Summary
Background
The preconception period is a window of opportunity to influence maternal and pregnancy outcomes. Inappropriate use of antibiotics results in gut dysbiosis and may affect host reproductive health through multiple dimensions. Animal studies demonstrate that antibiotic treatment profoundly affects ovarian functions and the estrous cycle, and it has a direct implication for infertility. Infertility was defined as the inability to conceive after 12 months of unprotected intercourse. However, whether antibiotic exposure in the preconception period influences female fertility, miscarriage, and congenital malformation remains obscure and controversial.
Methods
A systematic review and meta-analysis until April 20, 2024, was conducted by searching PubMed, Web of Science, Scopus, and Science Direct without restrictions to designs and language. The risk of bias was assessed by two independent reviewers using the Newcastle Ottawa Scale (NOS) and the Risk of Bias 2 (RoB-2) tools. The report followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Relative risks (RR), odds ratios (OR), and fecundability ratios (FR) with a 95% confidence interval (CI) were effect size measures determined with a random effect model. Heterogeneity across included studies was assessed using I2, T2, and H2. The review protocol is registered in PROSPERO, CRD42024515680.
Findings
Fifteen studies with a total of 1,206,583 participants were included. Preconception exposure to macrolides reduced the FR by 35% (FR: 0.65, 95% CI: 0.48, 0.88, P < 0.001). Sulfonamide users were also at 2.35 times (OR:2.35, 95% CI: 1.86, 2.97; P < 0.001) more risk of developing infertility. Using beta-lactams other than penicillin G reduced the odds of infertility by 64% (OR: 0.36, 95% CI: 0.26,0.50; P < 0.001). The possibility of infertility among quinolone users was 13% lower (OR: 0.87, 95% CI: 0.77, 0.99; P = 0.03) than non-users. Preconception antibiotics exposure increased the risk of spontaneous miscarriage by 34% (RR: 1.34, 95% CI: 1.16, 1.53; P < 0.001). Moreover, trimethoprim intake also increased the odds of congenital malformations by 85% (OR:1.85, 95% CI: 1.54, 2.23; P < 0.001).
Interpretation
Preconception antibiotics exposure in females increases the risk of infertility, miscarriage, and congenital anomalies. Macrolides, sulfonamides, and trimethoprim increase the risk of infertility, spontaneous miscarriage, and congenital malformation while beta-lactams and quinolones reduce the risk. Clinicians, pregnancy planners, and health care policymakers should be warranted for pregnancy needs and success. Further clinical and mechanistic studies are required to illustrate their specific functions and cause effects.
Funding
Funded by Leading Discipline Development Fund (No. 403947), The Second Affiliated Hospital of Shaanxi University of Traditional Chinese Medicine; and The Hong Kong Obstetrical and Gynaecological Trust Fund.
Keywords: Congenital birth defect, Female reproduction, Gut microbiome, Infertility, Miscarriage, Preconception antibiotics
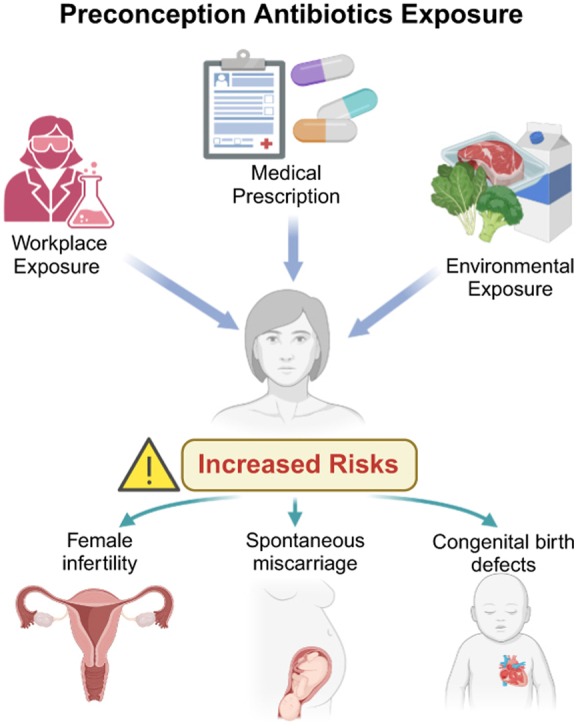
Graphical abstract
Preconception antibiotics exposure and reproduction risks (Created in https://BioRender.com).
Research in context.
Evidence before this study
Antibiotic exposure disrupts the gut microecology and affects systemic health issues. Lines of evidence showed antibiotic use during pregnancy is associated with various negative consequences, including preterm birth, fetal malformation, and miscarriage. The preconception period is a window of opportunity to affect both reproductive and pregnancy health in women. However, the specific risks of antibiotic exposure in the preconception period remain under investigation. Therefore, we searched PubMed, Web of Science, Scopus, and Science Direct electronic databases until September 2024, with no language and study design restrictions using systematically designed searching terms under contexts such as infertility, preconception antibiotics, miscarriage, and birth defects. Infertility was defined as the inability to conceive after 12 months of unprotected intercourse.
Added value of this study
We comprehensively reviewed 15 comparably higher-quality studies involving more than a million participants. Preconception exposure to macrolides reduces the fecundability ratio by 35%. Sulfonamide usage increases the odds of infertility by more than 2-fold. On the contrary, β-lactams and quinolones reduce the risk of infertility by 64% and 13% respectively. Preconception antibiotics administration also augments the risk of spontaneous miscarriage and congenital malformations by 34% and 85% respectively. Moreover, trimethoprim use was found to be linked with birth anomalies. Our findings suggest that certain antibiotics significantly increase the risk of infertility, miscarriage, and congenital anomalies, highlighting the importance of cautious antibiotic use in women planning to conceive.
Implications of all the available evidence
Our study suggests significant reproductive risks posed by preconception antibiotic exposure for women of reproductive age. It advocates for integrating antibiotic risk assessments into preconception care protocols to reduce adverse reproductive outcomes. Enhanced awareness and education about the risks of preconception antibiotic use is essential to promote reproductive health outcomes. Future studies are needed to uncover the mechanisms of how antibiotics influence reproductive health and explore potential preventive and therapeutic interventions to enhance female health.
Introduction
Preconception care in women before pregnancy emerges as a crucial time window in determining maternal and pregnancy health.1,2 Interventions in the preconception period such as folic acid supplementation and lifestyle modification substantially improve pregnancy outcomes.3 Enhancing preconception care,4 including maternal behavioural assessment, vaccination, genetic and infection screening, and weight management can effectively reduce neonatal mortality and increase antenatal care-seeking behaviour.5 On the contrary, exposure to risk factors in preconception, like smoking, alcohol abuse, and medication use, negatively impacts reproductive and pregnancy health.
Antibiotic abuse has emerged as a global public health concern. The antibiotic use rate is about 14.3 per 1000 population per day worldwide,6 where about 20% of adults’ antimicrobial use is self-medication.7 In addition to antibiotic resistance threats, growing studies demonstrated that inappropriate antibiotic exposure induces gut dysbiosis and is associated with intestinal barrier disruption, lipid metabolism dysfunction, and inflammation.8, 9, 10, 11 Though antibiotic treatments were accepted during pregnancy for certain clinical indications such as maternal group B-streptococcus colonization and chorioamnionitis infections,12 it has also negative effects on pregnancy outcomes and offspring health.13,14 A population-based cohort showed that antibiotic exposure increases the risk of preterm birth.15 Use of clindamycin, doxycycline, quinolones, macrolides, and phenoxymethylpenicillin during pregnancy can cause fetal malformations.16 Moreover, animal studies also suggested that antenatal antibiotic exposure is associated with various complications including miscarriage, low birth weight, and allergies in early and later life.17
However, compared to the known effects of antibiotic exposure in pregnancy, the impact of antibiotic usage in the preconception period is overlooked. The relative lack of investigations into this issue is primarily attributed to the unpredictability and unplanned nature of conception.18, 19, 20, 21 In addition, although antibiotic treatment significantly disrupts human gut and vagina microbiome homeostasis,8,22 it also exerts beneficial effects on female reproductive and gestational health in certain disease scenarios. For instance, antibiotic treatment is routinely prescribed for women with bacterial vaginosis (BV), which is substantially linked to subfertility, miscarriage, and preterm delivery.23 Therefore, given the existing controversy and inconclusive evidence,6,9,24 we summarized this systematic review aiming to comprehensively evaluate the association between preconception antibiotic exposure, including the differential association of specific antibiotics catalog, and reproductive outcomes in terms of 1) pregnancy rate, fecundability, and infertility; 2) spontaneous miscarriage, and 3) congenital malformation to the fetus.
