Dear Editor,
Bardet–Biedl syndrome (BBS) is a rare, autosomal recessive nonmotile ciliopathy due to BBS gene abnormalities and is characterized by retinal dystrophy and other multiorgan involvement [1]. BBS proteins are categorized into two groups: the heterooctameric BBSome complex, comprising BBS1, BBS2, BBS4, BBS5, BBS7, BBS8, BBS9, and BBS18, crucial for primary cilia development and homeostasis, and group II chaperonins family, including BBS6, BBS10, and BBS12 required for initial BBSome assembly [2]. The BBS10 gene is located on chromosome 12q21.2 and encodes a 723 amino acid BBS10, pivotal for both ciliogenesis and adipogenesis [2]. Mutations in the BBS10 gene can lead to photoreceptor dystrophy, resulting in central or peripheral vision impairment during the second or third decade of life [3]. There have been case reports of Korean siblings with BBS resulting from heterozygous mutations in the BBS10 gene. However, no reports exist on homozygous BBS10 mutations and their associated clinical characteristics [4].
This study was approved by the Institutional Review Board of Pusan National University Hospital (No. 2401-014-135). Written informed consent for publication of the research details and clinical images was obtained from the patient and the patient’s parent.
A 26-year-old woman presented with progressively worsening left visual acuity for 1 year and was referred for review by a retinal specialist. At presentation, her best-corrected visual acuity (BCVA) was hand motion on the right side and tunnel vision on the left side (20 / 160). She had polycystic kidney disease and polydactyly of both hands and feet. At age 5 years, she underwent strabismus surgery for 40-prism diopter exotropia. She presented with a 2-year developmental delay and began experiencing night blindness during school age. At 8 years of age, her right and left BCVA were 20 / 50 and 20 / 60, respectively, with no significant retinal abnormalities, diagnosed as amblyopia. Upon revisit at age 16 years, fundus examination revealed new circular depigmented lesions near the peripapillary and major vascular arcades, with right and left BCVA declining to 20 / 80 and 20 / 120, respectively, and <5° central visual field preserved in Goldmann perimetry. By age 21 years, her right BCVA further decreased to <20 / 1,000.
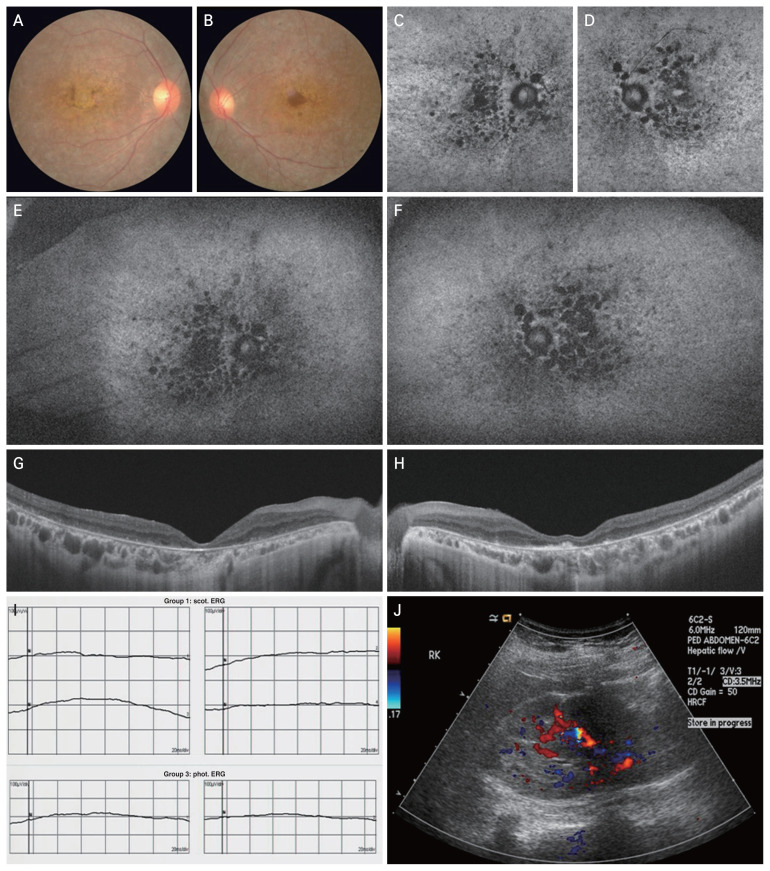
Fundus examination showed enlarged depigmentation and atrophy of the retinal pigment epithelium (Fig. 1A, 1B). A few bony spicules were observed in the mid-peripheral retina, with attenuated retinal arterioles and perivascular sheathing. Circular hypofluorescent lesions were scattered on the macula but rarely in the peripheral retina on fundus autofluorescence (Fig. 1C–1F). Swept-source optical coherence tomography revealed loss of photoreceptors and external limiting membranes and disruption of the outer plexiform layers. Hypertransmission was detected in the choroid, corresponding to the retinal pigment epithelium and outer retinal atrophy (Fig. 1G, 1H). Electroretinography was not recordable, corresponding to cone and rod cell dysfunction (Fig. 1I). Next-generation sequencing identified a homozygous frameshift mutation in BBS10:c.365dupA (NM_024685.4) leading to a protein change p.Asn-122LysfsTer30. The depth of coverage at this variant position was 173, and all reads confirmed the variant, indicating that the variant was homozygous [5]. This sequence alteration results in an early termination codon, designated p.Asn122Lysfs*30, in BBS10 gene, consequently causing premature termination of BBS10 protein translation. This mutation has been classified as likely pathogenic. Following the diagnosis of BBS10 (Online Mendelian Inheritance in Man [OMIM], 615987), additional referrals to the endocrinology, nephrology, and gynecology departments revealed stage 2 chronic kidney disease along with polycystic kidney disease (Fig. 1J).
