Abstract
Potato (Solanum tuberosum) plants were transformed with a cDNA encoding the 59-kD subunit of the potato tuber NAD-dependent malic enzyme (NADME) in the antisense orientation. Measurements of the maximum catalytic activity of NADME in tubers revealed a range of reductions in the activity of this enzyme down to 40% of wild-type activity. There were no detrimental effects on plant growth or tuber yield. Biochemical analyses of developing tubers indicated that a reduction in NADME activity had no detectable effects on flux through the tricarboxylic acid cycle. However, there was an effect on glycolytic metabolism with significant increases in the concentration of 3-phosphoglycerate and phosphoenolpyruvate. These results suggest that alterations in the levels of intermediates toward the end of the glycolytic pathway may allow respiratory flux to continue at wild-type rates despite the reduction in NADME. There was also a statistically significant negative correlation between NADME activity and tuber starch content, with tubers containing reduced NADME having an increased starch content. The effect on plastid metabolism may result from the observed glycolytic perturbations.
It is well established that the tricarboxylic acid (TCA) cycle functions universally in plant mitochondria in the breakdown of pyruvate derived from various metabolic processes to produce ATP, reducing equivalents, and biosynthetic precursors. There are three ways in which carbon can enter the cycle. First, phosphoenolpyruvate (PEP) produced in glycolysis can be converted into pyruvate for direct entry into the mitochondria. Second, PEP can be carboxylated through the action of PEP carboxylase to oxaloacetate (OAA), which can also enter the mitochondria (Ebbighausen et al., 1985; Kromer et al., 1996). Third, OAA can be reduced in the cytosol and the malate produced can enter the mitochondria. Once in the mitochondria pyruvate enters the TCA cycle via the action of the pyruvate dehydrogenase complex, whereas malate and OAA fulfill an anaplerotic role, supplementing mitochondrial pools of C4 acids (Hill, 1997). In addition, malate can be decarboxylated in the mitochondrial matrix through the action of NAD malic enzyme (NADME; EC 1.1.1.39) to produce pyruvate (Artus and Edwards, 1985), which is oxidized by the TCA cycle.
NADME occupies a key position in mitochondrial carbon metabolism, providing a means whereby import of C4 acids can be partitioned between replenishment of mitochondrial pools and complete oxidation. As such, NADME represents a potential regulatory site for mitochondrial carbon metabolism. Although the reaction catalyzed by NADME is reversible, the equilibrium position makes it unlikely that the reverse reaction has a significant role to play in metabolism (Wedding, 1989). 14CO2 labeling experiments have provided evidence that the decarboxylation of malate by NADME occurs in vivo (Bryce and ap Rees, 1985). However, the importance of this enzyme in determining respiratory flux is not known. Attempts to measure the extent of flux through NADME in vivo suggest that this varies greatly from one plant organ to another. In maize (Zea mays) kernels (Day and Hanson, 1977), the flux through NADME is approximately 30% of that through pyruvate kinase, whereas in maize root tips only 3% of the flux to pyruvate is via NADME (Dieuaide-Noubhani et al., 1995; Edwards et al., 1998).
NADME possesses a number of regulatory properties that may play a role in controlling flux from malate to pyruvate. It is activated by fumarate and coenzyme A (CoA; Day et al., 1984; Grissom et al., 1983) and is also potentially regulated via changes in aggregation state (Grover and Wedding, 1982). The enzyme consists of two subunits (Winning et al., 1994) that in potato (Solanum tuberosum) tuber are 59 and 62 kD in mass (Willeford and Wedding, 1987). These subunits have a similar primary sequence (65% amino acid identity; Winning et al., 1994) and in vitro studies have provided evidence that both subunits are required for activity (Grover and Wedding, 1984). The enzyme can exist in a range of oligomeric forms (heterodimer, heterotetramer, and heterooctamer) each of which has distinct kinetic properties (Grover and Wedding, 1982). In potato tubers, the tetramer is the most active, having a comparatively high Vmax and low Km (malate), and the presence of this form is favored when malate concentrations are high (Grover and Wedding, 1984). The significance of activation by effectors such as fumarate and changes in oligomeric state for the regulation of respiratory metabolism in vivo is not known. However, it has been recently demonstrated that changes in oligomeric state may exert an important influence on NADME activity in C4 photosynthesis (Dever et al., 1998).
The aim of this work was to explore the importance of NADME in the control and regulation of carbon entry into the TCA cycle in potato tubers. To do this we have produced and analyzed transgenic potato plants with reduced NADME activity through the expression of a cDNA encoding the 59-kD subunit in the antisense orientation. We have examined the effect of this reduction on respiratory metabolism and on carbohydrate metabolism in general in developing potato tubers.
RESULTS
Production of Transgenic Potato Plants with Reduced NADME Activity
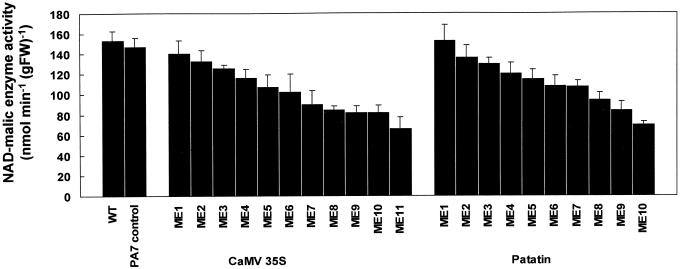
We previously have isolated cDNA clones encoding the 59- and 62-kD subunits of NADME from potato (Winning et al., 1994). To investigate the role of NADME in mitochondrial carbon metabolism, we transferred a cDNA encoding the 59-kD subunit in the antisense orientation into potato plants with the aim of specifically reducing NADME activity. The expression of the antisense RNA was under the control of either the CaMV 35S promoter for constitutive expression (Pietrzak and Hohn, 1986), or the class I patatin promoter (B33) for tuber-specific expression (Rocha-Sosa et al., 1989). For each construct approximately 15 independent transgenic lines were regenerated. Lines containing the antisense construct were confirmed by DNA gel-blot analysis (data not shown) and transferred to the greenhouse for the production of tubers. The activity of NADME in tuber extracts of these lines was determined and the results are shown in Figure 1. A range of reductions in NADME activity was observed with both constructs. In both cases, the maximum reduction was found to be to around 40% of the wild-type activity with tubers from the majority of lines displaying between 50% and 75% of wild-type activity. Multiple, independent harvests demonstrated that a reduction in NADME activity had no significant effects on plant morphology, tuber fresh weight per plant, or tuber number per plant (data not shown).
Figure 1.
Activity of NADME in tuber extracts from wild-type and transgenic lines. Activity of NADME was measured in tuber extracts from wild-type Desirée (WT), a vector-only transformed line (PA7 control), and a range of independent transgenic lines containing a cDNA encoding the 59-kD subunit of NADME in the antisense orientation, under the control of either the CaMV 35S or the potato tuber patatin promoter. Values are mean ± se (n = 3).
The following lines, representing a range of NADME activities, were selected for further analysis: the CaMV 35S promoter (35S-ME1, 35S-ME6, 35S-ME7, 35S-ME10, 35S-and ME11) and the B33 patatin promoter (PAT-ME1, PAT-ME5, PAT-ME8, PAT-ME9, and PAT-ME10).