Methods
Search strategy and eligibility criteria
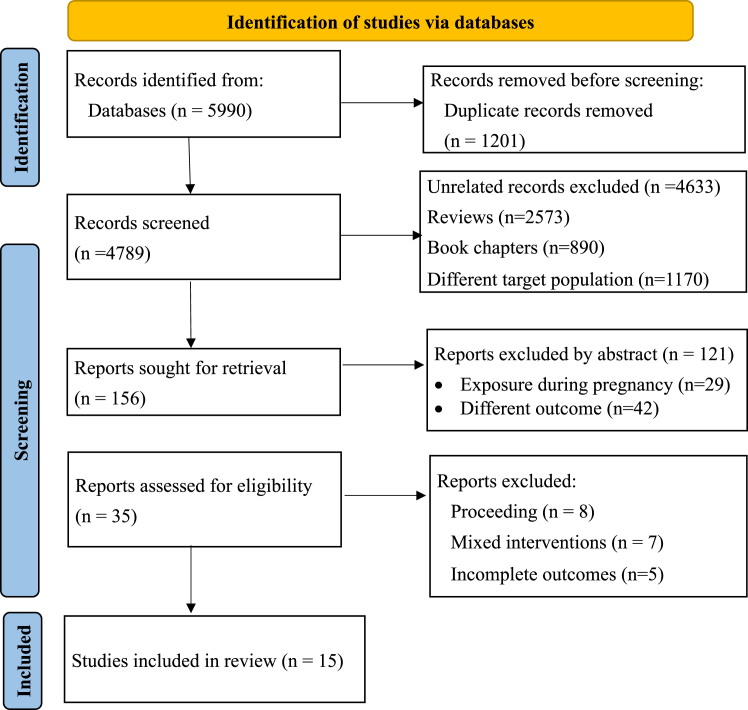
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA 2020) checklist.25 The protocol was registered in the International Prospective Register of Systematic Reviews, PROSPERO, CRD42024515680. The PECO (Populations, Exposure, Comparator, and Outcomes) strategy was followed to include all articles that met the following inclusion criteria. Population-includes female pregnancy planners, infertile women treated with invitro-fertilization or embryo transfer, and women with miscarriage and congenital malformation. The exposure includes any antibiotics before pregnancy (preconception exposure) and the comparators were non-antibiotic users. The outcomes measured in this review include fecundability, rate of clinical pregnancy, infertility, miscarriage, and congenital malformations following preconception antibiotics exposure. We included all studies irrespective of the study design, study setting, and publication language. The main inclusion criteria consisted of 1) papers that assessed the association of preconception antibiotics with women’s fertility, spontaneous miscarriage, and congenital birth defects; 2) primary studies; 3) any types of study design; and 4) published at any time. The exclusion criteria also involve 1) outcomes measured following antibiotics exposure during pregnancy, 2) review papers, and 3) male infertility (Fig. 1). Four popular electronic databases (PubMed, Web of Science, Scopus, and Science Direct) were systematically searched for articles published until April 20, 2024. Different search terms were used for each database in seven main contexts (Females, antibiotics, preconception, infertility, miscarriage, and congenital malformation) as detailed in Supplementary Table S1. Moreover, related reference lists and Supplementary files were retrieved to obtain further articles.
Fig. 1.
PRISMA flow diagram of study selection and inclusion.
Study selection and data extraction
Two independent authors (BKA and LQ) screened each article eligible for the review and extracted effect-measuring data using a standardized format that comprised study ID, first author and year, country, population, exposure, comparator, outcomes, conclusions made, type of antibiotics (if specified), indication of antibiotics (if any), sample size and effect size measures including FR, OR and RR. Ambiguities and conflicting issues during screening and data extraction were solved by discussion involving the 3rd reviewer (YW).
Outcome measures
Outcomes in this systematic review and meta-analysis include infertility, pregnancy rate, fecundability rate, miscarriage, and congenital malformation, which were measured by FR, OR, and RR. These effect measures were from different studies with different designs such as case controls, cohorts, and controlled trials. Moreover, outcomes were measured from varied population including women who were under follow-up for infertility treatment, health women, and women with unknown health status from the general population. Fecundability is the likelihood of conception in a menstrual cycle/one month.26 The FR was measured as the average per-cycle probability of conception comparing antibiotic users with nonusers. Thus, an FR < 1 indicates reduced fecundability for exposed participants.6 The risk or odds of infertility, miscarriage, and congenital birth defects with a 95% CI were also reported by included studies and we considered all of them as outcome measures. Infertility was defined as the inability to conceive after 12 months of unprotected intercourse.27 Each study reported a different antibiotic type. Some reported specific antibiotics while others reported chemical-based classes of antibiotics. Therefore, in this meta-analysis, each antibiotics were categorized into chemical classes like macrolides, quinolones, and sulfonamides. In case of no suitable class for categorization, antibiotics were categorized as “others”. Moreover, some papers reported just “antibiotics” which is not specified, and we used it as it is by considering it “non-specified”.
Statistical analysis
Stata 17 software (StataCorp LLC, TX, USA) was used for analysis and a random-effects model was fitted for all summary measures. Since multiple antibiotic effects were reported,6,21,28 we extracted the effect measure for each antibiotic reported separately and combined based on their drug class. Classes include macrolides, beta-lactams, sulfonamides, quinolones, and tetracyclines. Therefore, antibiotics from a single study were pooled in the meta-analysis based on their functional class. Some papers also reported “antibiotics” without specifying the type of antibiotics. In this case, we combined them and reported the pooled association of antibiotics and outcomes specified. In general, the reports here are presented with themes including the association of preconception antibiotics with A) infertility; B) miscarriage; and C) congenital malformation. The overall pooled results and the effect of each study were observed using forest plots. It was very imperative to conduct a subgroup analysis by study design, region of the study, population type (healthy and infertile), duration of preconception antibiotic exposure, and dosage. However, we did not perform it because of limited number of studies needed (n ≥ 2) for meta-analysis. The I2 (proportion of total variance between studies), T2 (a between-study variance), and H2 (communal variance) test statistics were used to assess the heterogeneity across studies while a P-value less than 0.05 to declare statistical significance. Publication bias was assessed using the asymmetry of funnel plots and Egger’s test. Sensitivity analyses were done to assess the effect of outlier studies that had an impact on the overall result estimation.
Quality assessment
The methodological quality of the included studies was assessed by two authors (BKA and YW) independently using the NOS for cohort and case–control studies.29 The RoB-2 tool was used to assess the included randomized controlled trials using the five domains.30 Potential confounders for consistency of assessment were discussed before assessment and result differences in each domain of ROB-2 were managed with discussion. The majority of cohort and case–control studies had good quality in the assessment of risk of bias. Only two studies were judged as “fair quality”.27,31 Of the RCTs included in this review, only one of the three RCTs was with a high risk of bias32 (Supplementary Table S2). Certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE). About 40% (n = 6) of included studies had high certainty while 60% (n = 9) were with moderate certainty (Table 1 and Supplementary Table S3).
Table 1.