Fig. 1.
Retinal and renal manifestations of Bardet–Biedl syndrome 10. (A,B) Fundus photography at presentation shows widespread depigmented and atrophic circular lesions on both maculae, accompanied by foveal atrophy in the right. (C–F) In fundus autofluorescence, multiple hypofluorescent regions are predominantly distributed in the macula and peripapillary region due to the retinal pigment epithelium atrophy. (G,H) Optical coherence tomography reveals disruption of photoreceptors and thinning of the outer plexiform layer. Hypertransmission is evident in the choroid region underlying the damaged retinal pigment epithelium. (I) Electroretinography shows a loss of photopic response in both eyes. (J) Additional sonographic evaluation of the kidney reveals numerous tiny anechoic foci at the corticomedullary junction, indicative of polycystic kidney disease.
BBS, the second most common syndromic retinal dystrophy following Usher syndrome, typically presents with early onset and severe retinal dystrophy [1,2]. The phenotypic expression varies, mainly including rod-cone dystrophy, but rarely cone-rod or cone dystrophy [2,3]. The fundamental mechanisms of BBS proteins in photoreceptor dystrophy remain unclear. Recent studies using murine models have suggested retinal degeneration may result from the accumulation of non-outer segment proteins rather than a failure to deliver them to the photoreceptor outer segment [3]. Additionally, the potential for gene therapy has been demonstrated as a subretinal delivery of an adeno-associated virus vector system with the BBS gene [1].
In summary, BBS10, associated with a homozygous frameshift mutation is characterized by rapidly progressive rod-cone dystrophy and is accompanied by renal abnormalities. This report presents the first case of BBS with a homozygous frameshift mutation in BBS10 in Korea. If rod-cone dystrophy involves the macula at a relatively young age, systemic genetic disorders, including BBS, should be considered, and genetic evaluation is recommended.
Acknowledgements
The authors thank the patient and her mother for permitting us to publish this information.
Footnotes
Conflicts of Interest:
None.
Funding:
None.
References
- 1.Weihbrecht K, Goar WA, Pak T, et al. Keeping an eye on bardet-biedl syndrome: a comprehensive review of the role of Bardet-Biedl syndrome genes in the eye. Med Res Arch. 2017;5 doi: 10.18103/mra.v5i9.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta N, D’Acierno M, Zona E, et al. Bardet-Biedl syndrome: the pleiotropic role of the chaperonin-like BBS6, 10, and 12 proteins. Am J Med Genet C Semin Med Genet. 2022;190:9–19. doi: 10.1002/ajmg.c.31970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grudzinska Pechhacker MK, Jacobson SG, Drack AV, et al. Comparative natural history of visual function from patients with biallelic variants in BBS1 and BBS10. Invest Ophthalmol Vis Sci. 2021;62:26. doi: 10.1167/iovs.62.15.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YH, Joo KS, Seong MW, et al. Retinitis pigmentosa associated with Bardet-Biedl syndrome with BBS9 gene mutation in a Korean patient. Korean J Ophthalmol. 2020;34:94–5. doi: 10.3341/kjo.2019.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rehm HL, Bale SJ, Bayrak-Toydemir P, et al. ACMG clinical laboratory standards for next-generation sequencing. Genet Med. 2013;15:733–47. doi: 10.1038/gim.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]