Abstract
Purpose
In March 2020, a 1-week ultrahypofractionated adjuvant breast radiation therapy schedule, 26 Gy in 5 fractions, and telehealth were adopted to reduce the risk of COVID-19 for staff and patients. This study describes real-world 1-year late toxicity for ultrahypofractionation (including a sequential boost) and patient perspectives on this new schedule and telehealth workflows.
Methods and Materials
Consecutive patients were enrolled between March and August 2020. Patient-reported outcome measures, including the presence of breast pain, swelling, firmness, and others, were recorded using the European Organisation for research and treatment of cancer quality of life questionairre (EORTC QLQ) BR45 at baseline, 3 months, 6 months, and 1 year. Virtual teleconferencing without video was used. Patients were invited to use video at 1 year for a physician-based assessment, including breast inspection. Patient-reported experience measures were also collected at 1 year to capture how a shortened schedule and telehealth influenced patient experience.
Results
In total, 121 of 135 patients completed at least 2 assessments, of which 33 (25%) received a sequential boost. The majority of patients reported no toxicity or mild toxicity at all 3 time points: 76% at 3 months, 76% at 6 months, and 82% at 1 year. When comparing 26 Gy in 5 fractions alone versus 26 Gy in 5 fractions followed by a sequential boost, there was no difference in toxicity reported at 1 year. A total of 94% felt supported by the medical team throughout their treatment course using telehealth-only consultations. Only 27% actually agreed to video consultation for the purpose of breast inspection when offered.
Conclusions
Ultrahypofractionated breast radiation therapy leads to acceptable late toxicity at 1 year, even when followed by a hypofractionated tumor bed boost. Patient satisfaction with ultrahypofractionated treatment and virtual consultations without video was high. Further investigation concerning the patient's acceptance of video consultations for a physician-based assessment, including breast inspection, is warranted.
Highlights
-
•
26 Gy in 5 fractions (+/−sequential boost) leads to no or mild 1-year late toxicity for the majority.
-
•
26 Gy in 5 fractions (+/−sequential boost) leads to moderate 1-year late toxicity for around 1/6 patients.
-
•
26 Gy in 5 fractions (+/−sequential boost) rarely leads to marked 1-year late toxicity (1%-2%).
-
•
Virtual consultations without video calls are acceptable for > 90% of patients with breast cancer.
-
•
Less than 1/3 of patients may consent to video calls for the purpose of breast inspection.
Introduction
Adjuvant breast radiation therapy (RT) using a moderately hypofractionated schedule is used to reduce the risk of local recurrence and improve overall survival.1 In March 2020, in response to the COVID-19 pandemic, both national and international guidelines supported the adoption of an ultrahypofractionated 1-week RT regimen for patients with node-negative breast cancer.2,3 The Fast Forward phase 3 trial demonstrated that a 1-week schedule of 26 Gy in 5 fractions was noninferior to standard moderately hypofractionated treatment, confirming equivalent breast cancer outcomes and normal tissue toxicity for patients with node-negative breast cancer at 5 years.4 In this trial, 25% of the patients received a sequential boost, which was conventionally fractionated.4 Further data regarding the late toxicity of ultrahypofractionated RT to the whole breast, including a sequential boost, may lead to more widespread acceptance and implementation of shorter treatment schedules.5 When assessing toxicity, the Fast Forward trialists reported both patient-reported outcome measures (PROMS) and physician-based assessments. Collecting both types of toxicity data is beneficial, as studies in breast cancer have reported differences in PROMS when compared with physician-based assessments.6 Physician-based assessments are seen as more objective; however, disease-specific PROMS are a better and more effective tool being used to drive improvement in services, inform commissioning, and promote choice.7
Another change in practice implemented around this time was the integration of telehealth workflows, encompassing virtual consultations using video and nonvideo approaches. Data on the effect of telehealth on patient experiences were lacking,5 but the goal of these transitions was to minimize hospital visits for patients, thereby reducing the potential risk of infection during the pandemic.3 In the context of a short 1- to 2-week treatment schedule and the integration of virtual consultations, there arises the possibility of diminished patient interactions with the radiation oncology team, potentially influencing the overall treatment experience. While virtual consultations, encompassing both video and nonvideo formats, have become increasingly prevalent, a more comprehensive exploration of oncology patients’ perspectives is warranted. Employing patient-reported experience measures (PREMs) serves as a valuable means to capture patients’ insights regarding their care experiences, offering insights into the quality of care received.8
This study aimed to report real-world 1-year late toxicity after ultrahypofractionated RT, including a sequential boost and patient perspectives on this new schedule and telehealth workflows.