Characteristics of included studies in the systematic review and meta-analysis.
| Author Year | Country | Study design | Population | Exposure, dose, and duration | Timing of antibiotics | Indications/Exposi ng factor | Comparator | Outcomes | Certainty |
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy | |||||||||
| Harrison e t al 197524 | UK | RCT | Women with primary infertility | Doxycycline 100 mg. per day for twenty- eight days | 1 year | Primary infertility anatomical factors excluded | Placebo and no treatment | Conception rate | Moderate |
| Schaumburg, 198933 | Denmark | Cohort | Female pharmacy assistants | Occupational antibiotics exposure (not specified type and dose) | 1 year | Occupation (worked in antibiotics station) | Same occupation with different workstation | Pregnancy rate | High |
| Grodstein et al., 199234 | USA | Case- control | Women who attended infertility clinics and pregnant women | Antibiotics (not specified type, used it for 6 months) | 6 months | NR | Not used any antibiotics | Clinical pregnancy rate | High |
| Peikrishvili et al., 200435 | France | RCT | Women for the IVF | Amoxicillin (1 g) + Clavulanic acid (125 mg) for 6 days | 6 days from ovum pickup | IVF | No antibiotics | Implantation rate | Moderate |
| Brook et al., 200632 | UK | RCT | Patients for IVF | Co-amoxiclav 750 mg for two different days | Before embryo transfer | NR | Not used any antibiotics | Clinical pregnancy rate and live birth | Moderate |
| Cicinelli et al., 201836 | Italy | Retrospective study | Women with unexplained infertility | Antibiotics (dose and type not specified) for a maximum of 3 cycles of menstruation | 1 year | Chronic endometriosis | Not used any antibiotics | Successful pregnancy | Moderate |
| Eskew et al., 202037 | USA | Prospective exploratory study | Subfertile women | Single dose prophylactic azithromycin | Prior to IVF | IVF | No azithromycin | FR | Moderate |
| Crowe et al., 20216 | USA/Cana da | Cohort | Female pregnancy planners | Penicillins, macrolides, nitrofurantoin, nitroimidazole, cephalosporins, sulfonamides, quinolones, tetracyclines or lincosamides (with not specified doses and duration) | 4 weeks | Infection | Not used any antibiotics | Pregnancy status | Moderate |
| Shao 202328 | China | Case- control | Women with and without infertility | Sulfonamides, tetracyclines, quinoxalines, and veterinary antibiotics (with not specified doses and duration) | 1 year | NR | Not used any antibiotics | Prolonged infertility | Moderate |
| Mikkelsen et al., 202321 | Denmark | Prospective cohort | Healthy females | Penicillins, sulfonamides, or macrolides (any dose by prescription, not specified duration) | 1 year | NR | Not used any antibiotics | FR | High |
| Duan et al., 202338 | China | Prospective cohort study | Patients with infertility underwent IVF | Antibiotics type dose and duration not specified. However, rather levels measured in urine | Before embryo transfer | Women treated with antibiotics for endometriosis | Women with no antibiotic treatment and endometriosis | Clinical pregnancy | High |
| Spontaneous miscarriage | |||||||||
| Peikrishvili et al., 200435 | France | RCT | Women for the IVF | Amoxicillin (1 g) + Clavulanic acid (125 mg) for 6 days | 6 days from ovum pickup | IVF | No antibiotics | Pregnancy loss rate | Moderate |
| Crowe et al., 20226 | USA/Canada | Cohort study | Female pregnancy planners | Penicillin, nitrofurantoin, cephalosporins, and macrolides (with not specified doses and duration) | 1 year | NR | No antibiotic use | SAB | Moderate |
| Duan et al. 202338 | China | Prospective cohort study | Patients with infertility underwent IVF/ICSI | Antibiotics type dose and duration not specified. However, rather levels measured from urine | Prior to embryo transfer | Endometriosis cured | No endometriosis | SAB | High |
| Gelder et al. 202339 | Netherlands | Cohort study | Females trying to conceive | Antibiotics type dose and duration not specified. | 4 weeks | Not specified | No antibiotic use | SAB | Moderate |
| A. Congenital malformation: | B. | ||||||||
| Andersen, TJ et al., 201340 | Denmark | Cohort study | Reproductive age women | Any dose of trimethoprim prescribed 12 weeks before conception | 12 weeks | Not specified | No- trimethoprim | Congenital malformation | High |
| Sun Y et al., 201431 | Denmark | case- time control | Babies of women in the cohort | Any dose of trimethoprim prescribed 1–3 months before pregnancy | 3 months | Not specified | Not used in 1–3 months pre-pregnancy | Congenital malformation | Moderate |
UK: United Kingdom; RCT: Randomized Controlled Trial; mg: milligram; USA: United States of America; NR: Not reported; FR: Fecundability Ratio; IVF/ICSI: In Vitro Fertilization/Intracytoplasmic Sperm Injection.
Ethics statement
This study is a systematic review and meta-analysis of publicly available data, and therefore, neither consent form nor ethics approval was required.
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
In this systematic review and meta-analysis, we identified a total of 5990 articles. After 1201 duplicate records were confiscated, we screened 4789 articles. In the final screening, approximately 35 articles were screened by reviewing the full texts. After full paper screening about 20 were excluded by eligibility criteria, and 15 articles were included in the qualitative synthesis of which 11 were in the meta-analysis (Fig. 1).
Study characteristics and risk of bias assessment
In the 15 included articles,6,21,24,28,31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 a total of 1,206,583 women were involved in measuring the association of preconception antibiotics exposure with the risk of infertility, miscarriage, and fetal congenital abnormalities. We searched for papers published from inception to September 2024, and we found studies published between 1975 and 2023. The majority of the included studies were from Europe (n = 9) (four from Denmark, two from the United Kingdom, and three from Italy, France, and the Netherlands (one from each)), the United States/Canada (n = 4), and China (n = 2). Different antibiotics and classes of drugs such as amoxicillin, clavulanic acid, penicillins, nitrofurantoin, cephalosporins, macrolides, nitroimidazole, quinolones, tetracyclines, sulfonamides, lincosamides, sulfamethoxazole, sulfaclozine, sulfamonomethoxine, chlortetracycline, ofloxacin, norfloxacin, cyadox, sulfamethazine, azithromycin, cefaclor, oxytetracycline, pefloxacin, sarafloxacin, enrofloxacin, and florfenicol had been investigated in the included studies (Table 1).
We summarized the association of preconception antibiotic exposure with the risk of infertility, spontaneous miscarriage, and any of the major congenital malformations. The risk of infertility was evaluated by rate of clinical pregnancy, implantation, fecundability, and odds of infertility (Table 2).
Table 2.
Association of preconception antibiotics with pregnancy, miscarriage, and birth defects in the included studies.
| Author Year | N in exposed/case | N non-exposed/control | FR/OR/RR/P | 95% CI/IQR | Conclusion |
|---|---|---|---|---|---|
| A. Pregnancy | |||||
| Harrison et al., 197524 | 30 | 58 | P (16%, 14% and 16%) | NR | No significant benefit in increasing the conception rate |
| Schaumburg, 198936 | 597 | 3833 | OR 1.34 To Delay | 1.0, 1.8 | A slightly increased risk of prolonged time to pregnancy |
| Grodstein et al., 199233 | 593 | 3833 | RR 1 | 0.7, 1.4 | No significant effect to prolong time to pregnancy |
| Peikrishvili et al., 200437 | 130 | 145 | P (36.9% and 36.5%) | NA | Implantation rate per transfer was similar in the two groups |
| Brook et al., 200632 | 178 | 172 | RR 1.01 | 0.81, 1.24 | Has no impact on clinical pregnancy rates |
| Cicinelli et al., 201832 | 53 | 42 | OR 14.48 | 4.50, 46.55 | Showed higher pregnancy rate and live birth rate |
| Eskew et al., 202037 | 12 | 14 | Median time to conception (25 and 18) | IQR (18, 48) and (8, 32) | Failure to achieve clinical pregnancy in the azithromycin group |
| Crowe et al., 20216 | 1432 | 8092 | FR 0.98 | 0.89, 1.07 | No significant difference between the two groups in terms of reduced fecundability |
| Shao 202328 | 302 | 302 | OR 1.29 | 0.78, 2.15 | Has no significant association with fecundability |
| Mikkelsen et al., 202321 | 1130 | 8332 | FR 0.86 | 0.76,0.99 | Antibiotics were associated with decreased fecundability compared with no-users |
| Duan et al., 202338 | 338 | 7962 | OR 0.83 | 0.66, 1.03 | No statistical difference in the occurrence of clinical pregnancy |
| B. Spontaneous miscarriage | |||||
| Peikrishvili et al., 200435 | 130 | 145 | P (33.3% and 20.8%) | NA (P-value = 0.15) | Antibiotic prescription for IVF cases didn’t cause significant pregnancy loss |
| Crowe et al., 20226 | 1537 | 6353 | HR 1.06 | 0.88, 1.28 | Not appreciably associated with SAB |
| Duan et al., 202338 | 338 | 7962 | OR: 1.49 | 1.01, 2.19 | Antibiotics increase the risk of SAB |
| Gelder et al., 202339 | 585 | 7305 | RR 1.34 | 1.11, 1.79 | Preconception antibiotics exposure increases the risk of SAB |
| C. Birth defects | |||||
| Andersen, TJ et al., 201340 | 402 | 520,865 | OR 2.01 | 1.45, 2.78 | Exposure to trimethoprim during the 12 weeks before conception increased the risk of heart and limb defects |
| Sun Y et al., 201431 | NR | NR | 1.66 | 1.10, 2.53 | Trimethoprim is a potential teratogen when used 3 months before pregnancy |
N: Sample size in the study; OR: Odds ratio; RR: Relative risk; FR: Fecundability ratio; p: Proportion; CI: Confidence interval; IQR: Interquartile range; NR: Not reported; NA: Not applicable; HR: Hazard ratio; IVF: In vitro fertilization, SAB: Spontaneous abortion; RCT: Randomized controlled trial.
