Abstract
Introduction
In 1960, Lazorthes and Campman introduced the concept of a ‘crisis of the third day’, which gained prominence in the field of traumatic brain injury (TBI), where it relates to neurological deterioration on the third day after injury. However, evidence regarding this phenomenon remains scarce.
Research question
This study aimed to analyze posttraumatic intracranial pressure (ICP) patterns in a large European cohort to investigate the existence of a third-day crisis and its impact on 12-month functional outcomes.
Materials and methods
Data were analyzed from the prospective Collaborative European Neurotrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. Patients with TBI admitted to ICUs in 65 European centers who received ICP monitoring were included. ICP measurements, averaged per day, were analyzed using mixed models. The association between ICP peak timing and functional outcome was examined with multivariable logistic regression.
Results
The study included 886 patients. Average ICP trajectories showed no significant changes over the first seven days post-injury, without elevation around the third day. Among 563 patients with ICP >20 during the first week, 45% reached their highest ICP after the third day. Elevated ICP (>20 mmHg) during the first week was associated with unfavorable 12-month outcomes, but the timing of ICP peak was not linked to functional outcomes.
Discussion and conclusion
This multicenter study challenges the ‘crisis of the third day’ concept. No distinct ICP or TIL elevations were observed around the third day. Elevated ICP remains a prognostic indicator, but ICP peak timing does not correlate with functional outcomes.
Keywords: Traumatic brain injury, Intracranial pressure, Therapy intensity level, Neurosurgery
Highlights
-
•
Average ICP and TIL values did not show significant changes or peaks around the third day post-TBI.
-
•
The timing of peak ICP was not linked to functional outcomes at 12 months.
-
•
A substantial share of patients had their ICP peak level after the third day, stressing the importance of continued monitoring.
1. Introduction
Traumatic brain injury (TBI) poses a profound global health challenge, contributing to both mortality and morbidity worldwide (Maas et al., 2017, 2022). The distinction between primary and secondary injury is central in understanding the disease process. Primary injury immediately occurs upon impact and is irreversible. Secondary injury develops through a complex interplay of biochemical, cellular, and pathophysiological processes, culminating among others in cerebral edema and raised intracranial pressure – a target point for medical and surgical interventions to optimize patient outcomes (Maas et al., 2022).
In 1960, Lazorthes and Campman described their observation of ‘a collection of signs which are revealed in a deterioration in the condition of the post-operative patient’ that seemed to occur around the third day after cranial surgery (Lazorthes and Campan, 1960). Terming this phenomenon ‘the crisis of the third day’, they attributed it to ‘pure reactive phenomena; most often due to cerebral edema which has developed at the operation site … ’.
Since then, the concept of a the third-day crisis has permeated neurosurgical practice, particularly within the context of TBI, where it denotes neurological deterioration on the third day after injury. This neurological deterioration is considered indicative of raised intracranial pressure (ICP) caused by, for instance, a delayed or increasing intracranial hematoma, diffuse edema, contusion expansion, cerebral ischemia or a combination of factors (Johnston et al., 1970; van Essen et al., 2022). In addition to neurological examinations to examine the patients’ condition, raised ICP can also be gauged through surgically inserted ICP monitors (Nattino et al., 2023). Current European consensus mandates intervention when ICP surpasses the 20 mmHg threshold and the Brain Trauma Foundation guidelines advise to initiate treatment when ICP exceeds 22 mmHg (Cnossen et al., 2017; Carney et al., 2017). However, treatment variation across centers exists and the BTF guidelines acknowledge insufficient evidence to support a level I or IIA recommendation (Cnossen et al., 2017; Carney et al., 2017).
In clinical practice, the third day after TBI is sometimes considered a critical period of maximum swelling and elevated ICP. However, the acceptance and clinical application of this notion may vary across countries and regions. A recent consensus statement indicated growing acceptance of removing ICP monitors after three days of acceptable ICP levels (Hawryluk et al., 2019). Also, multiple large trials have confined their ICP analyses to the first 72 h following injury (Maas et al., 2006; Knoller et al., 2002). Although ICP monitoring for clinical and research purposes should not be confused, habits in both fields may stem from the incorporation of the third-day crisis phenomenon into – at least part of – the neurosurgical community. Interestingly, the available literature on the topic is remarkably scarce. Only few studies have examined the course of posttraumatic ICP, with no subsequent investigation into the ‘crisis of the third day’ as described in the original French article (Güiza et al., 2015; Vik et al., 2008; Ai Åkerlund et al., 2020).
The primary aim of this study was to analyze the temporal course of posttraumatic ICP in a large European patient cohort and investigate the existence of the crisis of the third day after TBI. Secondly, different temporal patterns were linked to patient characteristics and functional outcome at 12 months after injury.
2. Materials and Methods
2.1. Study population
Data from the Collaborative European Neurotrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study were analyzed. CENTER-TBI was a prospective observational cohort study including patients presenting to one of 65 participating level-1 trauma centers across Europe and Israel between 2014 and 2018 who sustained TBI and had an indication for brain computed tomography (CT) scan (Maas et al., 2015). Patients with pre-existing cognitive impairments such as dementia, severely injured patients who died in the emergency room prior to imaging, and patients presenting more than 24 h after injury were excluded from CENTER-TBI (Steyerberg et al., 2019). For the current study, consecutive patients admitted to the intensive care unit (ICU) who received an ICP monitoring device – either an intraparenchymal monitor or an external ventricular drain – were included. CENTER-TBI was conducted in accordance with Good Clinical Practice (CPMP/ICH/135/95). Informed written or oral consent by patients or legal representatives was obtained according to local legislation. Ethical approval for the CENTER-TBI study was obtained from the medical ethics committees of all participating centers.
2.2. Data collection
Patient variables were collected by local clinical research teams who were notified upon arrival of potentially eligible patients at the emergency department. Data were collected on pre-specified timepoints and derived from the clinical chart after patients were admitted to the ICU. When patients received an ICP monitor, intracranial pressure was measured twice hourly on the ICU. While most participating centers used millimeters of mercury (mmHg) as their unit of measurement, two centers utilized mostly ventricular drains measuring centimeters of water (cmH2O). Central data curation converted all values to mmHg. The intensity of ICP targeted treatments was quantified as the Therapy Intensity Level (TIL) and was calculated daily as the sum of 38 different possible ICP-targeting therapies measured every 4 h (Maas et al., 2011; Zuercher et al., 2016; Maset et al., 1987). These therapies included patient (head) positioning, sedation and neuromuscular blockade, cerebral spinal fluid drainage, cerebral perfusion pressure management, ventilatory management, hyperosmolar therapy, temperature control, and surgery for intracranial hypertension, all designed to be provided in an escalating staircase pattern. Gathered data also included demographics (age and sex), pre-existing health status, severity of TBI expressed by the Glasgow Coma Scale (GCS), CT-scan characteristics, (timing of) neurosurgical interventions, duration of ICP monitoring, and 12-month functional outcome as expressed by the Extended Glasgow Outcome Score (GOSE). Data were entered into a web-based electronic case report form (Quesgen Systems Inc, Burlingame, USA) in an anonymized format. Data were hosted on the International Neuroinformatics Coordinating Facility (INCF) platform and extracted by a bespoke data management tool, Neurobot (RRID: SCR_01700) (INCF, Sweden). The core dataset CENTER-TBI version 3.0 was used for this study.