Methods and Materials
Patient population
A 1-week adjuvant breast RT schedule was implemented across 3 RT centers in March 2020.9 Suitable patients included those receiving adjuvant RT to the breast or chest wall.10 A comprehensive follow-up schedule was offered to all patients who commenced treatment between March and August 2020. The reporting of the results presented was approved by the Institutional Research Ethics Committee. All patients provided written informed consent for ultrahypofractionated RT, and follow-up was offered to all patients but was optional.
RT treating planning
All patients underwent a noncontrast computed tomography simulation in a supine position. Deep inspiration breath hold was considered for all patients less than 60 years old undergoing left-sided treatment or when the plan on a free-breathing scan did not meet organ-at-risk constraints. The heart and lungs were contoured as mandatory organs at risk. Plans were generated using opposed tangents with segmental fields used to minimize volume receiving >105% (V105%). The dose was prescribed according to ICRU 62 (International Commission on radiation units). Patients received a boost at the treating physician's discretion. Institutional guidelines considered a boost for patients <40 years or with a positive margin or patients aged 40 to 59 years with high-grade disease, lymphovascular invastion, or extensive intraductal component. For patients receiving a boost, the boost clinical target volume (CTV) consisted of clips and seroma with a 5 mm expansion, cropped from the chest wall and skin, with a 5 mm planning target volume (PTV) margin. A photon plan with either 3-dimensional conformal fields or mini tangents was generated, aiming to achieve coverage of PTV V95% > 95%.
Virtual consultations
Virtual teleconferencing without video was used at baseline, 3 months, 6 months, and 1 year. Eight physicians, including consultant and trainee radiation oncologists, were trained to carry out these consultations. The 3- and 6-month consultations were carried out using telephone calls, for which the patients were sent an appointment in advance. Patients were invited to use video teleconferencing at the 1-year assessment to facilitate a physician-based assessment. Initially, face-to-face consultations were planned for the 1-year assessment, but in 2021, COVID-19 restrictions were still in place, which prohibited face-to-face physical examination for this group; therefore, a decision was made to offer video conferencing. After patients consented to video consultation, the T-PRO clinic manager (T-PRO, version 1.18.7) was used to send a text message containing a direct link to initiate the video call without the need for application installation or downloads.11 The system was compatible with any mobile device equipped with video capabilities.
Toxicity assessment
PROMS were recorded at 3 months, 6 months, and 1 year following treatment completion. Patients who completed at least 2 of the 3 scheduled assessments were included in the analysis. The presence of breast pain, swelling, firmness, hypersensitivity and skin changes, arm or shoulder pain, restricted arm movement, and arm swelling were recorded using the EORTC QLQ BR45 (Appendix E1). Patient assessments used a 4-point scale (not at all, a little, quite a bit, and very much), which equates to none, mild, moderate, and marked toxicity (Appendix E1). Analysis was carried out on all patients who completed the 1 year and at least 1 other assessment.
Physician-assessed outcomes were recorded at 1 year for breast distortion, shrinkage, telangiectasia, edema, or discomfort using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.03 toxicity tool (Appendix E1). Breast induration was not recorded, as it was not possible to assess via a video system. Rates of ipsilateral recurrence, rib fracture, symptomatic lung fibrosis, and ischemic heart disease at 1 year were recorded.