Association of infertility with preconception antibiotics exposure
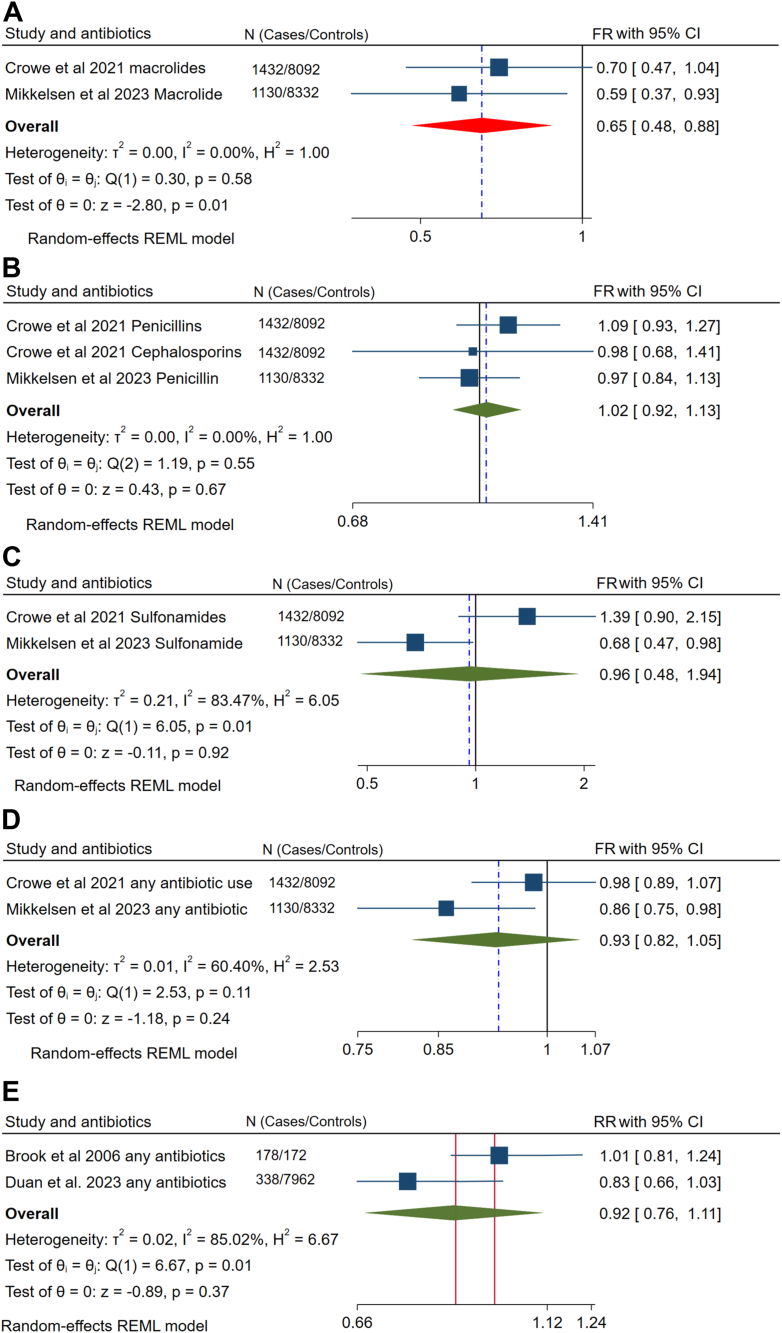
The FR is an average per-cycle probability of conception comparing antibiotic users with nonusers. Thus, an FR < 1 indicates reduced fertility for exposed participants. For the sake of different classes of antibiotics, we have conducted a meta-analysis for the association of macrolides, beta-lactams, sulfonamides, and other antibiotics with fecundability. Macrolide exposure in the preconception period delays the probability of getting pregnant by 35% (FR 0.65, 95% CI (0.48, 0.88)) (n = 2). However, evidence showed no significant association between fecundability and taking beta-lactams (FR 1.02, 95% CI (0.92, 1.13)) (n = 2); and sulfonamides (FR 0.96, 95% CI (0.48, 1.94)) (n = 2). Pre-pregnancy antibiotics exposure had no significant association with clinical pregnancy rates (RR 0.92, 95% CI (0.76, 1.11)) (Fig. 2 A–E). Though there was considerable heterogeneity in sulfonamides, we couldn’t perform sensitivity analysis due to the limited number of studies included.
Fig. 2.
The ratio of the average per-cycle probability of conception (fecundability) among women who took A) Macrolides, B) Betalactams, C) Sulfonamides, and D) Other antibiotics in the preconception period; and E) the association of preconception antibiotics with clinical pregnancy rate.
The odds of infertility were also evaluated among women exposed to beta-lactams, quinolones, sulfonamides, tetracyclines, and others in the preconception period. The odds of infertility among beta-lactam users were evaluated by combining three antibiotics with similar outcome measures and showed no significant association (OR: 0.62, 95% CI (0.22, 1.73)) (Table 3). Since there was substantial heterogeneity between included studies, egger’s test was done and showed no publication bias. Since the pooled data was from a single study, doing sensitivity analysis was found inappropriate. However, when we visually inspected the values in Table 3, Penicillin G seemed asymmetrically outlier, and we excluded it from the analysis and reperformed the final analysis which flashed a significant association between beta-lactam exposure and fertility (OR 0.36, 95% CI (0.26, 0.50)). Moreover, the use of quinolone also reduced the odds of infertility by 13% (OR 0.87, 95% CI (0.77, 0.99)). Since it showed a considerable heterogeneity (I2 = 94.88%) (Table 3), Egger’s test (P = 0.042) that highlighted a significant publication bias was computed. However, outlier study identification using sensitivity analysis was not conducted due to the limited number of studies in each analysis. Though the reports on sulfonamides were from a single study by Shao et al., 2023, the overall pooled effect size of three different antibiotics showed that preconception sulfonamide exposure increased the odds of infertility by 2.35 times more than those who have no such exposure (OR 2.35, 95% CI (1.86, 2.97)). Under the group tetracycline, chlortetracycline usage was reported to increase the risk of infertility. On the contrary, oxytetracycline was reported as protective. The overall risk of infertility due to tetracycline was also elevated. However, the pooled odds of infertility due to tetracyclines in the current study were not statistically significant (OR 1.95, 95% CI (0.39, 9.76)). Due to the limited number of studies (n = 2) included in this analysis, we didn’t perform tests of the between-study heterogeneity including sensitivity analysis. Antibiotics that did not suit to be classified under the abovementioned categories and a report which did not specify the type were pooled and reported as ‘others’. They exhibited no significant difference (OR 1.06, 95% CI (0.68, 1.65)) (Table 3).
Table 3.
