945 (Digenea: Allocreadiidae) from crustacean and fish hosts in Canterbury. New Zealand Journal of Zoology, 11, 1–8. [Google Scholar]
- Iglesias, R. , Paramá, A. , Alvarez, M. F. , Leiro, J. , Fernández, J. , & Sanmartín, M. L. (2001). Philasterides dicentrarchi (Ciliophora, Scuticociliatida) as the causative agent of scuticociliatosis in farmed turbot Scophthalmus maximus in Galicia (NW Spain). Diseases of Aquatic Organisms, 46(1), 47–55. 10.3354/dao046047 [DOI] [PubMed] [Google Scholar]
- Jackson, C. J. , Marcogliese, D. J. , & Burt, M. D. B. (1997). Role of hyperbenthic crustaceans in the transmission of marine helminth parasites. Canadian Journal of Fisheries and Aquatic Sciences, 54(4), 815–820. 10.1139/f96-329 [DOI] [Google Scholar]
- Jalenques, M. , Lair, S. , Schmidt‐Posthaus, H. , Jufer, M. , & Lamglait, B. (2021). Scuticociliate (Philasterides dicentrarchi) infection cluster in a multispecies marine aquarium system. Diseases of Aquatic Organisms, 22(144), 107–115. 10.3354/dao03580 [DOI] [PubMed] [Google Scholar]
- Jung, S. J. , Kitamura, S. I. , Song, J. Y. , Joung, I. Y. , & Oh, M. J. (2005). Complete small subunit rRNA gene sequence of the scuticociliate Miamiensis avidus pathogenic to olive flounder Paralichthys olivaceus . Diseases of Aquatic Organisms, 64, 159–162. 10.3354/Dao064159 [DOI] [PubMed] [Google Scholar]
- Kubiski, S. V. , Howerth, E. W. , Clauss, T. M. , Berliner, A. L. , & Camus, A. C. (2011). Pathology in practice. Journal of the American Veterinary Medical Association, 238(3), 301–303. 10.2460/javma.238.3.301 [DOI] [PubMed] [Google Scholar]
- Lai, C. C. , Crosbie, P. B. B. , Battaglene, S. C. , & Nowak, B. F. (2013). Effects of epitheliocystis on serum lysozyme activity and osmoregulation in cultured juvenile striped trumpeter Latris lineata (Forster). Aquaculture, 388–391, 99–104. [Google Scholar]
- Lieke, T. , Meinelt, T. , Hoseinifar, S. H. , Pan, B. , Straus, D. L. , & Steinberg, C. E. W. (2020). Sustainable aquaculture requires environmental‐friendly treatment strategies for fish diseases. Reviews in Aquaculture, 12, 943–965. [Google Scholar]
- Lynch, T. , Wong, L. , Fountain, T. , & Devine, C. (2017). Procedures and methods for establishment of captive breeding populations of spotted handfish. Report to the National Environmental Science Program (p. 62). Marine Biodiversity Hub. CSIRO. https://www.nespmarine.edu.au/document/proceduresand‐methods‐establishment‐captive‐breeding‐populationsspotted‐handfish [Google Scholar]
- Lynch, T. P. , Green, M. , Wong, L. S. C. , Bessell, T. J. , Cooper, A. , Valentine, J. , Barrett, N. , Ross, D. J. , McEnnulty, F. R. , & Foster, S. D. (2022). Assessment of conservations actions for the critically endangered spotted handfish (Brachionichthyidae), following curation of data collected by multiple investigators into a long‐term time‐series. Journal for Nature Conservation, 69, 126237. 10.1016/j.jnc.2022.126237 [DOI] [Google Scholar]
- Lynch, T. P. , Soo, L. , McEnnulty, F. , & Devine, C. (2022). Conservation of spotted handfish and their habitats—Annual report. Report to the National Environmental Science Program. CSIRO. [Google Scholar]
- Moustafa, E. M. M. , Tange, N. , Shimada, A. , & Morita, T. (2010). Experimental scuticociliatosis in Japanese flounder (Paralichthys olivaceus) infected with Miamiensis avidus: Pathological study on the possible neural routes of invasion and dissemination of the scuticociliate inside the fish body. Journal of Veterinary Medical Science, 72(12), 1557–1563. 10.1292/jvms.10-0214 [DOI] [PubMed] [Google Scholar]
- Munday, B. L. , O'Donoghue, P. J. , Watts, M. , Rough, K. , & Hawkesford, T. (1997). Fatal encephalitis due to the scuticociliate Uronema nigricans in sea‐caged, southern bluefin tuna Thunnus maccoyii . Diseases of Aquatic Organisms, 30(1), 17–25. 10.3354/dao030017 [DOI] [Google Scholar]
- Nowak, B. F. , & LaPatra, S. E. (2006). Epitheliocystis in fish. Journal of Fish Diseases, 29, 573–588. 10.1111/j.1365-2761.2006.00747.x [DOI] [PubMed] [Google Scholar]
- Ofelio, C. , Blanco, A. , Roura, Á. , Pintado, J. , Pascual, S. , & Planas, M. (2014). Isolation and molecular identification of the scuticociliate Porpostoma notata Moebius, 1888 from moribund reared Hippocampus hippocampus (L.) seahorses, by amplification of the SSU rRNA gene sequences. Journal of Fish Diseases, 37, 1061–1065. 10.1111/jfd.12207 [DOI] [PubMed] [Google Scholar]