Patient-reported experience measures
In order to gauge patients’ experiences, a questionnaire comprising inquiries pertaining to their comprehension, experience with, and advantages of ultrahypofractionated RT was devised, and patients were invited to engage in this survey during their 1-year consultation (Appendix E2). These questions were formulated by drawing from PREMs used in oncological patient care within various health care systems, such as the Netherlands, Germany, and the UK.12, 13, 14 The Likert scale was employed to capture patients’ attitudes, opinions, or perceptions, with the goal of evaluating how the treatment process influenced their overall experience.
Statistical analysis
The primary endpoint of the worst toxicity experienced by a patient was analyzed at each time point: 3 months, 6 months, and 1 year. Logistic regression was performed to assess the association between the grade of worst toxicity experienced at 1 year and the following dosimetric parameters: breast PTV V95%, breast PTV DMax, breast PTV V105% in cm3 body PTV V105% in cm3, breast PTV volume cm3, boost PTV Eval volume cm3, and boost PTV Dmax. Data were analyzed using SPSS software version 29 (IBM, SPSS Inc).
Results
Patient population
Between March 2020 and August 2020, 135 patients were included (Table 1). A previous publication presents acute toxicity for the same set of patients.9 The initial study focused on PROMS of acute toxicity using CTCAE v5 Scoring Criteria up to 1-month post-RT. The present study focuses on the PROMS of 3-month, 6-month, and 1-year toxicity using EORTC QLQ BR45, physician-based assessed toxicities at 1 year, and the results of a PREMs questionnaire. All patients completed the RT course prescribed. All of the RT plans met the predetermined planning objectives.8 Only 6% of patients were aged <50 years, 61% were aged 50 to 69 years, and 33% were aged >70 years. The majority of patients (97%) had T1 to T2 primary lesions, and 74% had grade 1 to 2 lesions. Five patients (4%) underwent a mastectomy. The majority of patients (93%) received adjuvant hormone therapy, and 18% of patients received chemotherapy in either the neo-adjuvant or adjuvant setting. Thirty-three patients (25%) underwent a sequential photon boost with 4 different dose fractionation schedules used: 10.68 Gy in 4 fractions (25/33), 12 Gy in 4 fractions (1/33), 13.35 Gy in 5 fractions (2/33), and 16 Gy in 8 fractions (5/33).
Table 1.
Patient, tumor, and treatment variables for patients who received 26 Gy in 5 fractions ± boost between March and August 2020
| Variable | No. patients | (%) | |||
|---|---|---|---|---|---|
| (a) | Patient | ||||
| Age, y at diagnosis | |||||
| <40 | 1 | 1 | |||
| 40-49 | 7 | 5 | |||
| 50-59 | 33 | 24 | |||
| 60-69 | 50 | 37 | |||
| 70-79 | 39 | 29 | |||
| 80+ | 5 | 4 | |||
| (b) | Tumor | ||||
| Breast cancer laterality | |||||
| Left | 64 | 47 | |||
| Right | 71 | 53 | |||
| Histology type | |||||
| Ductal | 105 | 78 | |||
| Lobular | 11 | 8 | |||
| Mixed | 8 | 6 | |||
| Other | 11 | 8 | |||
| Tumor stage | |||||
| Tis | 2 | 2 | |||
| T1 | 91 | 67 | |||
| T2 | 41 | 30 | |||
| T3 | 0 | 0 | |||
| T4 | 1 | 1 | |||
| No. of positive nodes | |||||
| pN0 | 117 | 87 | |||
| pN1 (mi) | 12 | 9 | |||
| pN1a | 5 | 3 | |||
| pN2 | 1 | 1 | |||
| Estrogen receptor status | |||||
| Positive | 126 | 93 | |||
| Negative | 9 | 7 | |||
| Her2 receptor status | |||||
| Positive | 12 | 9 | |||
| Negative | 123 | 91 | |||
| Tumor grade | |||||
| Grade 1 | 27 | 20 | |||
| Grade 2 | 73 | 54 | |||
| Grade 3 | 35 | 26 | |||
| Lymphovascular invasion | |||||
| Absent | 97 | 72 | |||
| Present | 38 | 28 | |||
| (c) | Treatment | ||||
| Type of surgery | |||||
| Mastectomy | 5 | 4 | |||
| Breast-conserving | 130 | 96 | |||
| Axillary staging | |||||
| Sentinel node biopsy | 133 | 98 | |||
| Axillary clearance | 1 | 1 | |||
| No axillary surgery | 1 | 1 | |||
| RT | |||||
| Whole breast only | 97 | 72 | |||
| Whole breast + sequential boost | |||||
| 10.68 Gy/4 f | 25 | 19 | |||
| 12 Gy/4 f | 1 | 1 | |||
| 13.35 Gy/5 f | 2 | 2 | |||
| 16 Gy/8 f | 5 | 3 | |||
| Chest wall | 5 | 3 | |||
| Deep inspiration breath hold | |||||
| Yes | 21 | 16 | |||
| No | 114 | 84 | |||
| All women | 135 | 100 | |||
Abbreviations: f = fraction; RT = radiation therapy.