The association of preconception antibiotics exposure with A. Fecundability ratio.
| Study and Antibiotics | Class | FR | CI | Pooled effect size | P-value | I2, T2, H2 |
|---|---|---|---|---|---|---|
| Crowe et al., 2021 lincosamides | Lincosamides | 1.58 | 0.96,2.60 | 0.94 (0.77, 1.14) | 0.520 | 10.48%, 0.01, 1.12 |
| Crowe et al., 2021 Nitrofurantoin | nitrofuran | 0.83 | 0.60,1.13 | |||
| Crowe et al., 2021 Nitroimidazole | Nitroimidazole | 0.9 | 0.60,1.34 | |||
| Crowe et al., 2021 Quinolones | Quinolone | 0.82 | 0.52,1.30 | |||
| Crowe et al., 2021 Tetracyclines | Tetracyclines | 0.9 | 0.55,1.48 |
| B. The odds of female infertility among different classes of antibiotics | ||||||
|---|---|---|---|---|---|---|
| Study and antibiotics | Class | OR | CI | Pooled effect size | P-value | I2, T2, H2 |
| Shao 2023 Penicillin G | Betalactams | 1.71 | 1.32, 2.21 | 0.62 (0.22, 1.73) | 0.360 | 95.17%, 0.79, 20.70 |
| Shao 2023 Cefaclor | 0.36 | 0.23, 0.56 | ||||
| Shao 2023 Amoxicillin | 0.36 | 0.22, 0.60 | ||||
| Shao 2023 Cefaclor | 0.36 | 0.23, 0.56 | 0.36 (0.26, 0.50) | <0.001 | 0.00%, 0.00, 1.00 | |
| Shao 2023 Amoxicillin | 0.36 | 0.22, 0.60 | ||||
| Shao 2023 Ofloxacin | Quinolones | 1.35 | 1.1, 1.66 | 0.87 (0.77, 0.99) | 0.030 | 94.88%, ---, 19.53 |
| Shao 2023 Norfloxacin | 1.3 | 1.02, 1.66 | ||||
| Shao 2023 Pefloxacin | 0.5 | 0.36,0.70 | ||||
| Shao 2023 Sarafloxacin | 0.49 | 0.33, 0.75 | ||||
| Shao 2023 Enrofloxacin | 0.33 | 0.24, 0.47 | ||||
| Shao 2023 Sulfamethoxazole | Sulfonamide | 1.88 | 1.21, 2.92 | 2.35 (1.86, 2.97) | <0.001 | 0.00%, 0.00, 1.00 |
| Shao 2023 Sulfaclozine | 2.36 | 1.64,3.40 | ||||
| Shao 2023 Sulfamonomethoxine | 2.86 | 1.87, 4.37 | ||||
| Shao 2023 Chlorotetracycline | TTC | 6.34 | 3.54, 11.37 | 1.95 (0.39, 9.76) | 0.420 | 94.92%, 1.28, 19.68 |
| Shao 2023 Oxytetracycline | 0.62 | 0.40, 0.95 | ||||
| Shao 2023 Lorfenicol | Others | 0.59 | 0.41,0.86 | 1.06 (0.68, 1.65) | 0.790 | 88.75%, 0.22, 8.89 |
| Schaumburg, 1989 | 1.34 | 1.0, 1.8 | ||||
| Shao 2023 non specified | 1.29 | 0.78, 2.15 | ||||
| Shao 2023 Azithromycin | 0.7 | 0.57, 0.87 | ||||
| Shao 2023 Cyadox | 2.01 | 1.35, 3.00 | ||||
FR: Fecundability ratio, CI: Confidence interval, I2, T2, H2: heterogeneity measures, OR: Odds ratio, TTC: Tetracycline.
The association of preconception antibiotics exposure with miscarriage and congenital malformations
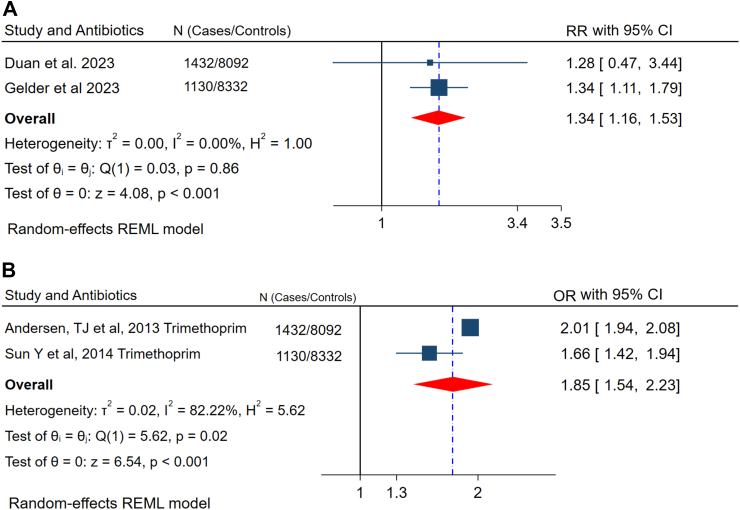
Maternal antibiotic exposure during pregnancy and prenatal is associated with negative outcomes, like preterm birth, miscarriage, birth defects, and long-term child health problems.42,43 Therefore, in this meta-analysis, besides the association of preconception antibiotics with fertility delay, we investigated its relationship with miscarriage and congenital anomalies even after successful conception. It significantly increases the risk of spontaneous miscarriage by 34% (RR 1.34, 95% CI (1.16, 1.53)). Similarly, the odds of congenital malformation among trimethoprim users in the preconception period was 85% higher than nonusers (OR 1.85, 95% CI (1.54, 2.23)) (Fig. 3 A and B).
Fig. 3.
The relationship between preconception antibiotic exposure and A) Spontaneous miscarriage, and B) Congenital malformations.
Discussion
Antibiotics are commonly applied in reproductive-age women for various indications such as urinary tract and sexually transmitted infections. Additionally, women may have the chance to be exposed to antibiotics in workplaces (e.g., hospitals and pharmaceutical factories), environmental pollution (like resident antibiotics in meat), and self-medication from private pharmacies. In this meta-analysis, by extracting data from fifteen studies that included over 1,200,000 subjects, we demonstrated antibiotic exposure in women at the preconception stage increased the risk of infertility, delayed fecundability, spontaneous miscarriage, and congenital malformation. Moreover, we further delineated the differential clinical impacts of specific antibiotics, which may facilitate the precision of clinical prescription practice. Our findings appeal to the critical need for careful consideration of antibiotic use in women of reproductive age to safeguard reproductive health and mitigate potential adverse events in pregnancy.
We first identified that exposure to macrolides and sulfonamides in the preconception phase negatively impacts female fertility. Despite current evidence remaining obscure to elucidate its effects, antibiotics may influent the women’s reproductive function through multiple mechanisms. First, macrolide and sulfonamides have been shown with anti-inflammation effects,44,45 which could impair the essential inflammatory process during embryo implantation.46 Another putative pathway is that antibiotics distribute gut microbiome equilibrium thereby affecting female reproductive health. Furthermore, gut microbiome has emerged as a cornerstone in orchestrating the host’s systemic health.47 Lines of evidence showed that antibiotic exposure is a potent modulator reshaping gut microbiome composition, reducing microbial diversity, and causing gut dysbiosis.48, 49, 50 Acute broad-spectrum antibiotic treatment leads to a 25% reduction of microbial diversity and deleting over 40% of core bacterial taxa.51 Interestingly, women with unexplained infertility were associated with downregulated gastrointestinal tight junction and decreased bacterial richness.52 In addition, there are group of enteric bacteria called estrobolome with the estrogen metabolization capacity,53,54 a vital hormone governing reproductive function.55 For instance, some lactic acid bacteria synchronize estrogen and progesterone to improve ovarian function and prevent polycystic ovarian syndrome.56 Antibiotic-triggered gut dysbiosis may result in estrobolome dysfunction which in turn causes estrogen-dependent disorders.57 Importantly, gut microbiota can directly implicate estrogenic metabolism and gestational-related hormone biosynthesis such as progesterone and progestins.58,59 Therefore, antibiotics-induced gut dysbiosis possibly disrupts endocrine haemostasis and fertility. Conversely, some beta-lactams and quinolones were found to be associated with a reduced risk of infertility. Such distinct effects of differential antibiotics on female reproduction require further discussion.
