Abstract
Type 2 diabetes (T2D) is a chronic metabolic disorder caused by defective insulin signaling, insulin resistance, and impairment of insulin secretion. Autophagy is a conserved lysosomal‐dependent catabolic cellular pathway involved in the pathogenesis of T2D and its complications. Basal autophagy regulates pancreatic β‐cell function by enhancing insulin release and peripheral insulin sensitivity. Therefore, defective autophagy is associated with impairment of pancreatic β‐cell function and the development of insulin rersistance (IR). However, over‐activated autophagy increases apoptosis of pancreatic β‐cells leading to pancreatic β‐cell dysfunction. Hence, autophagy plays a double‐edged sword role in T2D. Therefore, the use of autophagy modulators including inhibitors and activators may affect the pathogenesis of T2D. Hence, this review aims to clarify the potential role of autophagy inhibitors and activators in T2D.
Keywords: β‐cells, autophagyinsulin resistancetype 2 diabetes mellitus
Highlights
Basal autophagy provides protection against the development of pancreatic β‐cell dysfunction under normal physiological conditions.
Over‐activated autophagy induces the progression of type 2 diabetes by induction of pancreatic β‐cell apoptosis.
Autophagy modulators (activators or inhibitors) seem to have either protective or detrimental effects on pancreatic β‐cells.
Preclinical and clinical studies are warranted using autophagy modulators to manage type 2 diabetes patients.
1. INTRODUCTION
Type 2 diabetes (T2D) is one of the most common metabolic disorders primarily caused by a combination of two main factors; defective insulin secretion by pancreatic β‐cells and the failure of insulin‐sensitive tissues to respond to insulin. 1 T2D is the third leading cause of death worldwide and represents 90% of 537 million diabetes cases worldwide. 2 Of note, 50% of the population older than 65 years, which forms 40% of the general population, has a certain degree of glucose intolerance. 2 African Americans are more vulnerable to the development of T2D. 3 It has been shown that T2D is often associated with low‐grade inflammatory disorders due to hyperglycemia‐mediated oxidative stress and the release of proinflammatory cytokines. 4 Furthermore, environmental and genetic factors are involved with the initiation of chronic inflammation, insulin rersistance (IR), and the development of hyperglycemia in T2D 5 (Figure 1).
FIGURE 1.
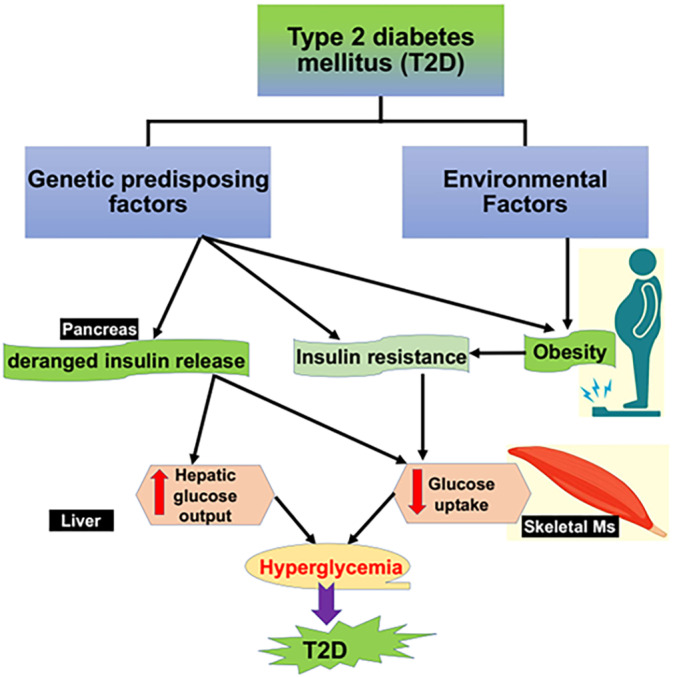
Pathophysiology of type 2 diabetes (T2D): Genetic and environmental factors induce the development of obesity, insulin resistance, and reduce insulin release, causing reduction in peripheral muscle glucose uptake and increasing of hepatic glucose output. These metabolic changes lead to chronic hyperglycemia and the development of T2D.