2.3. Statistical analysis
Descriptive statistics on data for continuous variables were presented as means with standard deviations (SDs) or medians with interquartile ranges (IQRs), depending on the normality of the distribution and the presence of outliers. Categorical data were presented as counts and frequencies. Percentages were rounded to the nearest integer. Standard statistical tests were used to compare means (T-tests) and medians (Mann-Whitney U or Kruksal Wallis tests). The temporal profiles of ICP and TIL over the 7-day period after TBI were analyzed and compared between subgroups of patients using linear mixed models with a random effect for participating center. ICP and TIL data were averaged each day after injury and analyzed accordingly. Individual average values per day for ICP and TIL were displayed using Lasagna plots (Swihart et al., 2010). The maximum value of average ICP per day was graphically cut off at 20 mmHg to enhance clarity and to reflect the clinically relevant threshold where treatment is usually initialized. The first day on which the maximum average ICP and TIL were reached was determined for all patients. For patients who sustained an episode of ICP >20 mmHg, the day on which the highest actual ICP occurred was calculated. Furthermore, the duration of consecutive ICP elevation was calculated, along with the total time of elevated ICP during the first week, regardless of whether the elevations were consecutive. Given the two-hourly measurements, intervals of raised ICP were rounded to the nearest hour. The association between ICP peak time and functional outcome at 12-months was analyzed using multivariable logistic regression with covariate adjustment for age, baseline GCS, pupillary reactivity, the day of maximum TIL, presence of extracranial injury requiring surgery and the occurrence of emergency intracranial surgery. All analyses were performed in R (version 4.0.3) or SPSS (version 29.0.2.0). A p-value ≤0.05 (two-sided) was considered statistically significant.
3. Results
3.1. Included patients and baseline characteristics
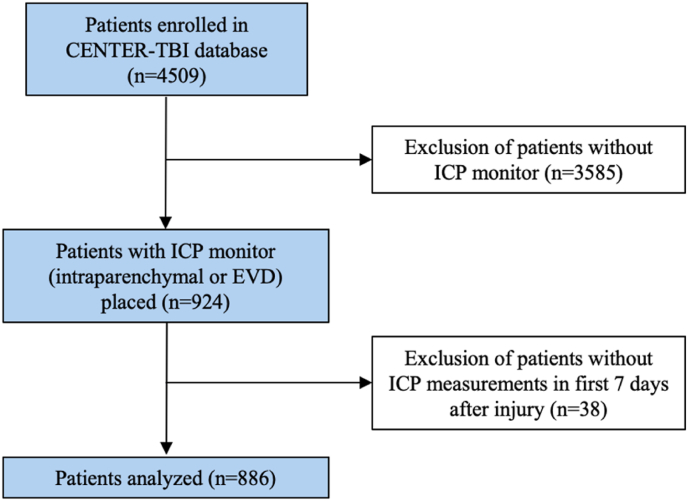
A total of 4509 patients were included in the CENTER-TBI database, of which 886 patients were included in this study (Fig. 1). Included patients were predominantly male (n = 653 [74%]) and had a median age of 47 years (IQR 28–62) (Table 1). Most patients were healthy before the injury (n = 509 [57%]) or had minor comorbidities (n = 252 [28%]). Anticoagulants (n = 37 [4%]), antiplatelets (n = 67 [8%]) or both (n = 4 [0.4%]) were used in a minority of patients. The majority of patients presented with severe TBI (n = 568 [64%]) while the other patients had sustained moderate (n = 179 [20%]) or mild (83 [9%]) TBI. A total of 613 (69%) patients had both pupils reacting to light at time of presentation, 79 (9%) patients had one light-reactive pupil and 142 (16%) patients had no light-reactive pupil. The first CT-scan showed a large acute subdural hematoma (ASDH) in 214 (24%) patients, a large epidural hematoma (EDH) in 53 (6%) patients, a large contusion in 136 (15%) patients, cortical subarachnoid hemorrhage in 373 (42%) patients, midline shift in 337 (38%) patients and a depressed skull fracture in 183 (21%) of patients.
Fig. 1.
Flowchart of included patients.
Abbreviations: CENTER-TBI, Collaborative European Neurotrauma Effectiveness Research in TBI; EVD, external ventricular drain; ICP, intracranial pressure; TBI, traumatic brain injury.
Table 1.
Baseline characteristics.
| Baseline characteristics (n = 886) | |
|---|---|
| Age (median (IQR)) | 47 (28–62) |
| Age group (n (%)) | |
| Pediatric (≤17) | 48 (5) |
| Adult (18–64) | 648 (73) |
| Older people (≥65) | 190 (21) |
| Sex (n (%)) | |
| Female | 233 (26) |
| Male | 653 (74) |
| ASA classification (n (%)) | |
| I – healthy | 509 (57) |
| II – mild systemic disease | 252 (28) |
| III – severe systemic disease | 78 (9) |
| IIII – severe systemic disease with constant threat to life | 4 (0) |
| Unknown/missing | 43 (5) |
| Anticoagulant or antiplatelet use | |
| No | 722 (82) |
| Yes, anticoagulants | 37 (4) |
| Yes, platelet aggregation inhibitors | 67 (8) |
| Yes, both | 4 (0) |
| Unknown/missing | 56 (6) |
| TBI severity (n (%)) | |
| Mild TBI (GCS 14–15) | 83 (9) |
| Moderate TBI (GCS 9–13) | 179 (20) |
| Severe TBI (GCS 3–8) | 568 (64) |
| Pupillary reaction (n (%)) | |
| Both reacting | 613 (69) |
| One reacting | 79 (9) |
| Both non-reacting | 142 (16) |
| Unknown/missing | 52 (6) |
| First CT-scan characteristics | |
| Acute subdural hematoma | |
| No | 307 (35) |
| Small | 307 (35) |
| Large | 214 (24) |
| Unknown/missing | 58 (7) |
| Epidural hematoma | |
| No | 678 (77) |
| Small | 95 (11) |
| Large | 53 (6) |
| Unknown/missing | 60 (7) |
| Contusion | |
| No | 232 (26) |
| Small | 451 (51) |
| Large | 136 (15) |
| Unknown/missing | 67 (8) |
| Subarachnoid hemorrhage | |
| No | 222 (25) |
| Basal | 82 (9) |
| Cortical | 373 (42) |
| Basal and cortical | 155 (18) |
| Unknown/missing | 54 (6) |
| Depressed skull fracture | |
| No | 648 (73) |
| Closed | 136 (15) |
| Open (compound) | 47 (5) |
| Unknown/missing | 55 (6) |
| Midline shift | |
| No | 493 (56) |
| Yes | 337 (38) |
| Unknown/missing | 56 (6 |
| Marshall classification of first CT-scan | |
| I - No visible pathology, cisterns present, MLS <5 mm | 18 (2) |
| II - Cisterns present, MLS <5 mm | 277 (31) |
| III - Cisterns compressed or absent, MLS <5 mm | 98 (11) |
| IV - MLS >5 mm, no mass lesion >25 cc | 18 (2) |
| V - Evacuated mass lesion | 1 (0) |
| VI - Non-evacuated mass lesion | 340 (38) |
| Unknown/missing | 134 (15) |
Abbreviations: ASA, American Society of Anaesthesiologist; CT, computed tomography; GCS, Glasgow Coma Scale; MLS, midline shift; SD, standard deviation; TBI, traumatic brain injury.