A Reduction in NADME Activity Does Not Result in Changes in the Maximum Catalytic Activity of Other Enzymes Involved in Carbohydrate and Respiratory Metabolism
To test whether the reduction in NADME activity was the only change in the transgenic lines we measured a range of enzyme activities in tuber extracts. Only values for lines with the lowest NADME activity are presented (Table I). We included enzymes from the TCA cycle, glycolysis, and starch synthesis. There were no significant differences between the activities in the transgenic lines and the wild type except for reductions in phosphofructokinase (ATP; line PAT-ME9) and phosphoglyceromutase (lines PAT-ME9 and PAT-ME10). However, these differences were not replicated in other harvests.
Table I.
Effect of reduced NADME activity on other enzyme activities in potato tubers
| Enzyme | Wild Type | 35S-ME11 | 35S-ME10 | PAT-ME9 | PAT-ME10 |
|---|---|---|---|---|---|
| nmol min−1 g−1 fresh wt | |||||
| Citrate synthase | 75 ± 5 | 69 ± 3 | 73 ± 2 | 80 ± 3 | 72 ± 4 |
| Isocitrate dehydrogenase | 26 ± 4 | 19 ± 2 | 22 ± 2 | 24 ± 5 | 31 ± 3 |
| Malate dehydrogenasea | 28 ± 3 | 28 ± 2 | 29 ± 3 | 30 ± 2 | 26 ± 1 |
| Phosphofructokinase (ATP) | 47 ± 1 | 52 ± 4 | 46 ± 1 | 35 ± 1b | 82 ± 21 |
| Phosphofructokinase (pyrophosphate) | 136 ± 32 | 143 ± 28 | 135 ± 21 | 160 ± 76 | 138 ± 9 |
| Phosphoglyceromutase | 2,058 ± 327 | 1,106 ± 393 | 1,414 ± 357 | 699 ± 52b | 792 ± 62b |
| Phosphoglycerate kinase | 456 ± 172 | 579 ± 91 | 603 ± 114 | 723 ± 205 | 823 ± 50 |
| Enolase | 111 ± 15 | 109 ± 35 | 126 ± 27 | 134 ± 27 | 106 ± 32 |
| PEP carboxylase | 75 ± 12 | 55 ± 15 | 78 ± 14 | 107 ± 47 | 46 ± 5 |
| Pyruvate kinase | 755 ± 40 | 719 ± 30 | 665 ± 56 | 712 ± 32 | 1,061 ± 76 |
| ADP-Glc pyrophosphorylase | 104 ± 19 | 98 ± 22 | 87 ± 17 | 92 ± 20 | nd |
| Total starch synthase | 126 ± 6 | 124 ± 3 | 116 ± 5 | 117 ± 15 | nd |
| Alkaline pyrophosphatase | 604 ± 156 | 442 ± 237 | 663 ± 210 | 587 ± 79 | nd |
| Starch phosphorylase | 212 ± 27 | 271 ± 24 | 234 ± 44 | 246 ± 28 | nd |
| Total amylolytic activity | 184 ± 41 | 102 ± 21 | 124 ± 50 | 127 ± 58 | nd |
The activity of the indicated enzymes was measured in tuber extracts from wild type and far transgenic lines. All values are from a single harvest, which is representative of at least one other harvest for each enzyme. Values are mean ± se (n = 3–5 tubers). nd, Not determined.
Malate dehydrogenase activities are expressed in units of μmol min−1 g−1 fresh wt.
Significantly different from wild type (P < 0.05; Student's t test).
A Reduction in NADME Activity Has No Discernible Effects on Mitochondrial Function
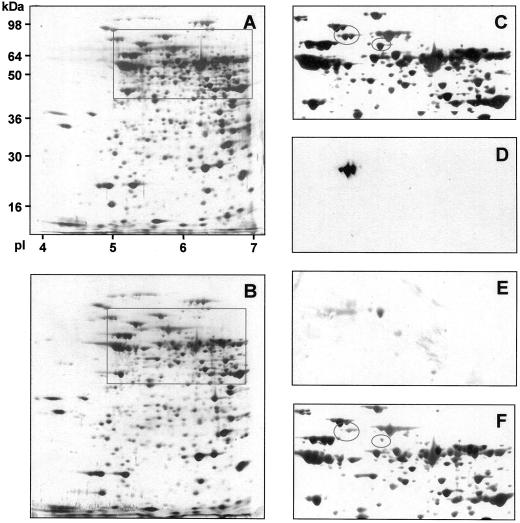
We further investigated the consequences of reduced NADME activity on the enzyme activities and protein composition of isolated mitochondria from tubers of wild type, 35S-ME10, and 35S-ME11 (Table II). Enzyme activities were corrected for mitochondrial yield by comparing the cytochrome c oxidase activity in the mitochondrial preparations with the whole cell extracts from the same tuber material and are expressed as grams per fresh weight. With the exception of NADME, no significant differences were found in the activity of any of the enzymes investigated. The percentage reduction in NADME activity observed in 35S-ME10 and 35S-ME11, relative to the wild type, was similar in isolated mitochondria (Table II) and whole tuber extracts (Fig. 1), which suggests that the residual activity is mitochondrial. To further characterize mitochondria in the transgenic lines we examined total mitochondrial protein composition by two-dimensional isoelectric focusing-SDS-PAGE (Fig. 2). Using this approach, we were able to resolve 379 separate spots in mitochondrial fractions from tubers of both wild type and 35S-ME11 (Fig. 2, A and B). Of these 379 spots, only nine have an intensity in the transgenic line (normalized to the total staining intensity of the gel) that is less than 40% that of the wild type; these are listed in Table III. Three of these nine spots react with antibodies raised against the two subunits of NADME (as confirmed by western blotting; Fig. 2, D and E), so that antisense reduction in NADME activity leads to only six other changes of any magnitude in the mitochondrial protein composition. It is interesting to note that antisense expression of a cDNA encoding the 59-kD subunit of NADME leads to a reduction in the content of both NADME subunits.
Table II.
Effect of reduced NADME activity on other mitochondrial enzyme activities in potato tubers
| Enzyme | Wild Type | 35S-ME10 | 35S-ME11 |
|---|---|---|---|
| nmol min−1 g−1 fresh wt | |||
| NADME | 1,629 ± 214 | 600 ± 60a | 746 ± 211a |
| Citrate synthase | 188 ± 17 | 159 ± 7 | 201 ± 16 |
| Isocitrate dehydrogenase | 228 ± 21 | 179 ± 73 | 163 ± 26 |
| Aconitase | 153 ± 23 | 120 ± 20 | 132 ± 33 |
| Fumarase | 253 ± 51 | 196 ± 52 | 172 ± 51 |
| Malate dehydrogenaseb | 30 ± 5 | 37 ± 14 | 37 ± 10 |
| Pyruvate dehydrogenase | 83 ± 5 | 65 ± 16 | 60 ± 14 |
Mitochondria were isolated from 8-week-old tubers. The enzyme activity measurements were corrected for mitochondrial yield by comparing the cytochrome oxidase activity in mitochondrial extracts and whole tuber extracts from the same tuber material. Values are mean ± se (n = 3).