PROMS
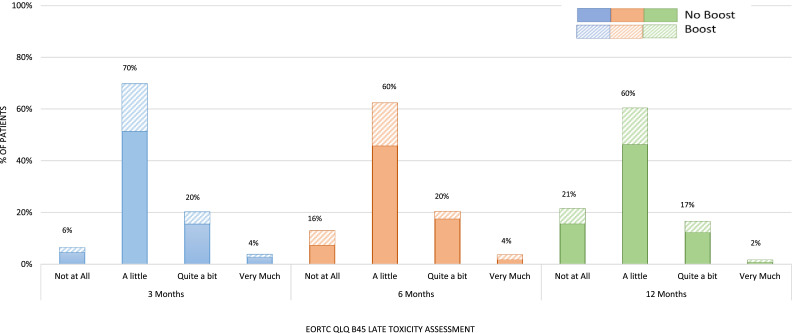
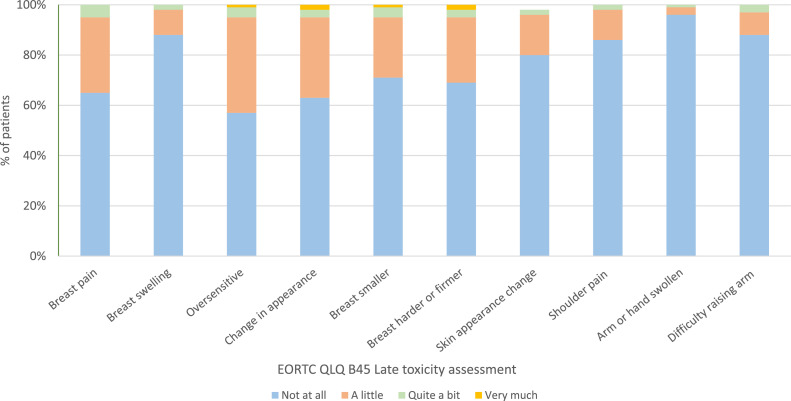
In total, 90% (121/135) of patients completed the 1-year assessment plus at least 1 other assessment. No toxicity or mild toxicity was reported by 76%, 76%, and 81% at 3, 6, and 12 months, respectively (Fig. 1). Moderate or marked toxicity was reported by 24% of participants at 3 and 6 months, and 19% reported an ongoing moderate or marked toxicity at 1 year. Seventeen percent (20/121) of patients reported moderate toxicity at 1 year, the most common being breast pain (Fig. 2). Other moderate toxicities reported included oversensitivity, smaller breasts, and harder breasts. Two patients (2%) reported marked toxicity at 1 year, both of whom reported multiple marked toxicities, including the appearance of breast firmness and skin changes.
Figure 1.
Worst patient-reported outcome measures for 121 patients following 26 Gy in 5 fractions to the whole breast (± sequential boost). Abbreviations; EORTC QLQ Br45 = European Organisation for research and treatment of cancer quality of life questionairre breast cancer 45
Figure 2.