3.2. Monitoring characteristics
The median time from injury to ICP monitor placement was 7 h [IQR 5–12] (Table 2). An intraparenchymal monitor was used in 717 (81%) patients while 20 (2%) and 102 (12%) patients received a ventricular drain with or without inbuilt sensor, respectively. The most common reasons for monitoring ICP were adherence to the guideline criteria (n = 300 [34%]), radiological signs of raised ICP (n = 234 [26%]) and clinical suspicion of raised ICP (n = 258 [29%]). During ICU stay, raised ICP was present in 437 (49%) patients and was refractory to treatment in 125 (14%) patients. The median duration of ICP monitoring was 6 days [IQR 3–11]. The main reported reasons for removing the ICP monitor were a stable ICP <20 mmHg (n = 437 [49%]) and an improved clinical condition (n = 156 [18%]).
Table 2.
ICP monitoring characteristics.
| ICP monitoring characteristics | |
|---|---|
| Timing of ICP monitor insertion after injury (hours) (median [IQR]) | 7 [5–12] |
| Timing of ICP monitor insertion after injury (n (%)) | |
| <1 h | 11 (1) |
| 1–3 h | 48 (5) |
| 3–6 h | 273 (31) |
| 6–12 h | 331 (37) |
| 12–24 h | 145 (16) |
| >24 h | 66 (7) |
| Unknown/missing | 12 (1) |
| Type of ICP monitor (n (%)) | |
| Ventricular | 102 (12) |
| Ventricular + inbuilt sensor | 20 (2) |
| Intraparenchymal | 717 (81) |
| Unknown/missing | 45 (5) |
| Reason for ICP monitoring on ICU (n (%)) | |
| Guideline criteria | 300 (34) |
| Radiological signs of raised ICP | 234 (26) |
| Clinical suspicion of raised ICP | 258 (29) |
| Anesthesia or mechanical ventilation required for extracranial injuries | 38 (4) |
| To inform surgical indication for mass lesion | 29 (3) |
| Unknown/missing | 26 (3) |
| Duration of ICP monitoring on ICU (days) (median [IQR]) | 6 [3–11] |
| Duration of ICP monitoring on ICU (n (%)) | |
| <1 day | 37 (4) |
| 1–4 days | 184 (21) |
| 4–7 days | 182 (21) |
| 7–10 days | 113 (13) |
| 10–14 days | 91 (10) |
| >14 days | 101 (11) |
| Unknown/missing | 178 (20) |
| Raised ICP on ICU (n (%)) | |
| No | 447 (51) |
| Yes, controlled | 312 (35) |
| Yes, refractory | 125 (14) |
| Unknown/missing | 2 (0) |
| Highest actual ICP for all patients (median [IQR]) | 24 (18–32) |
| First day of maximum average ICP for all patients | |
| Day 0, n (%) [cumulative %] | 109 (12) [12] |
| Day 1, n (%) [cumulative %] | 201 (23) [35] |
| Day 2, n (%) [cumulative %] | 177 (20) [55] |
| Day 3, n (%) [cumulative %] | 89 (10) [65] |
| Day 4, n (%) [cumulative %] | 79 (9) [74] |
| Day 5, n (%) [cumulative %] | 73 (8) [82] |
| Day 6, n (%) [cumulative %] | 71 (8) [90] |
| Day 7, n (%) [cumulative %] | 24 (3) [93] |
| Unknown/missing, n (%) | 63 (7) [100] |
| Highest actual ICP for patients with elevated ICP (median [IQR]) | 29 (24–39) |
| Duration (hours) of longest consecutive ICP elevation (median [IQR]) | 3 (1–7) |
| Duration (hours) of total ICP elevation | 7 (3–21) |
| First day of maximum average ICP for patients with elevated ICP | |
| Day 0, n (%) [cumulative %] | 49 (9) [9] |
| Day 1, n (%) [cumulative %] | 114 (20) [29] |
| Day 2, n (%) [cumulative %] | 76 (14) [43] |
| Day 3, n (%) [cumulative %] | 73 (13) [55] |
| Day 4, n (%) [cumulative %] | 75 (13) [69] |
| Day 5, n (%) [cumulative %] | 65 (12) [80] |
| Day 6, n (%) [cumulative %] | 81 (14) [95] |
| Day 7, n (%) [cumulative %] | 30 (5) [100] |
| First day of maximum average ICP for patients with refractory ICP elevation | |
| Day 0, n (%) [cumulative %] | 21 (19) [19] |
| Day 1, n (%) [cumulative %] | 28 (25) [43] |
| Day 2, n (%) [cumulative %] | 19 (17) [60] |
| Day 3, n (%) [cumulative %] | 11 (10) [70] |
| Day 4, n (%) [cumulative %] | 8 (7) [77] |
| Day 5, n (%) [cumulative %] | 9 (8) [85] |
| Day 6, n (%) [cumulative %] | 11 (10) [95] |
| Day 7, n (%) [cumulative %] | 6 (5) [100] |
| First day of average ICP >20 mmHg | |
| Day 0, n (%) [cumulative %] | 51 (28) [28] |
| Day 1, n (%) [cumulative %] | 48 (26) [54] |
| Day 2, n (%) [cumulative %] | 29 (16) [69] |
| Day 3, n (%) [cumulative %] | 9 (5) [74] |
| Day 4, n (%) [cumulative %] | 16 (9) [83] |
| Day 5, n (%) [cumulative %] | 10 (5) [88] |
| Day 6, n (%) [cumulative %] | 16 (9) [97] |
| Day 7, n (%) [cumulative %] | 6 (3) [100] |
| Reason for stop ICP monitoring, (n (%)) | |
| Clinically improved | 156 (18) |
| ICP stable and <20 mmHg | 437 (49) |
| Monitor/catheter failure | 27 (3) |
| Patient considered unsalvageable | 47 (5) |
| Patient died | 30 (3) |
| Other/unknown | 162 (18) |
Abbreviations:ICP, intacranial pressure; ICU, intensive care unit; IQR, interquartile range; TIL, therapy intensity level.