Indicates significantly different from the wild type (P < 0.05).
Malate dehydrogenase activities are expressed in units of μmol min−1 g−1 fresh wt.
Figure 2.
The effect of reduced NADME activity on mitochondrial protein composition. Total mitochondrial protein from tubers of wild-type and line 35S-ME11 was isolated and separated by two-dimensional SDS PAGE, and stained with silver. The complete gels for wild type and 35S-ME11 are shown in A and B, respectively. The boxes indicate the regions containing NADME subunits that are enlarged in C and F. The gel shown in A was transferred onto nitrocellulose and the resulting western blot probed with antibodies specific to the 59- or 62-kD subunits of NADME. Enlarged regions of these blots are shown in D (62-kD subunit) and E (59-kD subunit). The ovals in C and F indicate the position of the NADME subunits.
Table III.
Variation in mitochondrial protein abundance as a result of reduced activity of NADME
| Molecular Wt | Isoelectric Point | Notes |
|---|---|---|
| kDa | pl | |
| 110 | 5.2 | – |
| 62 | 5.50 | 62-kDa subunit of NADME |
| 62 | 5.55 | 62-kDa subunit of NADME |
| 60 | 6.0 | – |
| 59 | 5.7 | 59-kDa subunit of NADME |
| 38 | 5.6 | – |
| 35 | 6.6 | – |
| 34 | 6.6 | – |
| 31 | 5.0 | – |
The two-dimensional SDS-PAGE patterns shown in Figure 2, A and B, were analyzed using the Phoretix software package. Spot intensity was normalized relative to the total intensity of the gel. Spots are listed whose intensity in line 35S-ME11 is less than 40% of that in the wild type.
To investigate the effect of reduced NADME activity on the rate of respiration we measured the rate of CO2 production by intact, recently harvested, developing tubers using an infrared gas analyzer. This approach gives an estimate of the rate of pyruvate entry into the TCA cycle, although the values obtained are also influenced by the rate of CO2 fixation (via PEP carboxylase) and CO2 release from the oxidative pentose phosphate pathway. There was no statistically significant effect of reducing NADME activity on the rate of CO2 production by tubers (data not shown).
To investigate further any effect on respiration we measured the contents of metabolites involved in or derived from respiration in the transgenic tubers from two independent harvests. The values obtained for malate and citrate contents of tubers were comparable with those previously reported for developing potato tubers (Trethewey at al., 1998). No consistent significant differences were seen between the levels of citrate and malate in wild-type tubers and selected lines with reduced NADME activity (data not shown). HPLC analysis of basic fractions obtained from tuber extracts by ion-exchange chromatography provided data for 17 amino acids (data not shown). Although there were relatively minor differences between some of the transgenic lines and the wild type with respect to Lys, Pro, and Tyr, these differences were not displayed in all of the lines and their magnitude was not correlated with the extent of reduction of NADME activity.
Flux through the TCA cycle is coupled to the rate of electron transport and thus oxidative phosphorylation. Any major alteration in flux as a result of decreased NADME activity therefore may be reflected in the nucleotide levels of the transgenic tubers. Measurements of ATP, ADP, and ATP/ADP ratios revealed no significant differences between wild-type and transgenic tubers (data not shown).
Tubers with Reduced NADME Activity Have Increased Levels of the Glycolytic Intermediates 3-Phosphoglyceric Acid (3PGA) and PEP
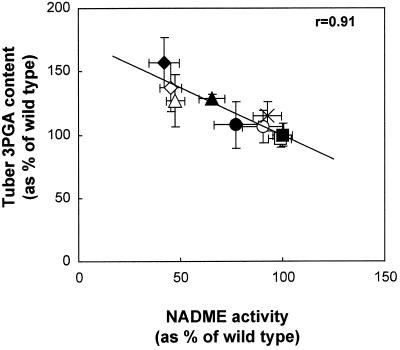
To investigate any effects of decreased NADME activity on carbohydrate oxidation, we measured the contents of various glycolytic intermediates in tubers from six independent batches of plants. The relationship between NADME activity and the content of the glycolytic metabolite 3PGA is shown in Figure 3. There is a statistically significant correlation between these parameters (P < 0.05). To pool the data from different harvests we expressed the 3PGA and PEP content data for each sample as a percentage of the mean wild-type content for the harvest from which the sample originated. We then used these normalized data to calculate mean 3PGA and PEP contents for each line for all six harvests (Table IV). This method allows us to compare the metabolite contents across the whole series of experiments, taking into account the variation in the wild-type values. In lines with reduced NADME activity there was a small but statistically significant increase in the content of 3PGA and PEP (Table IV).
Figure 3.
Relationship between 3PGA content and NADME activity in developing tubers from greenhouse-grown plants. NADME activity and 3PGA content were measured in tubers from a single harvest. The means of these parameters are plotted against each other for wild type and each transgenic line. ▪, Wild type; □, PAT-ME1; ×, 35S-ME1; ○, PAT-ME8; ●, PAT-ME10; ▴, PAT-ME9; ▵, 35S-ME11; ⋄, 35S-ME7; ♦, 35S-ME10. The line was fitted by linear regression and the significance of the relationship was analyzed using a 5% two-tailed significance test on the correlation coefficient.
Table IV.
The effect of reduced NADME activity on the 3PGA and PEP content of developing potato tubers
| Line | NADME Activity | 3PGA Content | PEP Content | Fisher's P Value (t Test, 3PGA Content versus Wild Type) | Fisher's P Value (t Test, PEP Content versus Wild Type) |
|---|---|---|---|---|---|
| % | |||||
| Wild type (n = 23) | 100 ± 2 | 100 ± 3 | 100 ± 4 | – | – |
| 35S-ME6 (n = 3) | 94 ± 9 | 96 ± 7 | 77 ± 7 | NS | <0.001 |
| 35S-ME7 (n = 13) | 57 ± 4 | 109 ± 16 | 111 ± 17 | <0.05 | <0.05 |
| 35S-ME10 (n = 14) | 61 ± 4 | 115 ± 7 | 117 ± 13 | <0.05 | <0.001 |
| 35S-ME11 (n = 12) | 51 ± 3 | 113 ± 6 | 112 ± 10 | <0.05 | <0.01 |
| PAT-ME1 (n = 11) | 96 ± 6 | 92 ± 5 | 78 ± 7 | NS | <0.001 |
| PAT-ME5 (n = 9) | 85 ± 3 | 117 ± 8 | 104 ± 7 | <0.05 | NS |
| PAT-ME8 (n = 9) | 89 ± 6 | 106 ± 10 | 109 ± 17 | NS | NS |
| PAT-ME9 (n = 21) | 77 ± 4 | 96 ± 12 | 92 ± 11 | NS | NS |
| PAT-ME10 (n = 9) | 71 ± 10 | 110 ± 12 | 90 ± 20 | <0.05 | NS |
For each harvest, tuber 3PGA and PEP content were expressed as a percentage of the mean value for the wild-type tubers in that harvest. The normalized value for each tuber from all of the harvests was then combined to give an overall mean (±se of the mean) for each line. A Student's t test was used to compare the starch content of each line (averaged across all six harvests) with that of the wild type; the Fisher's P values are shown. NS, Not significant (P > 0.05).