Furthermore, our result suggested preconception antibiotics (non-specified) exposure leads to a 34% higher risk of miscarriage in pregnancy. In agreement with our findings, lower microbial diversity was associated with the onset of spontaneous abortion in humans.60 Another meta-analysis of antibiotics exposure during pregnancy reported that macrolides, quinolones, and tetracyclines during pregnancy are strongly associated with spontaneous miscarriage.61 Furthermore, a register-based nationwide cohort also exhibited that clarithromycin use in early pregnancy increases the risk of miscarriage.62 Lathakumari et al. also revealed that the risk of miscarriage among macrolide users was higher.43 Several pathways shore up the linkage between antibiotic usage and pregnancy outcomes. First, gut microbiome orchestrates the immune response and immunological tolerance at the maternal–fetal interface.63 The microbial dysfunction increases the permeability of the lumen to lipopolysaccharides (LPS), which are endotoxins that induce inflammation and possibly lead to miscarriage.64,65 Antibiotics administered in pregnant mice disturb the immune response as evidenced by increased Th1 cells and decreased Th2 cells, thereby influencing the placental weight and leading to adverse pregnancy outcomes.66 Moreover, gut microbiome disruption in mothers also triggers the depletion of regulatory T cells, B cells, and follicular regulatory T cells, and alters transforming growth factor-β and interleukin-10 production,63,67 which may also contribute to pregnancy complications.
We also found that preconception antibiotics, particularly taking trimethoprim increase the risk of either of the commonest gross congenital anomalies.31,40,68,69 Preconception antibiotic exposure may also influence fetal structural and functional development through mediating microbiome-derived metabolites. For instance, Short-chain fatty acids (SCFA), folate, and trimethylamine N-oxide (TMAO) are typical gut microbiome-generated metabolites,70, 71, 72 which engage in fetal development. SCFA participates in mitochondrial energy metabolism to mediate fetal growth.73 TMAO impairs embryonic quality and influences fetal cardiovascular congenital abnormalities.74,75 Additionally, folate is an essential reagent contributing to embryonic development.76 A low folate condition during early pregnancy is associated with lipo-metabolic disturbance and causes neural tube defects in the fetus as compared to those with normal folate levels.41 Interestingly, accumulating evidence has proven that the administration of antibiotics remarkably reduced TMAO, SCFA, and folate levels in human circulation.77 Collectively, preconception antibiotic exposure increases the risk of congenital malformation possibly by disrupting the gut microbiome-produced metabolites during organogenesis and development.
This study possesses several noteworthy strengths. Foremost, it provides evidence that preconception antibiotic exposure in females is associated with an increased risk of infertility, spontaneous miscarriage, and birth defects in a large population. We employed a comprehensive search strategy, study selection, data extraction, and risk of bias assessment using a standard tool by two independent reviewers. The majority study included in our meta-analysis with high-quality designs. Moreover, our study encompasses data from over one million participants, enhancing the statistical robustness and generalizability of our findings. Overall, our findings illuminates the potential risk of preconception antibiotics on female reproductive health and prompts imperative attention from researchers, clinicians, pregnancy planners, and women of reproductive age regarding antibiotic treatment before conception. This study underscores the critical need to prioritize the preconception period in both research and clinical practice. The particular clinical implication of this paper is to embark on the importance of giving due attention to the preconception period to avoid the unforeseen effect of antibiotic exposure associated with reproductive and pregnancy outcomes. Clinicians can better guide patients in their family planning stages, potentially adjusting or timing antibiotic use to minimize risks. Moreover, this information is crucial not only for specialists in reproductive health but also for general practitioners who often prescribe these medications with less attention to the preconception phase. For clinical implications, though the message to reduce unnecessary antibiotic use is indeed not new, our study provides a targeted emphasis on the preconception period, which has been less studied in comparison to antibiotic use during pregnancy.
Despite these strengths, a considerable number of limitations are acknowledged. First, a limited number of included studies and pooling data from the same study across different antibiotic categories present major drawbacks. The insufficient number of studies in each meta-analysis precluded performance asymmetry tests for publication bias assessment. Furthermore, essential details concerning antibiotics exposure, such as dosage regimen, treatment frequency, pharmacokinetics concentration during the conception period, and the exact timing of fertilization were not addressed in this paper. Moreover, considering the lack of existing literature on this subject, we incorporated findings from animal studies to support our discussions. Additionally, we admitted that our study is unable to fully mitigate confounding factors like underlying infection status, which is intrinsically associated with negative reproductive health. Consequently, the findings should be carefully confined to a certain clinical context. The application of antibiotic therapy warrants nuanced consideration and further discussion based on specific clinical scenarios and needs. In some of the included studies, simultaneous multiple antibiotics exposure could happen. However, the data to show the extent of overlap of antibiotic use was limited in each included study to present in this meta-analysis. Lastly, there is an urgent need for more clinical studies to evaluate causality and to elucidate the underlying biological mechanisms by which preconception antibiotic exposure impacts female reproductive and pregnancy outcomes.
In conclusion, our results indicate critical insights into the potential adverse effects of antibiotic exposure on female reproductive health and pregnancy. Preconception antibiotic utilization, particularly for macrolides, sulfonamides, and trimethoprim, is linked with increased reproductive risks such as infertility, delayed fecundability, miscarriage, and congenital birth defects. On the other hand, using beta-lactams and quinolones is associated with reduced infertility. Pregnancy planners, preconception care providers, and all reproductive-age women shall carefully consider the risk of antibiotics treatment prior to conception. Of note, it is also essential to focus on the different effects of specific antibiotic perceptions, e.g., drug type, dosage, and timing, which are vital for developing evidence-based guidelines to inform clinical practice and optimize reproductive outcomes. Importantly, there is an urgent need to develop novel therapeutical strategies (e.g., probiotic supplementation) or alternative antimicrobial approaches to ensure the well-being of women. Future well-designed studies are essential to elucidate the causal relationship between preconception antibiotic exposure and women’s reproductive health and further reveal underlying molecular mechanisms.
Contributors
BKA and YW designed the review protocol, organized review terms, searched databases and analyzed the data. CCW revised the protocol and supervised the search. LLC and ZZY provided the initial idea based on animal experiments. LQ and YW obtained funding support. BKA, LLC, ZZY, and LQ synthesized the result and drafted the manuscript. CCW and YW supervised the result synthesis and data interpretation. All the authors accessed and verified the data, revised and approved the final version of the manuscript, and approved the final version of the manuscript.
Data sharing statement
Data in this systematic review and meta-analysis were extracted from published studies found in the mentioned databases. It is also accessible within the article and its Supplementary materials. The extracted data can be accessed upon reasonable request.
Declaration of generative AI and AI-assisted technologies in the writing process
During the revision period of this work, the author(s) used ChatGPT 4.0 to improve language and readability. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Declaration of interests
We declare no competing interests.
Acknowledgements
This work was funded by the Shaanxi Key Research and Development Program Project (2020SF-032), Xianyang Key Research and Development Program (2019k02-97), and The Hong Kong Obstetrical and Gynaecological Trust Fund. We would also like to thank Miss Siqi He from the University of Toronto, Canada, who provided comments and made edits for better readability of this manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102935.
Contributor Information
Qin Li, Email: liqin9800@sntcm.edu.cn.
Yao Wang, Email: yaowang1@cuhk.edu.hk.