3.3. Time course of ICP
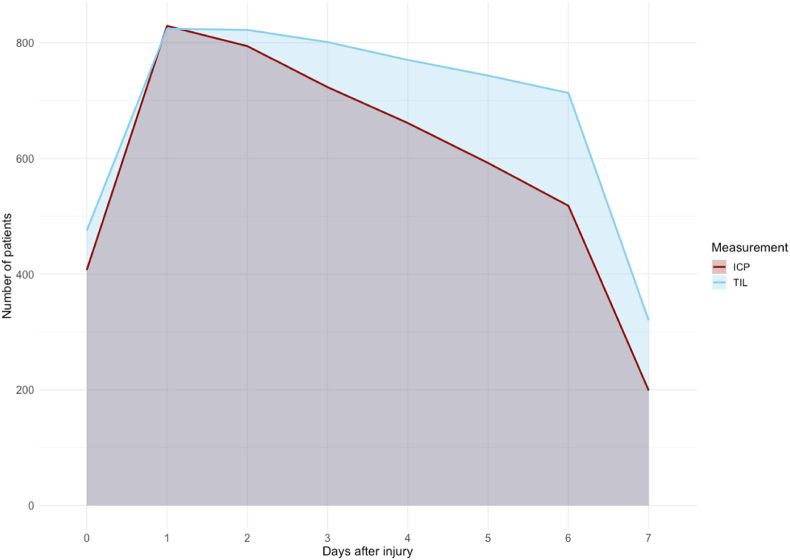
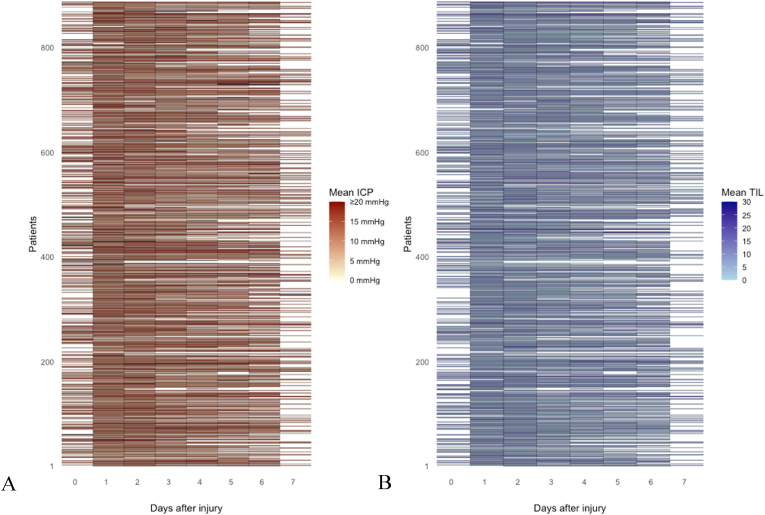
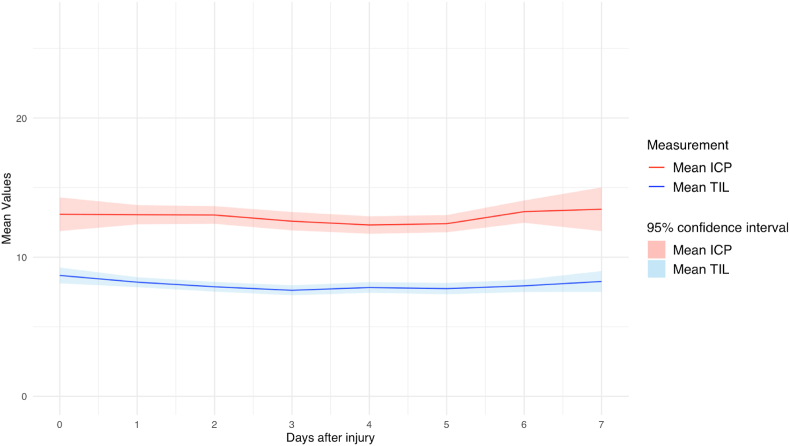
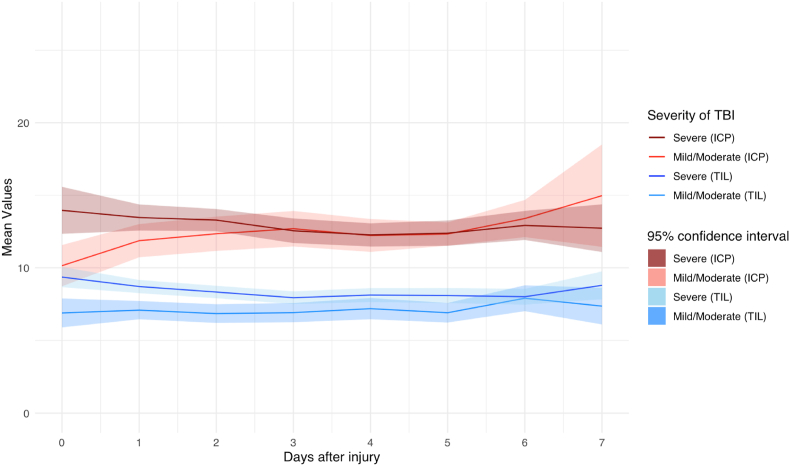
A total of 131.118 h of ICP data were recorded. The number of valid measurements for ICP was highest on day one after the injury (n = 829 [94%]) and gradually decreased to 199 (22%]) on day 7 (Fig. 2, Fig. 3A). Average ICP values varied between 12 and 13 during the first seven days post-injury, without a spike around day three (Fig. 4). Stratification by injury severity showed similar results (Fig. 5). For patients with severe TBI, average ICP was only significantly higher on the day of injury compared to patients with mild or moderate TBI (14 [SD 14] versus 10 [SD 7]), p < 0.01).
Fig. 2.
Number of valid ICP and TIL measurements over time.
Fig. 3.
Lasagna plot of mean ICP (A) and mean TIL (B) per day since TBI.
Fig. 4.
average ICP and TIL over time for all patients.
Fig. 5.
Average ICP and TIL over time stratified on injury severity.
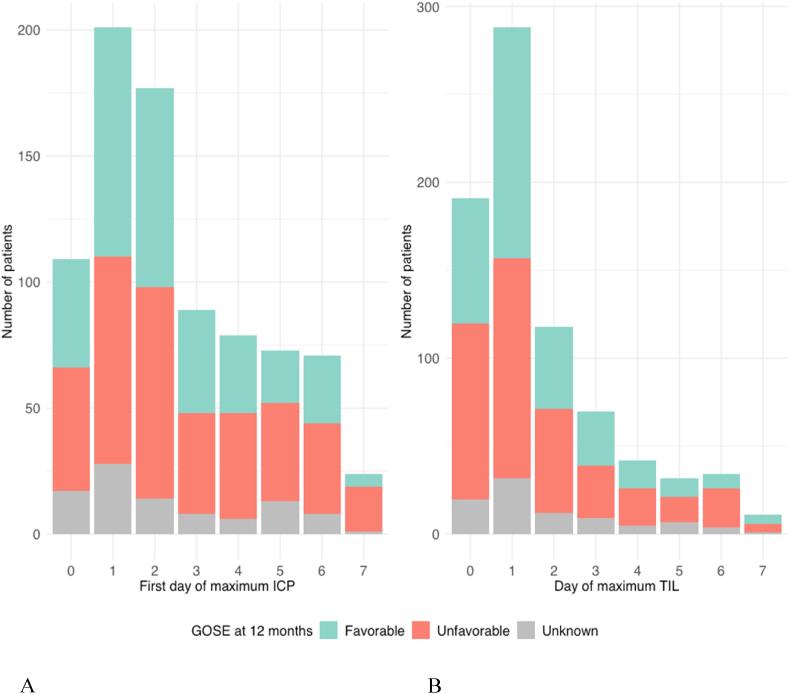
Most patients reached their maximum average ICP on day one after injury (201 [23%]), followed by day two after injury (177 [20%]) and the day of injury (day zero) (109 [12%]) (Table 2). During the first two days (zero and one) after injury, 35% (n = 310) of patients reached their maximum ICP, and another 30% (n = 266) during the next two days (two and three). After day three, 65% (n = 576) of patients had experienced their highest ICP value, indicating that the remaining 35% had their ICP peak at a later time (Fig. 6A).
Fig. 6.
Peak time of average ICP (A) and TIL (B) versus GOSE score at 12 months.