Patient-reported outcome measures at 1 year for 121 patients following 26 Gy in 5 fractions to the whole breast (± sequential boost). Abbreviations; EORTC QLQ Br45 = European Organisation for research and treatment of cancer quality of life questionairre breast cancer 45
Thirty patients received a sequential boost following 26 Gy in 5 fractions and completed at least two follow-up assessments. Eighty percent (24/30) of these patients reported mild or no toxicity at 1 year, 17% (5/30) reported moderate toxicity, and 3% (1/30) reported marked toxicity. When comparing 26 Gy in 5 fractions alone versus 26 Gy in 5 fractions followed by a sequential boost, there was no difference in toxicity. Toxicity reported at 1 year was none: 20.1% (19/91) versus 23.3% (7/30), mild 61.5% (56/91) versus 56.7% (17/30), moderate 16.5% (15/91) versus 16.7% (5/30), and marked 1.1% (1/91) versus 3.3% (1/30).
In our investigation of potential predictors of toxicity at the 1-year time point using logistic regression, we examined various independent variables, such as breast PTV V95%, breast PTV Dmax, breast PTV V105% in cc, body PTV V105% in cc, breast PTV volume cc, boost PTV Eval volume cc, and boost PTV Dmax. None of these variables exhibited a significant association with toxicity, as outlined in Table 2. In the previously reported paper describing acute toxicity, similarly, no statistically significant difference between the distribution of these same individual dosimetric parameters and acute grade 2 CTCAE v5 toxicity was found.
Table 2.
Logistic regression analysis on the association of T stage, chemotherapy received, and dosimetric parameters with toxicity reported at 1 year using EORTC QLQ BR45 for 121 patients following 26 Gy in 5 fractions treated
| 95% CI for odds ratio |
||||
|---|---|---|---|---|
| Variable | P value | Odds ratio | Lower | Upper |
| Breast PTV V95% | .522 | 1.083 | 0.849 | 1.382 |
| Breast PTV Dmax (%) | .404 | 1.323 | 0.685 | 2.557 |
| Breast PTV V105% (cm3) | .645 | 1.233 | 0.507 | 2.998 |
| Body V105% (cm3) | .644 | 0.814 | 0.340 | 1.946 |
| Breast PTV volume (cm3) | .553 | 1.000 | 0.999 | 1.001 |
| Boost PTV Eval volume (cm3) | .588 | 1.348 | 0.458 | 3.968 |
| Adjuvant chemotherapy | .799 | 0.812 | 0.163 | 4.051 |
| T stage | .364 | 0.571 | 0.171 | 1.912 |
Abbreviations: cm3 = centimeters cubed; Dmax = maximum dose; PTV = planning target volume; PTV Eval = evaluation PTV (PTV cropped from air and 5 mm from skin surface); V% = volume receiving X% of dose. EORTC QLQ Br45 = European Organisation for research and treatment of cancer quality of life questionairre breast cancer 45
Physician-reported assessment at 1 year
A cyber-attack at our institution15 delayed the 1-year assessment for some patients, but all were assessed within 18 months of completing treatment. Video consultation at 1 year was offered to all patients, but only 33 of 121 patients (27%) consented. Among these 33 patients at 1-year follow-up, no toxicity was recorded for 67% (22/33) of patients; mild toxicities were recorded for 24% (8/33) of patients, and moderate or marked toxicities were recorded across 9% (3/33) of patients. Compared with PROMS at 1 year, physician-reported assessment downgraded 45% (15/33) of patients, 6% (2/33) of patients had upgraded toxicity, and 49% (16/33) of patients were unchanged.
There was 1 reported ipsilateral recurrence at 1 year. There was 1 reported rib fracture and 2 reported ischaemic heard disease events, although only 1 of these patients actually received left-sided RT. This patient was aged 79, with underlying risk factors; her RT plan met optimal constraints, with a heart V5% of 14.5%.
Patient-reported experience measures
In total, 112 patients completed the patient-reported experience measures. Satisfaction with the use of telehealth was high. The majority of patients felt well-informed about their diagnosis, with 37.5% agreeing and 59.8% strongly agreeing (Table 3). The majority, 87.9%, felt well informed about side effects (27.7% agreed and 60.7% strongly agreed). Despite the exclusive use of telehealth for consultations, 94% (agreed or strongly agreed) stated that they felt well-supported by the medical team. Almost 50% of patients agreed or strongly agreed that they were open to virtual breast inspection with video. However, 6.25% strongly disagreed, and 28.6% disagreed with the idea of virtual breast inspection with video, and as described above, only 27% actually agreed to video consultation for the purposes of breast examination when offered. As part of this questionnaire, patients were asked about the perceived potential advantages of the 1-week schedule. Two main benefits were identified: 88 of 112 patients (78.6%) reported reduced overall treatment time as a benefit, and 66 of 112 (58.9%) reported infection control as a benefit.