Appendix ASupplementary data
References
- 1.World Health Organization . 2013. Preconception care: maximizing the gains for maternal and child health. [Google Scholar]
- 2.Dorney E., Black K.I. Preconception care. Austral J Gen Pract. 2018;47(7):424–429. doi: 10.31128/AJGP-02-18-4485. [DOI] [PubMed] [Google Scholar]
- 3.Benedetto C., Borella F., Divakar H., et al. FIGO Preconception Checklist: preconception care for mother and baby. Int J Gynecol Obstet. 2024;165(1):1–8. doi: 10.1002/ijgo.15446. [DOI] [PubMed] [Google Scholar]
- 4.Atrash H.K., Johnson K., Adams M., Cordero J.F., Howse J. Preconception care for improving perinatal outcomes: the time to act. Matern Child Health J. 2006;10:3–11. doi: 10.1007/s10995-006-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean S.V., Lassi Z.S., Imam A.M., Bhutta Z.A. Preconception care: closing the gap in the continuum of care to accelerate improvements in maternal, newborn and child health. Reprod Health. 2014;11:1–8. doi: 10.1186/1742-4755-11-S3-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowe H.M., Wesselink A.K., Wise L.A., et al. Antibiotics and fecundability among female pregnancy planners: a prospective cohort study. Hum Reprod. 2021;36(10):2761–2768. doi: 10.1093/humrep/deab173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira J.Q., Silva M.T., Galvão T.F. Use of antibiotics by adults: a population-based cross-sectional study. Sao Paulo Med J. 2018;136:407–413. doi: 10.1590/1516-3180.2018.0168060818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francino M.P. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6 doi: 10.3389/fmicb.2015.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan H., Yu L., Tian F., Zhai Q., Fan L., Chen W. Antibiotic-induced gut dysbiosis and barrier disruption and the potential protective strategies. Crit Rev Food Sci Nutr. 2022;62(6):1427–1452. doi: 10.1080/10408398.2020.1843396. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y., Wu Y., Zeng Z., et al. From the cover: exposure to oral antibiotics induces gut microbiota dysbiosis associated with lipid metabolism dysfunction and low-grade inflammation in mice. Toxicol Sci. 2016;154(1):140–152. doi: 10.1093/toxsci/kfw150. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q., Cheng L., Wang J., Hao M., Che H. Antibiotic-induced gut microbiota dysbiosis damages the intestinal barrier, increasing food allergy in adult mice. Nutrients. 2021;13(10):3315. doi: 10.3390/nu13103315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez de Tejada B. Antibiotic use and misuse during pregnancy and delivery: benefits and risks. Int J Environ Res Publ Health. 2014;11(8):7993–8009. doi: 10.3390/ijerph110807993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller J.E., Wu C., Pedersen L.H., de Klerk N., Olsen J., Burgner D.P. Maternal antibiotic exposure during pregnancy and hospitalization with infection in offspring: a population-based cohort study. Int J Epidemiol. 2018;47(2):561–571. doi: 10.1093/ije/dyx272. [DOI] [PubMed] [Google Scholar]
- 14.Andersson N.W., Olsen R.H., Andersen J.T. Association between use of macrolides in pregnancy and risk of major birth defects: nationwide, register based cohort study. BMJ. 2021;372 doi: 10.1136/bmj.n107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen M.H., Fornes R., Kamau N., et al. Antibiotic use during pregnancy and the risk of preterm birth: a population-based Swedish cohort study. J Antimicrob Chemother. 2022;77(5):1461–1467. doi: 10.1093/jac/dkac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muanda F.T., Sheehy O., Bérard A. Use of antibiotics during pregnancy and the risk of major congenital malformations: a population based cohort study. Br J Clin Pharmacol. 2017;83(11):2557–2571. doi: 10.1111/bcp.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alhasan M.M., Cait A.M., Heimesaat M.M., et al. Antibiotic use during pregnancy increases offspring asthma severity in a dose-dependent manner. Allergy. 2020;75(8):1979–1990. doi: 10.1111/all.14234. [DOI] [PubMed] [Google Scholar]
- 18.Bearak J., Popinchalk A., Ganatra B., et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8(9):e1152–e1161. doi: 10.1016/S2214-109X(20)30315-6. [DOI] [PubMed] [Google Scholar]
- 19.Finer L.B., Zolna M.R. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843–852. doi: 10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowe H.M., Hatch E.E., Wang T.R., et al. Periconceptional antibiotic use and spontaneous abortion: a prospective cohort study. Paediatr Perinat Epidemiol. 2023;37(3):179–187. doi: 10.1111/ppe.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikkelsen E.M., Ulrichsen S.P., Johannesen B.R., et al. Preconception use of antibiotics and fecundability: a Danish prospective cohort study. Fertil Steril. 2023;120(3 Pt 2):650–659. doi: 10.1016/j.fertnstert.2023.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Qi X., Yun C., Pang Y., Qiao J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microb. 2021;13(1) doi: 10.1080/19490976.2021.1894070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Oostrum N., De Sutter P., Meys J., Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum Reprod. 2013;28(7):1809–1815. doi: 10.1093/humrep/det096. [DOI] [PubMed] [Google Scholar]
- 24.Harrison R.F., Blades M., De Louvois J., Hurley R. Doxycycline treatment and human infertility. Lancet. 1975;305(7907):605–607. doi: 10.1016/s0140-6736(75)91885-1. [DOI] [PubMed] [Google Scholar]
- 25.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):1–11. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konishi S., Kariya F., Hamasaki K., Takayasu L., Ohtsuki H. Fecundability and sterility by age: estimates using time to pregnancy data of Japanese couples trying to conceive their first child with and without fertility treatment. Int J Environ Res Publ Health. 2021;18(10):5486. doi: 10.3390/ijerph18105486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health O. World Health Organization; 2013. International classification of diseases for oncology (ICD-O) [Google Scholar]
- 28.Shao S., Pan W., Wang B., et al. Association between antibiotic exposure and the risk of infertility in women of childbearing age: a case-control study. Ecotoxicol Environ Saf. 2023;249 doi: 10.1016/j.ecoenv.2022.114414. [DOI] [PubMed] [Google Scholar]
- 29.Luchini C., Stubbs B., Solmi M., Veronese N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World Journal of Meta-Analysis. 2017;5(4):80–84. [Google Scholar]
- 30.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y., Wu C.S., Olsen J. Trimethoprim use before pregnancy and risk of congenital malformation: reanalyzed using a case-crossover design and a case-time-control design. Pharmacoepidemiol Drug Saf. 2014;23(10):1076–1083. doi: 10.1002/pds.3691. [DOI] [PubMed] [Google Scholar]
- 32.Brook N., Khalaf Y., Coomarasamy A., Edgeworth J., Braude P. A randomized controlled trial of prophylactic antibiotics (co-amoxiclav) prior to embryo transfer. Hum Reprod. 2006;21(11):2911–2915. doi: 10.1093/humrep/del263. [DOI] [PubMed] [Google Scholar]
- 33.Schaumburg I., Olsen J. Time to pregnancy among Danish pharmacy assistants. Scand J Work Environ Health. 1989;15:222–226. doi: 10.5271/sjweh.1859. [DOI] [PubMed] [Google Scholar]
- 34.Grodstein F., Goldman M.B., Ryan L., Cramer D.W. Self-reported use of pharmaceuticals and primary ovulatory infertility. Epidemiology. 1993;4(2):151–156. doi: 10.1097/00001648-199303000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Peikrishvili R., Evrard B., Pouly J.L., Janny L. L’antibiothérapie prophylactique (amoxicilline+ acide clavulanique) avant transfert pour fécondation in vitro est inutile: résultats d’une étude randomisée. J Gynécol Obstétrique Biol Reproduct. 2004;33(8):713–719. doi: 10.1016/s0368-2315(04)96632-x. [DOI] [PubMed] [Google Scholar]
- 36.Cicinelli E., Matteo M., Trojano G., et al. Chronic endometritis in patients with unexplained infertility: prevalence and effects of antibiotic treatment on spontaneous conception. Am J Reprod Immunol. 2018;79(1) doi: 10.1111/aji.12782. [DOI] [PubMed] [Google Scholar]
- 37.Eskew A.M., Stout M.J., Bedrick B.S., et al. Association of the eukaryotic vaginal virome with prophylactic antibiotic exposure and reproductive outcomes in a subfertile population undergoing in vitro fertilisation: a prospective exploratory study. BJOG An Int J Obstet Gynaecol. 2020;127(2):208–216. doi: 10.1111/1471-0528.15951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan H., Li X., Hao Y., Shi J., Cai H. Risk of spontaneous abortion after antibiotic therapy for chronic endometritis before in vitro fertilization and intracytoplasmic sperm injection stimulation. Fertil Steril. 2022;118(2):337–346. doi: 10.1016/j.fertnstert.2022.04.026. [DOI] [PubMed] [Google Scholar]
- 39.van Gelder M., Lupattelli A., Nordeng H.M.E. Risk of spontaneous abortion after periconceptional medication use: time to tackle the methodological challenges. Paediatr Perinat Epidemiol. 2023;37:188–190. doi: 10.1111/ppe.12967. [DOI] [PubMed] [Google Scholar]