- Paramá, A. , Iglesias, R. , Álvarez, M. F. , Sanmartín, M. L. , & Leiro, J. (2004). Chemotactic responses of the fish‐parasitic scuticociliate Philasterides dicentrarchi to blood and blood components of the turbot Scophthalmus maximus, evaluated using a new microplate multiassay. Journal of Microbiological Methods, 58(3), 361–366. 10.1016/j.mimet.2004.04.018 [DOI] [PubMed] [Google Scholar]
- Penrith, M.‐L. , Bastianello, S. S. , & Penrith, M. J. (1994). Hepatic lipoidosis and fatty infiltration of organs in a captive African stonefish, Synanceja verrucosa Bloch & Schneider. Journal of Fish Diseases, 17, 171–176. [Google Scholar]
- Peters, L. , Spatharis, S. , Augusta, D. M. , Dwyer, T. , Roca Inaki, J. T. , Kintner, A. , Kanstad‐Hanssen, Ø. , Llewellyn, M. S. , & Praebel, K. (2018). Environmental DNA: A new low‐cost monitoring tool for pathogens in salmonid aquaculture. Frontiers in Microbiology, 9, article 3009. 10.3389/fmicb.2018.03009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, C. , Balli‐Garza, J. , Evans, D. , Nowak, B. F. , Bridle, A. R. , & Bott, N. J. (2019). Detection of Miamiensis avidus (Ciliophora: Scuticociliatia) and Cardicola spp. (Trematoda: Aporocotylidae) DNA in biofouling from southern Bluefin tuna, Thunnus Maccoyii Pontoons off Port Lincoln, South Australia. Aquaculture, 502, 128–133. 10.1016/j.aquaculture.2018.12.027 [DOI] [Google Scholar]
- Rossteuscher, S. , Wenker, C. , Jermann, T. , Wahli, T. , Oldenberg, E. , & Schmidt‐Posthaus, H. (2008). Severe Scuticociliate (Philasterides dicentrarchi) infection in a population of sea dragons (Phycodurus eques and Phyllopteryx taeniolatus). Veterinary Pathology, 45(4), 546–550. 10.1354/vp.45-4-546 [DOI] [PubMed] [Google Scholar]
- Salinas, I. , Anderson, S. A. , Wright, J. , & Webb, V. L. (2012). In vivo innate immune responses of groper (Polyprion oxygeneios) against Miamiensis avidus infection and lack of protection following dietary vitamin C administration. Fish & Shellfish Immunology, 32(1), 8–15. [DOI] [PubMed] [Google Scholar]
- Salinas, I. , Maas, E. W. , & Muñoz, P. (2011). Characterization of acid phosphatases from marine scuticociliate parasites and their activation by host's factors. Parasitology, 18, 1–12. [DOI] [PubMed] [Google Scholar]
- Spisni, E. , Tugnoli, M. , Ponticelli, A. , Mordentl, T. , & Tomasi, V. (1998). Hepatic steatosis in artificially fed marine telosts. Journal of Fish Diseases, 21, 177–184. [DOI] [PubMed] [Google Scholar]
- Steinel, N. C. , & Bolnick, D. I. (2017). Pigmented macrophage aggregate as a histological indicator of immune function in fish and other poikilotherms. Frontiers in Immunology, 8(10), 3389. 10.3389/fimmu.2017.00827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stidworthy, M. F. , Garner, M. M. , Bradway, D. S. , Westfall, B. D. , Joseph, B. , Repetto, S. , Guglielmi, E. , Schmidt‐Posthaus, H. , & Thornton, S. M. (2014). Systemic scuticociliatosis (Philasterides dicentrarchi) in sharks. Veterinary Pathology, 51, 628–632. [DOI] [PubMed] [Google Scholar]
- Stride, M. C. , Polkinghorne, A. , & Nowak, B. F. (2014). Chlamydial infections of fish: Diverse pathogens and emerging causes of disease in aquaculture species. Veterinary Microbiology, 171(1–2), 258–266. 10.1016/j.vetmic.2014.03.022 [DOI] [PubMed] [Google Scholar]
- Stuart‐Smith, J. , Edgar, G. J. , Last, P. , Linardich, C. , Lynch, T. , Barrett, N. , Bessell, T. , Wong, L. , & Stuart‐Smith, R. D. (2020). Conservation challenges for the most threatened family of marine bony fishes (handfishes: Brachionichthyidae). Biological Conservation, 252, 108831. 10.1016/j.biocon.2020.108831 [DOI] [Google Scholar]
- Wolf, J. C. , Baumgartner, W. A. , Blazer, V. S. , Camus, A. C. , Engelhardt, J. A. , Fournie, J. W. , Frasca, S., Jr. , Groman, D. B. , Kent, M. L. , Khoo, L. H. , Law, J. M. , Lombardini, E. D. , Ruehl‐Fehlert, C. , Segner, H. E. , Smith, S. A. , Spitsbergen, J. M. , Weber, K. , & Wolfe, M. J. (2015). Nonlesions, misdiagnoses, missed diagnoses, and other interpretive challenges in fish histopathology studies: A guide for investigators, authors, reviewers, and readers. Toxicologic Pathology, 43(3), 297–325. 10.1177/0192623314540229 [DOI] [PubMed] [Google Scholar]
- Wolf, J. C. , & Wolfe, M. J. (2005). A brief overview of nonneoplastic hepatic toxicity in fish. Toxicologic Pathology, 33(1), 75–85. 10.1080/01926230590890187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