Measurements of the Glc 6-phosphate contents of tubers revealed no significant differences between wild-type and transgenic lines (data not shown).
Tubers with Reduced NADME Activity Have an Increased Starch Content
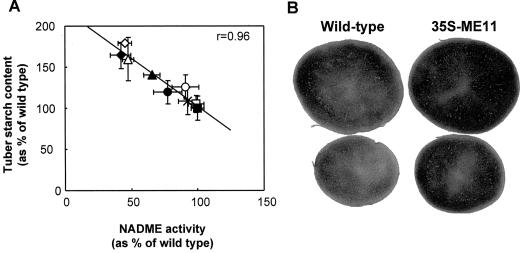
We found a statistically significant correlation between the NADME activity and tuber starch content (Fig. 4A), with tubers containing low NADME having an increased starch content (Fig. 4, A and B). Significant negative correlations between NADME activity and tuber starch content were observed in four out of six independent harvests (data not shown). In these harvests, tubers with the lowest NADME activity had a 20% to 45% increase in starch compared with wild-type tubers. To investigate this effect further, we measured the starch content in tubers from all six harvests, and combined the data as described above for 3PGA and PEP. In lines with reduced NADME activity there was a small, statistically significant increase in starch content (Table V).
Figure 4.
Relationship between starch content and NADME activity in developing tubers from greenhouse-grown plants. A, NADME activity and starch content were measured in tubers from a single harvest. The means of these parameters are plotted against each other for wild type and each transgenic line. ▪, Wild type; □, PAT-ME1; ×, 35S-ME1; ○, PAT-ME8; ●, PAT-ME10; ▴, PAT-ME9; ▵, 35S-ME11; ⋄, 35S-ME7; ♦, 35S-ME10. The line was fitted by linear regression and the significance of the relationship was analyzed using a 5% two-tailed significance test on the correlation coefficient. B, Tuber slices from freshly harvested developing tubers of wild type and line 35S-ME11 were stained with iodine solution to compare starch contents.
Table V.
The effect of reduced NADME activity on the starch content of developing potato tubers
| Line | NADME Activity | Starch Content | Fisher's P Value (t Test, Starch Content, versus Wild Type) |
|---|---|---|---|
| % | |||
| Wild type (n = 23) | 100 ± 2 | 100 ± 2 | – |
| 35S-ME6 (n = 4) | 94 ± 9 | 93 ± 9 | NS |
| PAT-ME1 (n = 13) | 96 ± 6 | 101 ± 6 | NS |
| PAT-ME8 (n = 11) | 89 ± 6 | 105 ± 6 | NS |
| 35S-ME1 (n = 3) | 87 ± 2 | 100 ± 2 | NS |
| PAT-ME5 (n = 11) | 85 ± 3 | 103 ± 3 | NS |
| PAT-ME9 (n = 20) | 77 ± 4 | 112 ± 4 | <0.01 |
| PAT-ME10 (n = 12) | 71 ± 4 | 109 ± 4 | <0.05 |
| 35S-ME10 (n = 15) | 61 ± 4 | 110 ± 4 | <0.05 |
| 35S-ME7 (n = 15) | 57 ± 4 | 110 ± 4 | <0.05 |
| 35S-ME11 (n = 16) | 51 ± 3 | 120 ± 3 | <0.001 |
For each harvest, NADME activity and tuber starch content were expressed as a percentage of the mean value for the wild-type tubers in that harvest. The normalized value for each tuber from all of the harvests was then combined to give an overall mean (±se of the mean) for each line. A Student's t test was used to compare the starch content of each line (averaged across all six harvests) with that of the wild type; the Fisher's P values are shown. NS, Not significant (P > 0.05).
We investigated the ADP-Glc content in tubers from two harvests. There was no significant difference in the content of ADP-Glc between the lines in either harvest (data not shown). Furthermore, there was no significant correlation between the concentration of ADPG and the activity of NADME or the starch content for either harvest (r values: ADPG content versus NADME activity 0.039 and −0.041 for harvest 1 and harvest 2, respectively; ADPG content versus starch content −0.267 and −0.056 for harvest 1 and harvest 2, respectively).
DISCUSSION
We have demonstrated that a reduction in NADME to 40% of wild-type levels has no detectable effect on respiratory metabolism in developing potato tubers. Our data show that there are no overall changes in the levels of malate, citrate, or adenylates in the transgenic tubers. The mitochondrial pools of these compounds account for a small percentage of total cellular pools (Stitt et al., 1989; ap Rees and Hill, 1994) so these individual analyses are a relatively crude representation of TCA cycle function. However, when considered together with the measurements of CO2 production by tubers and free amino acid contents, these results provide strong evidence that a significant decrease in NADME activity does not substantially alter flux through the TCA cycle in tubers. Although two-dimensional analysis of mitochondrial proteins revealed changes in abundance of six proteins other than NADME, our biochemical analyses of mitochondrial function indicate that there are no major pleiotropic effects resulting from these alterations.
The negligible impact of reduced NADME activity on respiratory metabolism may be explained in two ways. First, it is feasible that the residual NADME activity is sufficient to support respiratory flux under the growth conditions employed. The reduction in NADME may be compensated for by activation of the residual enzyme, for example, by changes in oligomeric state.
A second hypothesis is that other metabolic processes compensate for the reduction in pyruvate derived from malate via NADME. Pyruvate can also be supplied from PEP via the pyruvate kinase reaction in the cytosol. It is possible that an alteration in the partitioning of PEP between pyruvate kinase and PEP carboxylase allowed respiration to continue at wild-type rates. We detected no changes in the maximum catalytic activities of key glycolytic enzymes implying that any changes in metabolite partitioning must be brought about by mechanisms involving fine control.
Although NADME does not play a role in the control of TCA cycle flux under the growth conditions employed, it remains possible that the enzyme may be important in flux control during specific developmental stages or under specific stress conditions. It is plausible that plants with reduced NADME may have insufficient capacity to compensate for this reduction when they are subjected to conditions requiring high respiratory flux (Day and Hanson, 1977; Hill et al., 1994). For example, NADME may have an important role in bypassing the pyruvate kinase reaction during phosphate stress (Duff et al., 1989). An impact of reduced NADME on flux through the TCA cycle thus might be observed in phosphate-stressed plants even though there is no observable effect in plants grown under non-stressed conditions.
In lines with reduced NADME activity there was a small but statistically significant increase in starch content. In tubers with the lowest NADME activity this represented an increase of as much as 45% when compared with wild-type starch content. We found no significant change in the content of G6P. Because G1P, the direct precursor for of the committed pathway of starch synthesis in potato, is likely to be in equilibrium with G6P in tuber tissue (Merlo et al., 1993), this suggests that the increased starch content is not due to an increase in substrate concentration in the transgenic lines. There were no changes in the maximum catalytic activities of various enzymes involved in starch synthesis and degradation. We did, however, find an increase in the levels of 3PGA in lines with low NADME activity. 3PGA is a potent allosteric activator of AGPase (Sowokinos and Preiss, 1982), which plays an important role in the control of flux to starch in tubers (Geigenberger et al., 1999; Sweetlove et al., 1999). It has been demonstrated that changes in the concentration of cytosolic 3PGA can lead to the activation of AGPase in vivo in potato tubers (Geigenberger et al., 1997). We suggest, therefore, that increased 3PGA content in the tubers with reduced NADME activity may have stimulated AGPase, resulting in an increased rate of starch synthesis.