Table 3.
Patient-reported experience measures for 112 patients who received ultrahypofractionated breast radiation therapy and were reviewed using telehealth workflows between March and August 2020
| Strongly disagree (%) | Disagree (%) | Neither agree nor disagree (%) | Agree (%) | Strongly agree (%) | Do not know (%) | ||
|---|---|---|---|---|---|---|---|
| Felt well-informed about the diagnosis | 0 | 1.8 | 0.9 | 37.5 | 59.8 | 0 | |
| Felt well-informed about the 1 wk schedule | 0.9 | 4.5 | 3.6 | 33.9 | 56.2 | 0.9 | |
| Felt well-informed about the side effects | 1.8 | 3.6 | 5.3 | 27.7 | 60.7 | 0.9 | |
| Felt well-supported by medical teams | 0.9 | 3.6 | 1.8 | 21.4 | 72.3 | 0 | |
| Open to virtual breast inspection using video | 6.2 | 28.6 | 12.5 | 30.3 | 18.8 | 3.6 | |
| 1 wk RT schedule had a positive impact | 0.9 | 0 | 14.3 | 33.9 | 47.3 | 3.6 | |
Abbreviations: RT = radiation therapy.
Discussion
This report demonstrates the safety and tolerability at 1 year of ultrahypofractionated RT in our population. The majority of patients reported no toxicity or mild toxicity at all 3 time points. Ultrahypofractionation, followed by a boost, led to marked toxicity in <2% of our population. Furthermore, this data highlights the satisfaction of breast cancer patients with this shortened schedule and with the use of telehealth virtual consultations. Ninety-four percent felt supported by the medical team throughout their treatment course using telehealth-only consultations. Almost half of patients (49%) reported they would have been open to video consultations, but only 27% actually agreed to video consultations for the purpose of breast inspection when offered.
This report demonstrated moderate toxicity in 17% (20/121) for any symptom at 1 year. The most common moderate toxicity reported was breast pain in 6 patients. A number of other randomized and cohort studies have reported patient-reported late toxicities following ultrahypofractionated whole breast RT, including patients who received a boost. The Fast Forward trialists report a 5-year no or mild toxicity rate of 78% and a moderate or marked toxicity rate of 12% for any symptom, the most common being breast shrinkage.4 Twenty-five percent of patients in this trial had a conventionally fractionated boost, but toxicity was not published for comparison of boost versus no boost. Also of note, only a small number of patients in both this study and the Fast Forward trial received postmastectomy chest wall ultrahypofractionated RT, so it is difficult to draw conclusions for this particular population. Laughlin et al16 reported no change in moderate toxicity rates in a randomized trial of 25 Gy in 5 fractions compared with 40 Gy in 15 fractions in 107 patients. Ninety-one percent of patients in the ultrahypofractionated RT arm reported excellent or good cosmesis at 3 months, and a simultaneous integrated boost was delivered for 43% of patients (30 Gy in 5 fractions), with no difference in toxicity. Montero et al17 reported 6-month late toxicities for 383 patients who underwent ultrahypofractionation of 26 Gy in 5 fractions with no grade 3 toxicity. Seventy-one percent of patients received a simultaneous integrated boost of 29 Gy in 5 fractions, but a statistically significant correlation was reported between hyperpigmentation at 6 months and median PTV boost volume.17 Oulkadi et al18 reported prospectively collected 2-year late toxicity for 68 patients who underwent ultrahypofractionation of 26 Gy in 5 fractions, and 42 of these received a single fraction 6 Gy sequential boost. They reported moderate toxicities, the most common being sensitivity (13%), pain (9%), and skin changes and induration (9%) at 2 years. They found no significant difference between those who received a boost versus those who did not.18 In contrast, Othman et al19 found an increased risk of toxicity in those receiving a boost in 188 patients treated with ultrahypofractionation, 26 Gy in 5 fractions, but the boost dose used was not specified. Patient-reported late toxicities are described in various ways in the literature. While most studies have used the EORTC QLQ BR45, other studies do not specify the system used, making it challenging to compare results.