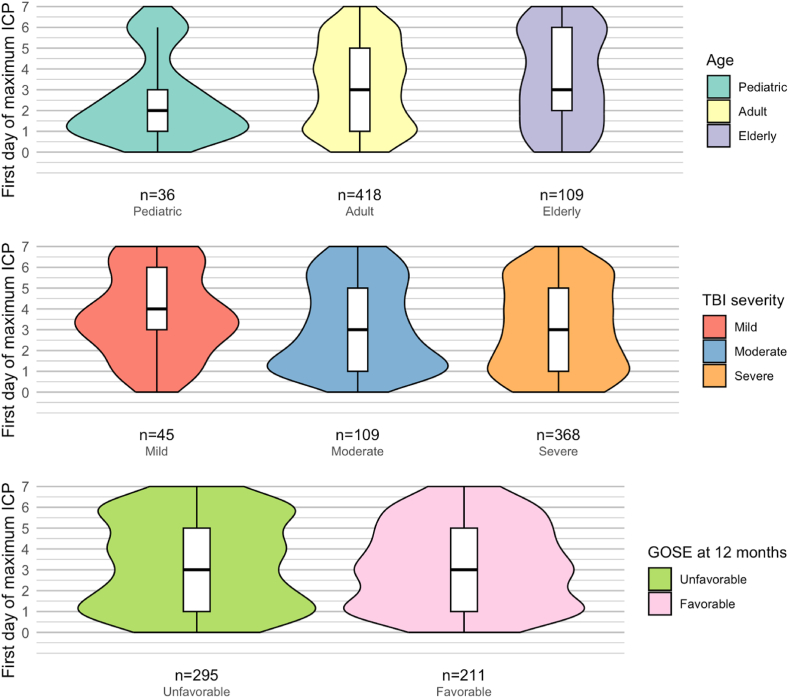
The median highest actual ICP was 24 [IQR 18–32] for all patients. For the 563 patients who experienced an ICP>20 mmHg at some point during the first week post-injury, the median highest ICP was 29 [IQR 24–39]. Of these patients, 163 (29%) patients reached their first highest ICP during the first two days and 149 (26%) patients during the next two days. After day three, 312 (55%) of patients had suffered their highest ICP. The median duration of the longest episode of elevated ICP was 3 h (IQR 1–7) and the median total duration of all episodes of raised ICP – whether consecutive or not – during the first week was 7 h (IQR 3–21). Within the raised ICP group, the median first day of the highest ICP was earlier for pediatric patients (2 [IQR1-3]) compared to adults or elderly (3 [IQR 1–5]) (p = 0.04). No significant difference in median ICP peak timing was found between levels of injury severity (mild 4 [IQR 2.5–6], moderate 3 [1–5], severe 3 [1–5]. (p = 0.15). Similarly, the median first day of highest ICP was not different between patients with unfavorable (3 [IQR 1–5]) or favorable outcome (3 [IQR 1–5) at 12 months after injury (p = 0.58).
A total of 185 patients experienced an average ICP >20 mmHg at some day during the first week post-injury. The first occurrence of an average daily ICP >20 mmHg was mostly early after trauma on day 0 (51 [28%]), day 1 (48 [26%]) or day 2 (29 [16%]) while 9 patients (5%) experienced their first average ICP>20 mmHg at day 3. In 48 (26%) patients, the first day of average ICP >20 mmHg occurred after day 3.
3.4. TIL and treatment characteristics
The number of valid TIL measures declined from 824 [93%] on day 1–320 [36%] on day seven (Fig. 2, Fig. 3B). Average TIL was highest (9) on the day of injury and remained stable around eight over the course of the next seven days, without a spike around the third day (Fig. 4). Like ICP, the first day of maximum TIL was day one post-injury in 303 (34%) patients, day two post-injury in 123 (14%) patients and the day of injury (day zero) in 199 (23%) patients (Table 3). For patients who sustained an ICP >20 during the first week after TBI, the first day of maximum TIL was also day one post-injury in 170 (30%) patients, followed by day zero in 120 (21%) patients and day two in 80 (14%) of patients.
Table 3.
Treatment and functional outcome.
| Treatment and functional outcome | |
|---|---|
| First day of maximum TIL for all patients | |
| Day 0, n (%) [cumulative %] | 199 (23) [23] |
| Day 1, n (%) [cumulative %] | 303 (34) [57] |
| Day 2, n (%) [cumulative %] | 123 (14) [71] |
| Day 3, n (%) [cumulative %] | 74 (8) [79] |
| Day 4, n (%) [cumulative %] | 43 (5) [84] |
| Day 5, n (%) [cumulative %] | 40 (5) [89] |
| Day 6, n (%) [cumulative %] | 45 (5) [94] |
| Day 7, n (%) [cumulative %] | 12 (1) [95] |
| Unknown/missing, n (%) | 47 (5) [100] |
| First day of maximum TIL for patients with elevated ICP | |
| Day 0, n (%) [cumulative %] | 120 (21) [21] |
| Day 1, n (%) [cumulative %] | 170 (30) [52] |
| Day 2, n (%) [cumulative %] | 80 (14) [66] |
| Day 3, n (%) [cumulative %] | 46 (8) [74] |
| Day 4, n (%) [cumulative %] | 32 (6) [80] |
| Day 5, n (%) [cumulative %] | 36 (6) [86] |
| Day 6, n (%) [cumulative %] | 35 (6) [92] |
| Day 7, n (%) [cumulative %] | 11 (2) [94] |
| Unknown/missing, n (%) | 33 (6) [100] |
| Third line medical therapy for lowering ICP (n (%)) | |
| Intensive hypocapnia | 64 (7%) |
| Hypothermia <35 Celsius | 98 (11%) |
| Metabolic suppression | 305 (34%) |
| Emergency intracranial surgerya (n (%)) | |
| No | 570 (64) |
| Yes | 308 (35) |
| Unknown/missing | 8 (1) |
| Type of emergency intracranial surgery (n (%)) | |
| Craniotomy for hematoma/contusion | 164 (53) |
| Decompressive craniectomy | 94 (31) |
| Depressed skull fracture | 15 (5) |
| Other intracranial procedure | 34 (11) |
| Unknown/missing | 0 (0) |
| Intracranial surgery for progressive mass lesion (n (%)) | |
| Day 0 | 52 (33) |
| Day 1 | 61 (39) |
| Day 2 | 17 (11) |
| Day 3 | 5 (3) |
| Day 4 | 9 (6) |
| Day 5 | 5 (3) |
| Day 6 | 5 (3) |
| Day 7 | 3 (2) |
| Emergency extracranial surgery (n (%)) | |
| No | 745 (84) |
| Yes | 135 (15) |
| Unknown/missing | 6 (1) |
| Type of emergency extracranial surgery (n (%)) | |
| Damage control thoracotomy | 14 (2) |
| Damage control laparotomy | 13 (2) |
| Extraperitoneal pelvic packing | 3 (0) |
| External limb fixation | 37 (4) |
| Cranio-maxillo-facial reconstruction | 10 (1) |
| Other extracranial procedure | 58 (7) |
| Unknown/missing | 0 (0) |
| Status at ICU discharge | |
| Dead, n (%) | 151 (17) |
| Alive, n (%) | 727 (82) |
| Unknown/missing, n (%) | 8 (1) |
| GOS-E at 12 months | |
| Upper good recovery, n (%) | 97 (11) |
| Lower good recovery, n (%) | 59 (7) |
| Upper moderate disability, n (%) | 113 (13) |
| Lower moderate disability, n (%) | 89 (10) |
| Upper severe disability, n (%) | 49 (6) |
| Vegetative state or lower severe disability, n (%) | 152 (17) |
| Dead, n (%) | 225 (25) |
| Unknown/missing, n (%) | 102 (12) |
Abbreviations: GOS-E, Glasgow Outcome Scale Extended; ICP, intracranial pressure; ICU, intensive care unit.