We found that not all tubers displaying decreased NADME activity and increased starch content have an increased 3PGA content. This does not, however, rule out the 3PGA stimulation of AGPase as an explanation of the increased starch content in tubers. First, it is possible that the increases in 3PGA were too small to be detected as statistically significant above the background biological variation in 3PGA content. Second, it is the ratio of 3PGA:Pi, rather than the absolute level of 3PGA within the plastid, which is important in regulating AGPase (Sowokinos, 1981). If small changes in 3PGA are matched by reciprocal changes in Pi, the impact upon AGPase activity can be substantial. Third, our measurements reflect only the total cellular levels of 3PGA. If an increase in 3PGA in one subcellular compartment is matched by a decrease in another, these changes will not be reflected in measurements of total 3PGA. Our measurements also reflect only the amount of 3PGA at the time of harvest, whereas starch is laid down over a long developmental period. Differences between wild-type and transgenic plants in 3PGA content may be more substantial at earlier stages of tuber development.
It remains possible that 3PGA activation of AGPase is not the sole mechanism responsible for the increased starch content of tubers with reduced NADME activity. There is no significant difference in the content of ADPG between the transgenic lines. Furthermore, there is no significant correlation between the concentration of ADPG and the activity of NADME or the starch content for either harvest. This suggests that, in addition to the postulated activation of AGPase by an increase in the concentration of 3PGA in some lines, there is a second mechanism activating starch synthesis in response to a reduction in NADME activity. This second mechanism may involve reactions downstream of AGPase, such as the transfer of the glucosyl unit from ADPG to starch, and may either be a direct consequence of the reduced NADME activity, independent of AGPase activation, or a secondary effect of AGPase activation. Current work is focusing on the elucidation of this second mechanism.
Despite the universal presence of NADME in higher plants and its position in mitochondrial metabolism, this work demonstrates that under defined conditions plants are able to cope with substantial reductions in the activity of this enzyme without any detrimental effects on growth and development. This is not entirely unexpected when it is considered that NADME is not the sole source of pyruvate in plant cells. A surprising discovery was that tubers with reduced NADME activity have an increased starch content. The mechanism for this increase has not been elucidated but this work highlights the complex interactions that exist between different metabolic pathways and suggests novel approaches for manipulation of starch synthesis in potato tubers.
MATERIALS AND METHODS
Materials
Virus-free potato (Solanum tuberosum L. cv Desirée) stocks were obtained from the Department of Agriculture and Fisheries for Scotland (East Craigs, Edinburgh). Chemicals were obtained either from Sigma (Poole, Dorset, UK) or BDH (Poole, Dorset, UK). Enzymes and cofactors were from Boehringer Mannheim (Lewes, Sussex, UK). Plants were grown from seed tubers in 150-mm-diameter pots in potting compost. Growth was in a greenhouse at 25°C, with supplementary lighting to give a 16-h-light/8-h-dark photoperiod.
Plasmid Construction and Plant Transformation
Modified versions of pBin19 (Bevan, 1984) containing the octopine synthase terminator together with either the CaMV 35S promoter (pBinAR) or the type I patatin promoter (pBinB33) were kindly supplied by Prof. Lothar Willmitzer (Max Planck Institute for Molecular Plant Physiology, Golm, Germany). pBinAR was constructed using the 35S promoter, derived as a 529-bp EcoRI/KpnI-fragment (nucleotides 6,909–7,437) from the plasmid pDH51 (Pietrzak and Hohn, 1986), and cloned into pUC18 giving rise to pUC18-35S. The 192-bp octopine synthase terminator was excised from pAGV40 (Herrera-Estrella et al., 1983) with HindIII and PvuII and cloned, after addition of SphI linkers to the PvuII site, into the SphI and HindII sites of pUC18-35S. Of the resulting plasmid the entire polylinker was excised with EcoRI and HindIII and cloned into pBin19 (Bevan, 1984). For pBinB33, the patatin promoter was cloned as a DraI Fragment (nucleotides −1,512–+14; Rocha-Sosa et al., 1989) into the blunted SstI site of pUC19. This was then cloned as an EcoRI/SmaI fragment into the correspondingly cut pBinAR. The cDNA encoding the 59-kD subunit of NADME (Winning et al., 1994) was cut out from pBluescript using the SalI site that is 91 bp from the 5′ end of the cDNA and the SalI site in the polylinker and the SalI fragment cloned into the SalI site of either pBinAR or pBinB33. Plasmids containing the cDNA in the antisense orientation were identified by digestion with restriction endonuclease and transformed into Agrobacterium tumefaciens (LBA4404) using the method of Höfgen and Willmitzer (1988). Potato cv Desirée stem explants were transformed using the method of Twell and Ooms (1987), with the modifications of Sheerman and Bevan (1988).
Preparation of Tuber Extracts for Measurement of Enzyme Activities
Developing tuber material was harvested and frozen immediately in liquid N2. Frozen tuber tissue (500–1,000 mg) was ground to a fine powder under liquid N2 and added, together with 200 mg insoluble polyvinylpyrrolidone, to 3 volumes of extraction buffer (see below). After centrifugation at 10,000g for 10 min the supernatant was desalted by passage through a Sephadex G-25 column and used immediately for the determination of enzyme activity. The extraction buffer for NADME, malate dehydrogenase, phosphoglycerate mutase, phosphoglycerate kinase, enolase, PEP carboxylase, and pyruvate kinase consisted of 25 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-NaOH (pH 6.9), 1 mm MgSO4, 0.5 mm EDTA, 1 mm dithiothreitol (DTT), 5% (v/v) glycerol, 0.25% (w/v) bovine serum albumin (BSA), 0.25% (w/v) polyvinylpyrrolidone-40, and 0.01% (v/v) Triton X-100. The extraction buffer for NAD isocitrate dehydrogenase was as for NADME, except that Triton X-100 was omitted, the glycerol concentration was increased to 25% (v/v), and 100 mm sodium citrate was included. The extraction buffer for citrate synthase, ATP-dependent phosphofructokinase, PPi-dependent phosphofructokinase, alkaline pyrophosphatase, and starch phosphorylase consisted of 30 mm HEPES-NaOH (pH 7.3), 10 mm DTT, 1 mm MgSO4, 0.5 mm EGTA, 0.5% (w/v) BSA, 0.5% (w/v) polyvinylpyrrolidone-40, and 10 mm Cys. The extraction buffer for ADP-Glc pyrophosphorylase, starch synthase, and total amylolytic activity was as for citrate synthase except that the pH was 6.9 and the Cys was omitted. The extraction buffer for cytochrome c oxidase consisted of 300 mm mannitol, 50 mm TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid]-KOH (pH 7.5), 1% (w/v) BSA, 20 mm ascorbate, and 0.05% (v/v) Triton X-100.