- 40.Andersen J.T., Petersen M., Jimenez-Solem E., et al. Trimethoprim use prior to pregnancy and the risk of congenital malformation: a register-based nationwide cohort study. Obst Gynecol Int. 2013;2013 doi: 10.1155/2013/364526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crowe H.M., Hatch E.E., Wang T.R., et al. Periconceptional antibiotic use and spontaneous abortion: a prospective cohort study. Paediatr Perinat Epidemiol. 2023;37(3):179–187. doi: 10.1111/ppe.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gestels T., Vandenplas Y. Prenatal and perinatal antibiotic exposure and long-term outcome. Pediatr Gastroenterol Hepatol Nutr. 2023;26(3):135. doi: 10.5223/pghn.2023.26.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan H., Li L., Wijlaars L., Gilbert R.E. Associations between use of macrolide antibiotics during pregnancy and adverse child outcomes: a systematic review and meta-analysis. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0212212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ianaro A., Ialenti A., Maffia P., et al. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000;292(1):156–163. [PubMed] [Google Scholar]
- 45.Ottonello L., Dapino P., Scirocco M.C., Balbi A., Bevilacqua M., Dallegri F. Sulphonamides as anti-inflammatory agents: old drugs for new therapeutic strategies in neutrophilic inflammation? Clin Sci (Lond) 1995;88(3):331–336. doi: 10.1042/cs0880331. [DOI] [PubMed] [Google Scholar]
- 46.Griffith O.W., Chavan A.R., Protopapas S., Maziarz J., Romero R., Wagner G.P. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci USA. 2017;114(32):E6566–E6575. doi: 10.1073/pnas.1701129114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durack J., Lynch S.V. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019;216(1):20–40. doi: 10.1084/jem.20180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choo J.M., Martin A.M., Taylor S.L., et al. The impact of long-term macrolide exposure on the gut microbiome and its implications for metabolic control. Microbiol Spectr. 2023;11(4) doi: 10.1128/spectrum.00831-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDonnell L., Gilkes A., Ashworth M., et al. Association between antibiotics and gut microbiome dysbiosis in children: systematic review and meta-analysis. Gut Microb. 2021;13(1) doi: 10.1080/19490976.2020.1870402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lathakumari R.H., Vajravelu L.K., Satheesan A., Ravi S., Thulukanam J. Antibiotics and the gut microbiome: understanding the impact on human health. Med Microecol. 2024;20 [Google Scholar]
- 51.Panda S., El khader I., Casellas F., et al. Short-term effect of antibiotics on human gut microbiota. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0095476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azpiroz M.A., Orguilia L., Palacio M.I., et al. Potential biomarkers of infertility associated with microbiome imbalances. Am J Reprod Immunol. 2021;86(4) doi: 10.1111/aji.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plottel C.S., Blaser M.J. Microbiome and malignancy. Cell Host Microbe. 2011;10(4):324–335. doi: 10.1016/j.chom.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pai A.H.-Y., Wang Y.-W., Lu P.-C., Wu H.-M., Xu J.-L., Huang H.-Y. Gut microbiome–estrobolome profile in reproductive-age women with endometriosis. Int J Mol Sci. 2023;24(22) doi: 10.3390/ijms242216301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rumi M.A.K., Singh P., Roby K.F., et al. Defining the role of estrogen receptor β in the regulation of female fertility. Endocrinology. 2017;158(7):2330–2343. doi: 10.1210/en.2016-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He Y., Wang Q., Li X., et al. Lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota. Food Funct. 2020;11(6):5192–5204. doi: 10.1039/c9fo02554e. [DOI] [PubMed] [Google Scholar]
- 57.Tracy Tranchitella N.D. 2023. The estrobolome: the bidirectional relationship between gut microbes and hormones. [Google Scholar]
- 58.McCurry M.D., D’Agostino G.D., Walsh J.T., et al. Gut bacteria convert glucocorticoids into progestins in the presence of hydrogen gas. Cell. 2024;187(12):2952–2968.e13. doi: 10.1016/j.cell.2024.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coombes Z., Yadav V., McCoubrey L.E., et al. Progestogens are metabolized by the gut microbiota: implications for colonic drug delivery. Pharmaceutics. 2020;12(8):760. doi: 10.3390/pharmaceutics12080760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao B., Zhao X., Liu X., et al. Imbalance of the gut microbiota may be associated with missed abortions: a perspective study from a general hospital of hunan province. J Immunol Res. 2021;2021(1) doi: 10.1155/2021/5571894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omranipoor A., Kashanian M., Dehghani M., Sadeghi M., Baradaran H.R. Association of antibiotics therapy during pregnancy with spontaneous miscarriage: a systematic review and meta-analysis. Arch Gynecol Obstet. 2020;302:5–22. doi: 10.1007/s00404-020-05569-4. [DOI] [PubMed] [Google Scholar]
- 62.Andersen J.T., Petersen M., Jimenez-Solem E., et al. Clarithromycin in early pregnancy and the risk of miscarriage and malformation: a register based nationwide cohort study. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0053327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu X., Shi Z., Jiang L., Zhang S. Maternal gut microbiota in the health of mothers and offspring: from the perspective of immunology. Front Immunol. 2024;15 doi: 10.3389/fimmu.2024.1362784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu J., Jin J., Qi Q., et al. The association of gut microbiome with recurrent pregnancy loss: a comprehensive review. Drug Discov Ther. 2023;17(3):157–169. doi: 10.5582/ddt.2023.01010. [DOI] [PubMed] [Google Scholar]
- 65.George L., Ramasamy T., Sirajudeen K.N.S., Manickam V. LPS-induced apoptosis is partially mediated by hydrogen sulphide in RAW 264.7 murine macrophages. Immunol Invest. 2019;48(5):451–465. doi: 10.1080/08820139.2019.1566355. [DOI] [PubMed] [Google Scholar]
- 66.Faas M.M., Liu Y., Wekema L., Weiss G.A., van Loo-Bouwman C.A., Silva Lagos L. The effect of antibiotics treatment on the maternal immune response and gut microbiome in pregnant and non-pregnant mice. Nutrients. 2023;15(12):2723. doi: 10.3390/nu15122723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y., Chen X., Chen P., et al. Alteration of the gut microbiota in missed abortion. Indian J Microbiol. 2023;63(1):106–119. doi: 10.1007/s12088-023-01063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.World Health O, Centers for Disease C, Prevention . 2020. Birth defects surveillance: quick reference handbook of selected congenital anomalies and infections. [Google Scholar]
- 69.Ovung A., Bhattacharyya J. Sulfonamide drugs: structure, antibacterial property, toxicity, and biophysical interactions. Biophys Rev. 2021;13(2):259–272. doi: 10.1007/s12551-021-00795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y., Huang Y., He Q., et al. From heart to gut: exploring the gut microbiome in congenital heart disease. Imeta. 2023;2(4) doi: 10.1002/imt2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang T., Chen L., Huang P., et al. Association of maternal gut microbiota and plasma metabolism with congenital heart disease in offspring: a multi-omic analysis. Sci Rep. 2021;11(1):5339. doi: 10.1038/s41598-021-84901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gholami H., Chmiel J.A., Burton J.P., Maleki Vareki S. The role of microbiota-derived vitamins in immune homeostasis and enhancing cancer immunotherapy. Cancers. 2023;15(4):1300. doi: 10.3390/cancers15041300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen X.-F., Chen X., Tang X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin Sci. 2020;134(6):657–676. doi: 10.1042/CS20200128. [DOI] [PubMed] [Google Scholar]
- 74.Nagy R.A., Homminga I., Jia C., et al. Trimethylamine-N-oxide is present in human follicular fluid and is a negative predictor of embryo quality. Hum Reprod. 2020;35(1):81–88. doi: 10.1093/humrep/dez224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schiattarella G.G., Sannino A., Toscano E., et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. 2017;38(39):2948–2956. doi: 10.1093/eurheartj/ehx342. [DOI] [PubMed] [Google Scholar]
- 76.Seelan R.S., Mukhopadhyay P., Philipose J., Greene R.M., Pisano M.M. Gestational folate deficiency alters embryonic gene expression and cell function. Differentiation. 2021;117:1–15. doi: 10.1016/j.diff.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zarrinpar A., Chaix A., Xu Z.Z., et al. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun. 2018;9(1):2872. doi: 10.1038/s41467-018-05336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.