Defined as directly scheduled for an intracranial operation on presentation to the emergency room.
The difference in TIL between severe and moderate or mild TBI patients was largest at the day of injury (9 [SD 5] versus 6 [SD 5], p < 0.01) but remained significant until day six after injury (all p < 0.05) (Fig. 5). The most aggressive medical treatments to lower ICP were metabolic suppression with high dose barbiturates or propofol (305 [34%]), hypothermia below 35 °C (98 [11%]) and intensive hyperventilation (hypocapnia PaCO2 <4.0 kPa (30 mmHg)) (64 [7%]).
An emergency neurosurgical procedure was performed immediately after hospital admission in 35% (n = 308) of patients and mostly consisted of a craniotomy for a hematoma or contusion (n = 164 [53%]) or a decompressive craniectomy (n = 94 [31%]) (Table 3). A total of 157 (18%) patients underwent a neurosurgical procedure for progressive mass lesion during the first seven days after injury that was not scheduled at admission. Most of these delayed intracranial surgeries took place on the day of injury (n = 52 [33%]) or the first day post-injury (n = 61 [39%]) and gradually decreased after that without a spike on the third day. Emergency extracranial surgery occurred in 135 (15%) patients and consisted of, among others, external limb fixation (n = 37 [4%]), damage control thoracotomy (n = 14 [2%]), damage control laparotomy (n = 13 [2%]), extraperitoneal pelvic packing (n = 3 [0%]) and cranio-maxillo-facial reconstruction (n = 10 [1%]).
3.5. Functional outcome
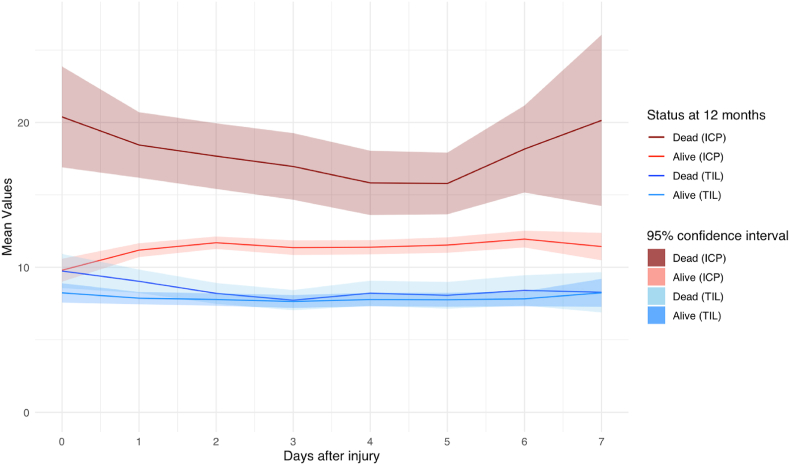
Patients who died after injury had significantly higher average ICP values during the entire zero to seven-day period after TBI compared to patients who were still alive at 12 months (p < 0.01) (Fig. 7). Comparing 12-month survivors with non-survivors, TIL values were only significantly different on day 0 (10 [SD 6] versus 8 [SD 5], p=0.03) and day 1 after injury (9 [SD 6] versus 8 [SD 5], p=0.01).
Fig. 7.
Average ICP and TIL over time stratified on status at 12 months.
Patients who sustained an ICP >20 at any timepoint during the zero to seven-day period post-injury had a lower likelihood of survival (OR 0.4 [CI 0.2–0.5]) or favorable outcome (OR 0.6 [0.5–0.9]) outcome at 12 months, which remained present after covariate adjustment (Table 4). Patients who sustained refractory ICP elevation had an even lower likelihood of survival (OR 0.3 [CI 0.1–0.5]) and favorable outcome (OR 0.3 [CI 0.2–0.6]).
Table 4.
Likelihood of 12-month survival and favorable outcome based on ICP peak times.
| Unadjusted odds ratio (OR) (95%CI) | Adjusted odds ratio (OR)b (95%CI) | |
|---|---|---|
| 12-month survival | ||
| First day of maximum average ICP (0–7) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) |
| Early (day 0–1) intermediate (day 2–3) or late (day 4–7) ICP peak | 1.0 (0.8–1.2) | 1.1 (0.9–1.5) |
| Very early (day 0) ICP peak versus later peak | 0.5 (0.3–0.8)a | 0.6 (0.3–1.1) |
| Very late (day 7) ICP peak versus earlier peak | 0.4 (0.2–1.0)a | 0.4 (0.2–1.1) |
| Average ICP peak on third day versus other peak moments | 0.9 (0.5–1.4) | 1.0 (0.5–1.9) |
| ICP >20 at any timepoint | 0.4 (0.2–0.5)a | 0.3 (0.2–0.4)a |
| 12-month favorable outcome | ||
| First day of maximum average ICP (0–7) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) |
| Early (day 0–1) intermediate (day 2–3) or late (day 4–7) ICP peak | 0.8 (0.7–0.9)a | 0.9 (0.7–1.1) |
| Very early (day 0) ICP peak versus later peak | 1.0 (0.7–1.6) | 1.4 (0.8–2.4) |
| Very late (day 7) ICP peak versus earlier peak | 0.3 (0.1–0.8)a | 0.3 (0.1–1.0) |
| Average ICP peak on third day versus other peak moments | 1.2 (0.8–1.9) | 1.2 (0.7–2.1) |
| ICP >20 at any timepoint | 0.6 (0.5–0.9)a | 0.5 (0.4–0.8)a |
Abbreviations: ICP, intracranial pressure; OR, odds ratio.
Statistically significant (p ≤ 0.05).
The odds ratio was adjusted for age, baseline GCS, pupillary reactivity, the day of maximum TIL, the occurrence of emergency intracranial surgery and polytrauma requiring extracranial surgery.
The timing of the highest value of ICP in the zero to seven-day period showed no association with mortality or unfavorable outcome. Sensitivity analyses categorizing ICP as (very) early (day 0–1), intermediate (day 2–3) or (very) late (day 4–7), and examining peak time as a continuous measure, underscored the robustness of these findings, showing no overall effect of ICP peak timing on 12-month outcome (Table 4). Additionally, the timing of ICP peaks did not significantly differ among patients based on their GOSE score at 12 months (p=0.33) (Fig. 6, Fig. 8).
Fig. 8.
Medians and distributions of first day of maximum average ICP grouped by 12-month outcome.
4. Discussion
Our study challenges the existence of a ‘crisis of the third day’ following TBI. Despite analyzing ICP monitoring data in a substantial cohort of 886 patients, we found no evidence supporting this phenomenon. Instead, the timing of the highest ICP typically occurred on the first day after injury and was not associated with functional outcome.