Measurement of Enzyme Activities
All assays were carried out at 25°C. The reaction conditions for each assay were optimized for pH, the amount of extract, the concentration of all components, and linearity with time. We confirmed that there were no losses during extraction by performing recovery experiments or, where necessary, tissue recombination experiments. NADME was assayed using a modification of the method of ap Rees et al. (1983). NADME activity was measured as the rate of pyruvate production from malate. The assay was carried out in the following mixture: 25 mm HEPES-NaOH (pH 6.9), 4 mm NAD, 50 μm CoA, and 10 mm MgSO4. The reaction was started by the addition of 20 mm malate, and stopped after a fixed time period by boiling. After centrifugation at 10,000g for 30 s, the pyruvate content of an aliquot of the supernatant was determined in the following mixture: 125 mm HEPES-NaOH (pH 7.5) and 0.7 mm NADH. Pyruvate was measured as the change in A340 following the addition of 10 units of lactate dehydrogenase. Citrate synthase was assayed using a modification of the method of Stitt et al. (1989). The reaction mixture contained 82 mm triethanolamine buffer (pH 7.9), 2.8 mm malate, 0.2 mm acetylpyridine adenine nucleotide, 0.05 (v/v) Triton X-100, and 12 units of malate dehydrogenase. The mixture was allowed to come to equilibrium before addition of extract followed by 0.17 mm acetyl CoA. The rate of absorbance change at 365 nm was monitored. Isocitrate dehydrogenase was assayed using a modification of the method of Cox and Davies (1969). The rate of change in A340 was monitored in the following mixture: 40 mm Tris-HCl (pH 7.6), 1.5 mm NAD, 6.3 mm MnCl2, and 0.05% (v/v) Triton X-100. The reaction was started by the addition of 15 mm isocitrate.
Malate dehydrogenase activity was assayed by monitoring the rate of change in A340 in the following mixture: 50 mm TES-NaOH (pH 7.2), 5 mm MgCl2, 0.2 mm NAD, and 0.05% (v/v) Triton X-100. The reaction was started by the addition of 1 mm OAA. Aconitase was assayed using a modification of the method of MacDougall and ap Rees (1991). The rate of change in A340 was monitored in the following mixture: 80 mm HEPES-NaOH (pH 7.5), 0.5 mm NADP, 0.42 mm MnCl2, 0.2 units NADP-isocitrate dehydrogenase, and 0.05% (v/v) Triton X-100. The reaction was started by the addition of 8 mm aconitate. Fumarase was assayed using a modification of the method of MacDougall and ap Rees (1991). The rate of absorbance change at 240 nm was monitored in the following mixture: 70 mm KH2PO4-NaOH (pH 7.7) and 0.05% (v/v) Triton X-100. The reaction was started by the addition of 50 mm fumarate. Pyruvate dehydrogenase complex was assayed according to Millar et al. (1998). Cytochrome c oxidase activity was assayed using a modification of the method of Neuberger et al. (1982). The assay was carried out in the following mixture: 300 mm mannitol, 10 mm TES-KOH (pH 7.5,) 3 mm MgSO4, 10 mm NaCl, 5 mm KH2PO4, and 0.1% (w/v) BSA. The following reagents were added sequentially to the final concentration stated: 5 mm ascorbate, 0.04 mm cytochrome c, 0.05% (v/v) Triton X-100, and 0.4 mm KCN. ATP-phosphofructokinase and PPi-phosphofructokinase were assayed according to Hajirezaei and Stitt (1991). Phosphoglyceromutase, phosphoglycerate kinase, enolase, and pyruvate kinase were assayed according to Burrell et al. (1994). PEP carboxylase was assayed according to Merlo et al. (1993). ADP-Glc pyrophosphorylase, soluble starch synthase, alkaline pyrophosphatase, and starch phosphorylase were assayed according to Sweetlove et al. (1996a). Total amylolytic activity was assayed according to Sweetlove et al. (1996b).
Isolation of Mitochondria from Potato Tubers
Unless otherwise stated, all isolation procedures were carried out at 4°C. Single tubers (5–20 g) were freshly harvested and homogenized using a mortar and pestle in 10 mL of prechilled grinding buffer consisting of 0.3 m mannitol, 50 mm TES-NaOH (pH 7.5), 0.5% (w/v) BSA, 0.5% (w/v) polyvinylpyrrolidone-40, 2 mm EGTA, and 20 mm Cys. The resulting suspension was filtered through four layers of muslin to give an unfractionated homogenate that was centrifuged for 1 min at 1,000g. The supernatant was layered onto a Percoll step gradient consisting of 50%, 28%, and 20% (v/v) Percoll in wash buffer (0.3 m mannitol, 10 mm TES-NaOH [pH 7.5], and 0.1% [w/v] BSA). Subsequent centrifugation at 39,200g for 45 min yielded a mitochondrial fraction at the interface between 28% and 50% (v/v) Percoll. This mitochondrial fraction was removed and diluted to approximately 40 mL with wash buffer. Subsequent centrifugation for 15 min at 31,000g yielded a mitochondrial pellet that was resuspended in a further 40 mL of wash buffer and the centrifugation repeated. The final pellet was resuspended in 200 μL wash buffer, frozen in liquid nitrogen, and stored at −80°C until use.
Separation of Mitochondrial Proteins by Two-Dimensional SDS-PAGE
Aliquots of mitochondria (250 μg protein) isolated from wild-type and 35S-ME11 tubers were resuspended in 200 μL of water in 1.5-mL capped tubes and placed on ice. Acetone (800 μL), cooled to −80°C, was added and the solution vortexed for 20 s. After storage at −20°C for 3 h, samples were centrifuged at 20,000g for 15 min and the supernatant discarded. Proteinaceous pellets were dissolved in 330 μL of isoelectric focusing sample buffer containing 6 m urea, 2 m thiourea, 2% (w/v) CHAPS {3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid}, 0.3% (w/v) DTT, 0.5% (v/v) Pharmacia immobilized pH gradient buffer 4–7L, and 0.0001% (w/v) Bromphenol blue. Each sample was then imbibed along the total length of a Pharmacia 4–7 pH 110-mm IPG dry strip in a reswelling tray for 15 h at 25°C. Isoelectric focusing was carried out using a MultiPhor II flat-bed gel apparatus according to the manufacturers instructions. SDS-PAGE second dimension separation was carried out under denaturing, reducing conditions on 0.1% (w/v) SDS and 12% (w/v) polyacrylamide gels according to Laemmli (1970). For staining, gels were fixed in 10% (w/v) trichloroacetic acid and 50% (v/v) methanol and then silver stained according to a standard oxidizing protocol using formaldehyde for image development. For immunoblotting, proteins from two-dimensional gels were transferred onto nitrocellulose membranes and blocked in 5% (w/v) casein. Membranes were incubated in phosphate-buffered saline (120 mm NaCl, 3 mm Na2HPO4, and 1 mm KH2PO4) with 0.1% (v/v) Tween 20 and supplemented with 1:5,000 dilutions of polyclonal antibodies raised to the á or â subunit of NADME from potato (Winning et al., 1994). Chemiluminescence was used for detection of horseradish peroxidase-conjugated secondary anti-rabbit antibodies.
Measurement of Tuber Starch Content
Tuber starch content was determined exactly as described by Sweetlove et al. (1996b).