Virtual consultations have been used intermittently in the past in medicine but were not widely adopted in oncology until the onset of the COVID-19 pandemic. A systematic review of virtual consultations found that although clinical care was not compromised, patients did report nervousness and reluctance to communicate with physicians, which could lead to emotional distance between patients and care providers.20 Lewis et al21 report on the long-term experience of using telehealth in radiation oncology and suggest it may not completely replace in-person but acts as a good screening tool to assess who needs it in person. It may also not be appropriate for those with impaired vision or hearing, and it is necessary to screen for these issues in advance.22 In a survey of oncology patient experience in Ireland with virtual consultations, overall satisfaction levels were high.23 The majority (67%) of patients felt comfortable discussing symptoms over the phone. The majority of patients (82%) felt there was a role for virtual clinics after the pandemic. A notable finding of this study is the relatively low participation rate in the video assessment at 1 year. The low uptake of video consultation may be due to a reluctance to have a physical examination, including breast inspection, during video consultations. Tenfelde et al24 looked at patient factors, physician factors, and technology factors that impact video conferencing; however, the acceptability of clinical examination has not been explored. A more in-depth exploration is needed to better understand the reasons behind some patients’ hesitancy to participate in video-based virtual consultations. Another factor to consider is that the reasons may vary across different cultures and societies. While the focus in the present study is the assessment of toxicity, it is important to acknowledge that induration cannot be assessed on inspection, nor can a thorough breast clinical exam be undertaken for detection of recurrence using telehealth.
A strength of this study is the prospective collection of data up to 1 year for the use of ultrahypofractionation followed by a sequential hypofractionated boost where indicated. A limitation is the short follow-up period of 1 year to capture late toxicity, as some changes may further develop over time. Another limitation is the inherent variability in patient-reported outcomes; there is variability in patient's subjective views of their symptoms on a particular day.16 The authors also acknowledge that there are other methods for collecting PROMS, including electronic options and clinical photographs, which may increase compliance in future projects. Also, the type of boost received ranged from 4 to 8 fractions, so this significant difference may alter patients’ responses and experiences of this regime.
In conclusion, ultrahypofractionated whole breast RT leads to acceptable late toxicity rates at 1 year, even when followed by a hypofractionated tumor bed boost. Patient satisfaction with ultrahypofractionated treatment and virtual consultations without video was high. Further investigation concerning the patient's acceptance of video consultations for a physician-based assessment, including breast inspection, is warranted.
Disclosures
None.
Footnotes
Sources of support: J.N. received funding from the St Luke's Institute of Cancer Research, Dublin, Ireland (SLICR 2023/7542).
Data Sharing Statement: Research data are not available at this time.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2024.101668.
Appendix. Supplementary materials
References
- 1.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 2.Coles CE, Aristei C, Bliss J, et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol (R Coll Radiol) 2020;32:279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Library HSE. HSE library guides: COVID-19 HSE clinical guidance and evidence: Radiation oncology - NCCP advice for medical professionals on the management of patients undergoing breast cancer radiotherapy in response to COVID-19. Accessed February 22, 2021. https://hse.drsteevenslibrary.ie/c.php?g=679077&p=4865893
- 4.Brunt AM, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunt AM, Haviland JS. Hypofractionation: The standard for external beam breast irradiation. Breast. 2023;69:410–416. doi: 10.1016/j.breast.2023.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behroozian T, Milton L, Zhang L, et al. How do patient-reported outcomes compare with clinician assessments? A prospective study of radiation dermatitis in breast cancer. Radiother Oncol. 2021;159:98–105. doi: 10.1016/j.radonc.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 7.T-Pro Clinical Manager, version 1.18.7. Accessed January 08 2024. https://info.tpro.io/solutions/dictation.