4.1. Temporal course of ICP
The temporal trajectory of average ICP over the zero to seven-day period post-injury showed no significant fluctuations and no discernible rise on the third day after injury, despite our large sample size and accurate timing parameters. Although heterogeneity in TBI between and within subgroups could theoretically result in inverse temporal ICP patterns, leading to a net zero effect, it is improbable for a structural phenomenon to be entirely nullified. Additionally, our subgroup analyses did not provide any indication of this. Pediatric patients were found to experience their maximum ICP a median of one day earlier compared to adults and elderly patients, possibly due to limited intracranial space causing mass lesions to become symptomatic sooner. However, this could also be due to random variation given the relatively small sample.
Within our patient cohort, 35% of patients reached their maximum average ICP during days zero and one, while another 30% did so over days two and three post-injury. Approximately 35% of patients reached their highest ICP value after the third day, with 25% doing so after the fourth day. For patients who experienced an ICP >20 mmHg at any timepoint during the first week after TBI, an even larger percentage of patients (45%) suffered their highest ICP after the third day. These findings not only fail to support the theory of a crisis occurring on the third day, but also challenge the concept of a critical window closing after this period. Therefore, the assumption that ICP stabilized after the third day and the ICP monitor can thus be safely removed may often be premature and unwarranted. Similarly, clinical trials restricting their ICP analyses to the first three days post-injury may overlook a significant proportion of cases with elevated pressures (Maas et al., 2006; Knoller et al., 2002). By shedding light on the temporal course of ICP over a relatively extended median period of six days, our study underscores the necessity of sustained ICP monitoring beyond the third day.
Our findings align with previous smaller-scale studies that have demonstrated variability in the temporal patterns of ICP elevation after TBI. For instance, a cohort study conducted in 201 patients reported that one-third of patients reached their highest average ICP during the first two days, another third over day three and four, with 80% reaching their peak by day five (corresponding to day four in our study), indicating that 20% reached their peak after that time point (Stocchetti et al., 2007). Another study categorized ICP rise into early (day 0–1), intermediate (day 2–4) and late (after day 4) phases based on the first day the highest average ICP was reached. It found an early rise in ICP in 32% of patients, an intermediate rise in 34%, and a late rise in 34% of patients (Bremmer et al., 2010). Yet another study observed a relatively flat time course of average ICP with minor peaks from 48 to 72 h and at 216 h (Adams et al., 2017). Finally, one study reported that 17% of included patients experienced a late increase in ICP (beginning after day three), many of whom had no sustained increase in ICP for the first three days post-injury (O'Phelan et al., 2009). In their cohort, 20% of patients experienced their highest average ICP after day four.
Collectively, these retrospectively conducted single center studies in relatively small patient cohorts indicate that the temporal patterns of ICP elevation after TBI are variable, and the underlying pathophysiology remains incompletely understood. Notably, TIL was not considered in these studies, which could have obscured post-traumatic ICP fluctuations and may have led to partial reporting of a treatment effect rather than the natural course of ICP.
4.2. Therapy intensity level
The average TIL was highest on the day of injury and maintained a relatively stable course over the subsequent seven days, mirroring the trend observed in average ICP. Elevated levels over days 0–5 were observed among patients with severe TBI, indicating a greater need for intensive treatment to manage ICP within normal ranges. Among the third-line medical interventions employed, hyperventilation was least frequently utilized, consistent with findings from prior studies (Aarabi et al., 2006; Huijben et al., 2021). Although the BTF guidelines advocate for a gradual implementation of TIL, our data showed that maximum TIL was predominantly achieved early on (days zero to two) in the post-trauma period. Similarly, ‘delayed’ neurosurgical procedures were most commonly performed on the day of injury or within the subsequent day. This proactive approach to TIL administration suggests that the stepwise approach recommended by the guidelines may not always be followed, aligning with prior research reporting a tendency for early adoption of higher-tier therapies without a systemic progression in intensity, potentially complicating the interpretation of TIL (Huijben et al., 2021).
4.3. Functional outcome
The presence of increased ICP >20 mmHg at any timepoint during the zero to seven-day period after TBI was consistently linked to unfavorable and fatal outcomes at 12 months, in line with prior evidence and underscoring the pivotal role of elevated ICP as a contributor to, or an indicator of, secondary brain injury (Güiza et al., 2015; Vik et al., 2008; Ai Åkerlund et al., 2020; Adams et al., 2017; Stein et al., 2013). Interestingly, we did not observe an independent association between the timing of peak ICP within the first week after TBI and functional outcome of 12 months. Previous studies have indicated a correlation between a late rise in ICP, defined variably from 1-3 days to 1–2 weeks post-injury, and unfavorable outcomes (Johnston et al., 1970; Bremmer et al., 2010; O'Phelan et al., 2009; Stein et al., 2013; Unterberg et al., 1993). Although unadjusted analyses in our cohort suggested similar associations between a very late ICP peak (day seven) and mortality or unfavorable outcome, these associations lost significance after covariate adjustment. Hence, our results emphasize the importance of both early and late monitoring of ICP, as patients experiencing ICP peaks at any stage may be at risk for unfavorable outcomes.
4.4. Strengths and limitations
This study has several strengths, including a robust, large patient cohort from 65 centers across Europe, and accurately recorded injury and ICP timing parameters. Additionally, incorporating TIL adds depth to our observations, offering more insight into the natural progression of posttraumatic ICP beyond treatment effects.
However, certain limitations warrant consideration. Firstly, the exclusive focus on level-1 trauma centers with neurosurgical capabilities may restrict the generalizability of our findings to non-neurosurgical settings. Additionally, while TIL offers valuable insights, its implementation has been reported to vary markedly across centers, complicating its interpretation (Huijben et al., 2021). Moreover, our study did not include sufficient data on intracranial pressure dose – an integrative measure combining intensity and duration of ICP- or autoregulation, as this was not central to our primary aim of identifying a third-day crisis post-TBI. ICP dose and autoregulation could, however, be relevant contributors to the relationship between elevated ICP and functional outcome (Maas et al., 2022; Ai Åkerlund et al., 2020). Lastly, decisions to start or remove ICP monitoring are often based on clinical judgment regarding the injury's severity and the patient's salvageability, potentially leading to self-fulfilling prophecies and incomplete data for some patients, since they could then no longer contribute to observations of later ICP peaks.