Measurement of Free Amino Acid Content of Tuber Tissue
Frozen tuber tissue was extracted three times in boiling 80% (v/v) ethanol. The resulting ethanol soluble fractions were pooled, evaporated to dryness under vacuum, and resuspended in a known volume of water. The equivalent to 120 mg of tissue was loaded onto a cationic exchange column and the basic components were eluted (Quick et al., 1989). The basic fraction was freeze dried and then resuspended in 100 μL of water. An aliquot of extract was mixed with an equal volume of internal standard of Nor-Leu (200 pmol μL−1). Samples were derivatized with phenylisothiocyanate prior to analysis. Free amino acid contents were determined by measuring A254 following separation by HPLC (PTC C-18 column, 2.1 × 220 mm; Perkin Elmer Applied Biosystems, Warrington, UK). Mobile phases were phase A, 150 mm sodium acetate (pH 5.5); and phase B, 70% (v/v) acetonitrile-water. The gradient employed involved equilibration with 7% (phase A) followed by a linear gradient to 32% (phase A) over 10 min. The percentage (phase A) was then increased to 62% over 10 min and then further increased to 100% over 5 min. The flow rate was maintained at 300 μL min−1.
Measurement of Metabolite Contents of Tuber Tissue
Frozen tuber tissue was extracted in trichloroacetic acid and diethyl ether according to Weiner et al. (1987). The amounts of 3PGA and PEP in extracts were determined using the method of Burrell et al. (1994). Glc 6-phosphate was assayed according to the method of Michal (1988). Malate was measured according to the method of Stitt et al. (1989). Citrate content was determined using a commercial kit from Boehringer Mannheim UK Ltd. according to the manufacturer's instructions. To check for losses of metabolites during extraction and analysis, small representative amounts of each metabolite were added to replicate samples of tissue prior to extraction and the recovery determined. Recoveries were in excess of 90% of that added.
Measurement of ADP-Glc and Nucleotide Contents of Tuber Tissue
Frozen tuber tissue was extracted in trichloroacetic acid and diethyl ether according to Weiner et al. (1987). The amounts of ADP-Glc, ADP, and ATP were determined by measuring A254 after separation from other nucleotides and nucleotide sugars by HPLC with a Partisil-SAX anion-exchange column. ADP-Glc, ADP, and ATP were identified and quantified by cochromatography with authentic standards. For HPLC, mobile phases were: phase A, 40 mm KH2PO4 (pH 3.0); and phase B, 200 mm KH2PO4 and 750 mm KCl (pH 2.8). After injection of 100 μL onto the column ADP-Glc was eluted using a flow rate of 1 mL min−1 with 100% (phase A) for 2 min, followed by a linear gradient to 65% (phase A) and 35% (phase B) for 18 min. Under these conditions ADP-Glc eluted as a single peak after 12.8 min, ADP after 25.7 min, and ATP after 41.2 min. To check for losses of these compounds during extraction and analysis, small representative amounts of ADP-Glc, ADP, and ATP were added to replicate samples of tissue prior to extraction and the recovery determined. Recovery for all three compounds was in excess of 85% of that added.
Measurement of CO2 Production by Tubers
Freshly harvested tubers from individual plants were placed in a darkened air-tight chamber at 25οC. CO2-free air was passed through the chamber at 200 mL min−1 and the CO2 concentration of the out-flowing air was determined using an infrared gas analyzer.
ACKNOWLEDGMENTS
We thank Mrs. Gülsen Akgun and Mr. Tony Willis for excellent technical assistance. Thanks to Dr. Lee Sweetlove for many helpful discussions, to Dr. Alisdair Fernie for help in establishing the HPLC method for adenylate measurements described in this paper, and to Dr. Sam Zeeman and Prof. Alison Smith for critical reading of the manuscript.
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council of the UK (Biochemistry of Metabolic Regulation in Plants grant to C.J.L. and studentship to H.L.J.), by the Human Frontiers Science Programme (fellowship to A.H.M.), and by Zeneca Agrochemicals (grant to C.J.L. and S.A.H.).
LITERATURE CITED
- ap Rees T, Bryce JH, Wilson PM, Green JH. Role and location of NAD malic enzyme in thermogenic tissues of Araceae. Arch Biochem Biophys. 1983;227:511–521. doi: 10.1016/0003-9861(83)90480-0. [DOI] [PubMed] [Google Scholar]
- ap Rees T, Hill SA. Metabolic control analysis of plant metabolism. Plant Cell Environ. 1994;17:587–599. [Google Scholar]
- Artus N, Edwards G. NAD-malic enzyme from plants. FEBS Lett. 1985;182:225–233. [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce JH, ap Rees T. Rapid decarboxylation of the products of dark fixation of CO2 in roots of Pisum and Plantago. Phytochemistry. 1985;24:1635–1638. [Google Scholar]
- Burrell MM, Mooney PJ, Blundy M, Carter D, Wilson F, Green J, Blundy KS, ap Rees T. Genetic manipulation of 6-phosphofructokinase in potato tubers. Planta. 1994;194:5–101. [Google Scholar]
- Cox GF, Davies DD. The effects of pH and citrate on the activity of nicotinamide adenine dinucleotide-specific isocitrate dehydrogenase from pea mitochondria. Biochem J. 1969;113:813–820. doi: 10.1042/bj1130813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Hanson JB. Pyruvate and malate transport and oxidation in corn mitochondria. Plant Physiol. 1977;59:630–635. doi: 10.1104/pp.59.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Neuberger M, Douce R. Activation of NAD-linked malic enzyme in intact mitochondria by exogenous CoA. Arch Biochem Biophys. 1984;231:233–234. doi: 10.1016/0003-9861(84)90383-7. [DOI] [PubMed] [Google Scholar]
- Dever LV, Pearson M, Ireland RJ, Leegood RC, Lea PJ. The isolation and characterization of the C4 plant Amaranthus edulis deficient in NAD malic enzyme activity. Planta. 1998;206:649–656. [Google Scholar]
- Dieuaide-Noubhani M, Raffard G, Canioni P, Pradet A, Raymond P. Quantification of compartmented metabolic fluxes in maize root tips using isotope distribution from 13C- or 14C-labeled glucose. J Biol Chem. 1995;270:13147–13159. doi: 10.1074/jbc.270.22.13147. [DOI] [PubMed] [Google Scholar]
- Duff SM, Moorhead GB, Lefebvre DD, Plaxton WC. Phosphate starvation inducible “bypasses” of adenylate and phosphate-dependent glycolytic enzymes in Brassica nigra suspension cells. Plant Physiol. 1989;90:1275–1278. doi: 10.1104/pp.90.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbighausen H, Chen J, Heldt HW. Oxaloacetate translocator in plant mitochondria. Biochim Biophys Acta. 1985;810:184–199. [Google Scholar]