- 8.Kingsley C, Patel S. Patient-reported outcome measures and patient-reported experience measures. BJA Educ. 2017;17:137–144. [Google Scholar]
- 9.Nugent K, Quinlan E, Cleary S, et al. Implementation of 26 Gy in five fractions over 1 week adjuvant radiotherapy for breast cancer: Prospective report of acute skin toxicity and consideration of resource implications. Breast. 2023;67:55–61. doi: 10.1016/j.breast.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Cancer Control Programe. Treatment of patients with breast cancer: Radiation Oncology. Accessed October 30 2023. https://www.hse.ie/eng/services/list/5/cancer/profinfo/guidelines/breast/
- 11.Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs) Health Serv Insights. 2013;6:61–68. doi: 10.4137/HSI.S11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkinson C, Coulter A, Bruster S. The picker patient experience questionnaire: Development and validation using data from in-patient surveys in five countries. Int J Qual Health Care. 2002;14:353–358. doi: 10.1093/intqhc/14.5.353. [DOI] [PubMed] [Google Scholar]
- 13.Al-Abri R, Al-Balushi A. Patient satisfaction survey as a tool towards quality improvement. Oman Med J. 2014;29:3–7. doi: 10.5001/omj.2014.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeves R, Seccombe I. Do patient surveys work? The influence of a national survey programme on local quality-improvement initiatives. Qual Saf Health Care. 2008;17:437–441. doi: 10.1136/qshc.2007.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faul C, Robinson J, Carey J, et al. Effect of the Cyberattack Targeting the Irish Health System in May 2021 on Radiation Treatment at St. Luke’s Radiation Oncology Network. Adv Radiat Oncol. 2022;7(5):100993. doi: 10.1016/j.adro.2022.100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laughlin BS, Corbin KS, Toesca DAS, et al. Physician- and patient-reported outcomes of the MC1635 phase 3 trial of ultrahypofractionated versus moderate hypofractionated adjuvant radiotherapy after breast-conserving surgery. Int J Radiat Oncol Biol Phys. 2024;118:1049–1059. doi: 10.1016/j.ijrobp.2023.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Montero A, Ciérvide R, Cañadillas C, et al. Acute skin toxicity of ultra-hypofractionated whole breast radiotherapy with simultaneous integrated boost for early breast cancer. Clin Transl Radiat Oncol. 2023;41 doi: 10.1016/j.ctro.2023.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oulkadi R, Amrouch S, Dabach A, et al. Ultrahypofractionated radiation therapy for breast cancer: two-year normal tissue effects. Eur J Cancer. 2022;175(suppl 1):S46. [Google Scholar]
- 19.Othman H, Koch A, Purdie TG, et al. Early institutional experience of ultra-hypofractionated breast radiotherapy in a large academic cancer center. Int J Radiat Oncol Biol Phys. 2022;114:e21. [Google Scholar]
- 20.Shirke MM, Shaikh SA, Harky A. Tele-oncology in the COVID-19 era: The way forward? Trends Cancer. 2020;6:547–549. doi: 10.1016/j.trecan.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis GD, Hatch SS, Wiederhold LR, Swanson TA. Long-term institutional experience with telemedicine services for radiation oncology: A potential model for long-term utilization. Adv Radiat Oncol. 2020;5:780–782. doi: 10.1016/j.adro.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitamura C, Zurawel-Balaura L, Wong RK. How effective is video consultation in clinical oncology? A systematic review. Curr Oncol. 2010;17:17–27. doi: 10.3747/co.v17i3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Reilly D, Carroll H, Lucas M, et al. Virtual oncology clinics during the COVID-19 pandemic. Ir J Med Sci. 2021;190:1295–1301. doi: 10.1007/s11845-020-02489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenfelde K, Bol N, Schoonman GG, Bunt JEH, Antheunis ML. Exploring the impact of patient, physician and technology factors on patient video consultation satisfaction. Digit Health. 2023;9 doi: 10.1177/20552076231203887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.