5. Conclusions
Average ICP and TIL values showed no significant fluctuations during the first week following trauma, especially no elevation around the third day. As such, there were no signs of a ‘crisis of the third day’ following TBI. The presence of ICP >20 mmHg during the first week after trauma was linked to unfavorable outcomes at 12 months, but no convincing association was found between the timing of the ICP peak and functional outcome. A significant proportion (45%) of patients who sustained elevated ICP during the first week after TBI experienced their highest ICP levels after the third day, emphasizing the importance of continued monitoring beyond this timeframe.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Dr W Peul
References
- Aarabi B., Hesdorffer D.C., Ahn E.S., Aresco C., Scalea T.M., Eisenberg H.M. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J. Neurosurg. 2006;104(4) doi: 10.3171/jns.2006.104.4.469. [DOI] [PubMed] [Google Scholar]
- Adams H., Donnelly J., Czosnyka M., Kolias A.G., Helmy A., Menon D.K., et al. Temporal profile of intracranial pressure and cerebrovascular reactivity in severe traumatic brain injury and association with fatal outcome: an observational study. PLoS Med. 2017;14(7) doi: 10.1371/journal.pmed.1002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai Åkerlund C., Donnelly J., Zeiler F.A., Helbok R., Holst A., Cabeleira M., et al. Impact of duration and magnitude of raised intracranial pressure on outcome after severe traumatic brain injury: a CENTER-TBI high-resolution group study. PLoS One. 2020;15(12 December) doi: 10.1371/journal.pone.0243427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer R., De Jong B.M., Wagemakers M., Regtien J.G., Van Der Naalt J. The course of intracranial pressure in traumatic brain injury: relation with outcome and ct-characteristics. Neurocrit Care. 2010;12(3) doi: 10.1007/s12028-009-9329-2. [DOI] [PubMed] [Google Scholar]
- Carney N., Totten A.M., O'Reilly C., Ullman J.S., Hawryluk G.W.J., Bell M.J., et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1) doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- Cnossen M.C., Huijben J.A., van der Jagt M., Volovici V., van Essen T., Polinder S., et al. Variation in monitoring and treatment policies for intracranial hypertension in traumatic brain injury: a survey in 66 neurotrauma centers participating in the CENTER-TBI study. Crit. Care. 2017;21(1) doi: 10.1186/s13054-017-1816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güiza F., Depreitere B., Piper I., Citerio G., Chambers I., Jones P.A., et al. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med. 2015;41(6) doi: 10.1007/s00134-015-3806-1. [DOI] [PubMed] [Google Scholar]
- Hawryluk G.W.J., Aguilera S., Buki A., Bulger E., Citerio G., Cooper D.J., et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle international severe traumatic brain injury consensus conference (SIBICC) Intensive Care Med. 2019;45:1783–1794. doi: 10.1007/s00134-019-05805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijben J.A., Dixit A., Stocchetti N., Maas A.I.R., Lingsma H.F., van der Jagt M., et al. Use and impact of high intensity treatments in patients with traumatic brain injury across Europe: a CENTER-TBI analysis. Crit. Care. 2021;25(1) doi: 10.1186/s13054-020-03370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston I.H., Johnston J.A., Johnston I.H., Johnston J.A., Jennett B. INTRACRANIAL-PRESSURE changes following head injury. Lancet. 1970;296(7670) doi: 10.1016/s0140-6736(70)90054-1. [DOI] [PubMed] [Google Scholar]
- Knoller N., Levi L., Shoshan I., Reichenthal E., Razon N., Rappaport Z.H., et al. Dexanabinol (HU-211) in the treatment of severe closed head injury: a randomized, placebo-controlled, phase II clinical trial. Crit. Care Med. 2002;30(3) doi: 10.1097/00003246-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Lazorthes G., Campan L. La «Crise du troisième jour» en Chirurgie du cerveau. Acta Neurochir. 1960;8(1) [Google Scholar]
- Maas A.I.R., Murray G., Henney H., Kassem N., Legrand V., Mangelus M., et al. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006;5(1) doi: 10.1016/S1474-4422(05)70253-2. [DOI] [PubMed] [Google Scholar]
- Maas A.I.R., Harrison-Felix C.L., Menon D., Adelson P.D., Balkin T., Bullock R., et al. Standardizing data collection in traumatic brain injury. J. Neurotrauma. 2011;28(2) doi: 10.1089/neu.2010.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A.I.R., Menon D.K., Steyerberg E.W., Citerio G., Lecky F., Manley G.T., et al. Collaborative European NeuroTrauma effectiveness research in traumatic brain injury (CENTER-TBI) Neurosurgery. 2015;76(1) doi: 10.1227/NEU.0000000000000575. [DOI] [PubMed] [Google Scholar]
- Maas A.I.R., Menon D.K., David Adelson P.D., Andelic N., Bell M.J., Belli A., et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16 doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- Maas A.I.R., Menon D.K., Manley G.T., Abrams M., Åkerlund C., Andelic N., et al. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022;21 doi: 10.1016/S1474-4422(22)00309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maset A.L., Marmarou A., Ward J.D., Choi S., Lutz H.A., Brooks D., et al. Pressure-volume index in head injury. J. Neurosurg. 1987;67(6) doi: 10.3171/jns.1987.67.6.0832. [DOI] [PubMed] [Google Scholar]
- Nattino G., Gamberini L., Brissy O., Carrara G., Chesnut R., Chiarini V., et al. Comparative effectiveness of intracranial pressure monitoring on 6-month outcomes of critically ill patients with traumatic brain injury. JAMA Netw. Open. 2023;6(9) doi: 10.1001/jamanetworkopen.2023.34214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Phelan K.H., Park D., Efird J.T., Johnson K., Albano M., Beniga J., et al. Patterns of increased intracranial pressure after severe traumatic brain injury. Neurocrit Care. 2009;10(3) doi: 10.1007/s12028-008-9183-7. [DOI] [PubMed] [Google Scholar]
- Stein D.M., Brenner M., Hu P.F., Yang S., Hall E.C., Stansbury L.G., et al. Timing of intracranial hypertension following severe traumatic brain injury. Neurocrit Care. 2013;18(3) doi: 10.1007/s12028-013-9832-3. [DOI] [PubMed] [Google Scholar]
- Steyerberg E.W., Wiegers E., Sewalt C., Buki A., Citerio G., De Keyser V., et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18(10) doi: 10.1016/S1474-4422(19)30232-7. [DOI] [PubMed] [Google Scholar]
- Stocchetti N., Colombo A., Ortolano F., Videtta W., Marchesi R., Longhi L., et al. Time course of intracranial hypertension after traumatic brain injury. J. Neurotrauma. 2007;24(8) doi: 10.1089/neu.2007.0300. [DOI] [PubMed] [Google Scholar]
- Swihart B.J., Caffo B., James B.D., Strand M., Schwartz B.S., Punjabi N.M. Lasagna plots: a saucy alternative to spaghetti plots. Epidemiology. 2010;21 doi: 10.1097/EDE.0b013e3181e5b06a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterberg A., Kiening K., Schmiedek P., Lanksch W. Long-term observations of intracranial pressure after severe head injury. The phenomenon of secondary rise of intracranial pressure. Neurosurgery. 1993;32(1) doi: 10.1227/00006123-199301000-00003. [DOI] [PubMed] [Google Scholar]
- van Essen T.A., Lingsma H.F., Pisică D., Singh R.D., Volovici V., den Boogert H.F., et al. Surgery versus conservative treatment for traumatic acute subdural haematoma: a prospective, multicentre, observational, comparative effectiveness study. Lancet Neurol. 2022;21(7) doi: 10.1016/S1474-4422(22)00166-1. [DOI] [PubMed] [Google Scholar]
- Vik A., Nag T., Fredriksli O.A., Skandsen T., Moen K.G., Schirmer-Mikalsen K., et al. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J. Neurosurg. 2008;109(4) doi: 10.3171/JNS/2008/109/10/0678. [DOI] [PubMed] [Google Scholar]
- Zuercher P., Groen J.L., Aries M.J.H., Steyerberg E.W., Maas A.I.R., Ercole A., et al. Reliability and validity of the therapy intensity level scale: analysis of clinimetric properties of a novel approach to assess management of intracranial pressure in traumatic brain injury. J. Neurotrauma. 2016;33(19) doi: 10.1089/neu.2015.4266. [DOI] [PubMed] [Google Scholar]