- Edwards S, Nguyen BT, Do B, Roberts JKM. Contribution of malic enzyme, pyruvate kinase, phosphoenolpyruvate carboxylase and the Krebs cycle to respiration and biosynthesis and to intracellular pH regulation during hypoxia, in maize root tips observed by nuclear magnetic resonance imaging and gas chromatography-mass spectrometry. Plant Physiol. 1998;116:1073–1081. doi: 10.1104/pp.116.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Müller-Röber B, Stitt M. Contribution of adenosine 5 '-diphosphoglucose pyrophosphorylase to the control of starch synthesis is decreased by water stress in growing potato tubers. Planta. 1999;209(3):338–345. doi: 10.1007/s004250050641. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Reimholz R, Geiger M, Merlo L, Canale V, Stitt M. Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta. 1997;201:502–518. [Google Scholar]
- Grissom CB, Canellas PF, Wedding RT. Allosteric regulation of the NAD malic enzyme from cauliflower: activation by fumarate and coenzyme A. Arch Biochem Biophys. 1983;220:133–144. doi: 10.1016/0003-9861(83)90394-6. [DOI] [PubMed] [Google Scholar]
- Grover SD, Wedding RT. Kinetic ramifications of the association-dissociation behavior of NAD-malic enzyme. Plant Physiol. 1982;70:1169–1172. doi: 10.1104/pp.70.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover SD, Wedding RT. Modulation of the activity of NAD-malic enzyme from Solanum tuberosum by changes in oligomeric state. Arch Biochem Biophys. 1984;234:418–425. doi: 10.1016/0003-9861(84)90288-1. [DOI] [PubMed] [Google Scholar]
- Hajirezaei M, Stitt M. Contrasting roles for pyrophosphate:fructose 6-phosphate phosphotransferase during aging of tissue slices from potato tubers and carrot storage tissues. Plant Sci. 1991;77:177–183. [Google Scholar]
- Herrera-Estrella L, Depicker A, Van Montagu M, Schell J. Expression of chimeric genes transferred into plant cells using a Ti-plasmid vector. Nature. 1983;303:209–213. [PubMed] [Google Scholar]
- Hill SA. Carbon metabolism in mitochondria. In: Dennis DT, Turpin DH, Lefebvre DD, Layzell DB, editors. Plant Metabolism. London: Addison Welsey Longman; 1997. pp. 181–199. [Google Scholar]
- Hill SA, Bryce JH, Leaver CJ. Pyruvate metabolism in mitochondria from cucumber cotyledons during early seedling development. J Exp Bot. 1994;45:1489–1491. [Google Scholar]
- Höfgen R, Willmitzer L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 1988;16:9877. doi: 10.1093/nar/16.20.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer S, Gardestrom P, Samuelsson G. Regulation of the supply of cytosolic oxaloacetate for mitochondrial metabolism via phosphoenolpyruvate carboxylase in barley leaf protoplasts: I. The effect of covalent modification on PEPC activity, pH response and kinetic properties. Biochim Biophys Acta. 1996;1289:343–350. doi: 10.1016/0304-4165(95)00164-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacDougall AJ, ap Rees T. Control of the Krebs cycle in Arum spadix. J Plant Physiol. 1991;137:683–690. [Google Scholar]
- Merlo L, Geigenberger P, Hajirezaei M, Stitt M. Changes in carbohydrates, metabolites and enzyme activities in potato tubers during development and within a single tuber along the stolon-apex gradient. J Plant Physiol. 1993;142:392–402. [Google Scholar]
- Michal G. D-glucose-1-phosphate. In: Bergemeyer HE, editor. Methods in Enzymatic Analysis 6. Weinheim, Germany: Verlag Chemie; 1988. pp. 185–197. [Google Scholar]
- Millar AH, Knorrp C, Leaver CJ, Hill SA. Plant mitochondrial pyruvate dehydrogenase complex:purification and identification of catalytic components of potato. Biochem J. 1998;334:571–576. doi: 10.1042/bj3340571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M, Journet EP, Bligny R, Carde JP, Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of percoll. Arch Biochem Biophys. 1982;217:312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Pietrzak M, Hohn T. The pol coding region of the cauliflower mosaic-virus is expressed as a part of large polyprotein-precursor homology to retroviruses. Experientia. 1986;42(6):691–691. [Google Scholar]
- Quick P, Neuhaus E, Feil R, Stitt M. Fluoride leads to an increase of inorganic pyrophosphate and an inhibition of photosynthetic sucrose synthesis in spinach leaves. Biochim Biophys Acta. 1989;973:263–271. [Google Scholar]
- Rocha-Sosa M, Sonnewald U, Frommer W, Stratmann M, Schell J, Willmitzer L. Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J. 1989;8:23–29. doi: 10.1002/j.1460-2075.1989.tb03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheerman S, Bevan MW. A rapid transformation method for Solanum tuberosum using binary Agrobacterium tumefaciens vectors. Plant Cell Rep. 1988;7:13–16. doi: 10.1007/BF00272967. [DOI] [PubMed] [Google Scholar]
- Sowokinos JR. Pyrophosphorylases in Solanum tuberosum. II:Catalytic properties and regulation of ADP-glucose and UDP-glucose pyrophosphorylase activities in potatoes. Plant Physiol. 1981;68:924–929. doi: 10.1104/pp.68.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos JR, Preiss J. Pyrophosphorylases in Solanum tuberosum: III. Purification, physical and catalytic properties of ADPglucose pyrophosphorylase in potatoes. Plant Physiol. 1982;69:1459–1466. doi: 10.1104/pp.69.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Lilley RM, Gerhardt R, Heldt HW. Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol. 1989;174:518–552. [Google Scholar]
- Sweetlove LJ, Burrell MM, ap Rees T. Characterization of transgenic potato (Solanum tuberosum) tubers with increased ADP glucose pyrophosphorylase. Biochem J. 1996a;320:487–492. doi: 10.1042/bj3200487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove LJ, Burrell MM, ap Rees T. Starch metabolism in tubers of transgenic potato (Solanum tuberosum) tubers with increased ADP glucose pyrophosphorylase. Biochem J. 1996b;320:493–498. doi: 10.1042/bj3200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove LJ, Müller-Röber B, Willmitzer L, Hill SA. The contribution of adenosine 5′-diphosphoglucose pyrophosphorylase to the control of starch synthesis in potato tubers. Planta. 1999;209:330–337. doi: 10.1007/s004250050640. [DOI] [PubMed] [Google Scholar]
- Trethewey R, Geigenberger P, Riedel K, Hajirezaei M, Sonnewald U, Stitt M, Riesmeier J, Willmitzer L. Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and the stimulation of glycolysis. Plant J. 1998;15:109–118. doi: 10.1046/j.1365-313x.1998.00190.x. [DOI] [PubMed] [Google Scholar]
- Twell D, Ooms G. The 5′ flanking DNA of a patatin gene directs tuber specific expression of a chimeric gene in potato. Plant Mol Biol. 1987;9:345–375. doi: 10.1007/BF00014911. [DOI] [PubMed] [Google Scholar]
- Wedding RT. Malic enzymes of higher plants. Plant Physiol. 1989;90:367–371. doi: 10.1104/pp.90.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H, Stitt M, Heldt HW. Compartmentation of pyrophosphate and alkaline pyrophosphatase in leaves. Biochim Biophys Acta. 1987;893:13–21. [Google Scholar]
- Willeford KO, Wedding RT. Evidence for a multiple subunit composition of plant NAD-malic enzyme. J Biol Chem. 1987;262:8423–8429. [PubMed] [Google Scholar]
- Winning BM, Bourguignon J, Leaver CJ. Plant mitochondrial NAD-dependent malic enzyme. J Biol Chem. 1994;269:4780–4786. [PubMed] [Google Scholar]