Moreover, glucolipotoxicity and inflammatory cytokines contribute in the development of pancreatic β‐cell dysfunction through induction of endoplasmic reticulum (ER) stress. 6 In response to these pathological changes in the pancreatic β‐cell dysfunction, autophagy pathway is activated 7 which is the major cellular pathway for elimination of misfolded proteins and damaged organelles. 8 Autophagy is a conserved and evolutionarily lysosomal‐dependent catabolic cellular pathway through which abnormal cytoplasmic components including damaged/injured organelles, lipid droplets, and protein aggregates are degraded, and their constituents recycled. 9 Different signaling proteins involved in the regulation of autophagy flux; for example, Beclin 1, which is a critical component of the class III PI3 kinase complex (PI3KCIII), can induce the formation of autophagosomes. 10 Triggers of autophagy activate the upregulation of Beclin 1 which promotes PI3KCIII to form Beclin 1–PI3KCIII complex. Therefore, Beclin 1 is required for autophagy differentiation and activation, and depletion of Beclin 1 inhibit autophagy and activate apoptosis. 10
Moreover, formation of autophagosomes required autophagy‐related genes (ATG) which produce six functional protein groups, including the ATG1 (ULK in mammals) kinase complex, the ATG9 vesicle, the ATG14‐containing PtdIns 3‐kinase complex, the ATG2–ATG18 complex, the ATG12 conjugation system, and the ATG8 conjugation system. 11 Mammalian ATG8 homologs are called microtubule‐associated protein 1 light chain 3 (LC3) and gamma‐aminobutyric acid receptor–associated protein (GABARAP), which are collectively referred to as ATG8 (s). The ATG8 and ATG12 systems constitute ubiquitin‐like covalent conjugation systems. In the ATG12 system, the most C‐terminal glycine of the ubiquitin‐like protein ATG12 is activated by ATG7, an E1‐like enzyme, in an ATP‐dependent manner, and then sequentially forms thioester intermediates with ATG7 and the E2‐like enzyme ATG10. Finally, ATG12 is conjugated to the acceptor lysine residue in ATG5 via an isopeptide bond. Two sets of ATG12–ATG5 conjugates form a complex with the ATG16(L) dimer. In the ATG8 system, ATG8, another ubiquitin‐like protein, is first synthesized as a proform, whose C‐terminal region is cleaved by ATG4 family enzymes to expose a glycine residue. This processed ATG8 is activated by ATG7 (shared with ATG12), transferred to its specific E2‐like enzyme ATG3, and conjugated to the head group of phosphatidyl ethanolamine (PE). ATG8–PE is present on autophagic membranes. 12 In contrast to the irreversible ATG12 conjugation, ATG8–PE can be deconjugated again by ATG4. Of note, the ATG12–ATG5 conjugate acts as an E3‐like enzyme to promote ATG8–PE conjugation, which is mediated by an interaction between ATG12 and ATG3. Although ATG16 is not required for the lipidation reaction of ATG8, the membrane binding of ATG16L1 determines the site of ATG8 lipidation. ATG8 lipidation could occur on nonautophagic membranes, but it is efficiently corrected by ATG4‐mediated deconjugation that can be regulated by ATG1 and ULK1. The processed unlipidated ATG8 and ATG8–PE are called ATG8‐I and ATG8‐II, respectively (e.g., LC3‐I and LC3‐II). ATG8 is the most commonly used autophagosome marker, and ATG5 and ATG7 have been frequently used in knockout mouse studies. 11 , 12
Therefore, autophagy plays a crucial role in maintaining and preserving of cellular homeostasis in response to the different intracellular stressors. 9 However, efficiency and functional activity of autophagy process is highly reduced during aging and by over‐nutrition which interferes with autophagic flux. 9 As well, obesity, IR, T2D, and other metabolic disorders which are linked with aging are often associated with increment of intracellular stress result in more deterioration of cellular homeostasis. Aging‐mediated autophagy dysfunction further aggravates IR and T2D. 9 , 13 In addition, autophagy contributes in cellular nutrition during starvation, improves pancreatic β‐cells, and increases peripheral insulin sensitivity. 13 Indeed, basal autophagy and normal autophagy response are regulated by various intracellular nutrient‐sensing pathways including AMP–activated protein kinase (AMPK), mechanistic target of rapamycin complex 1 (mTORC1), and sirtuin 1 (SIRT1). 14 Both AMPK and SIRT1 activate autophagy, whereas mTORC1 inhibits autophagy under physiological conditions. For example, caloric restriction which increases the expression of AMPK and SIRT1, improves autophagy process with subsequent reduction of IR and T2D development. 15 However, aberrant over‐expression of mTORC1 induces metabolic disorders by inhibiting autophagy flux. 15 Basal autophagy under normal physiological conditions seems to be protective against the development of pancreatic β‐cell dysfunction. 14 Conversely, over‐activated autophagy increases apoptosis of pancreatic β‐cells. 15 Thus, autophagy plays a double‐edged sword role in T2D. 16 Therefore, use of autophagy modulators including inhibitors and activators may affect the pathogenesis of T2D. 17 Therefore, this review aims to clarify the potential role of autophagy inhibitors and activators in T2D.
2. AUTOPHAGY IN T2D
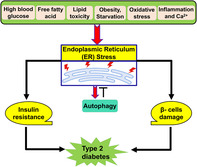
Hyperglycemia, glucolipotoxicity, ER stress, oxidative stress, inflammation, and Ca2+ dyshomeostasis contribute to the development of pancreatic β‐cell dysfunction, IR, and the development of T2D. 18 , 19 , 20 , 21 , 22 , 23 Prolong ER stress triggers activation of autophagy, which also inhibits ER stress 24 (Figure 2).
FIGURE 2.
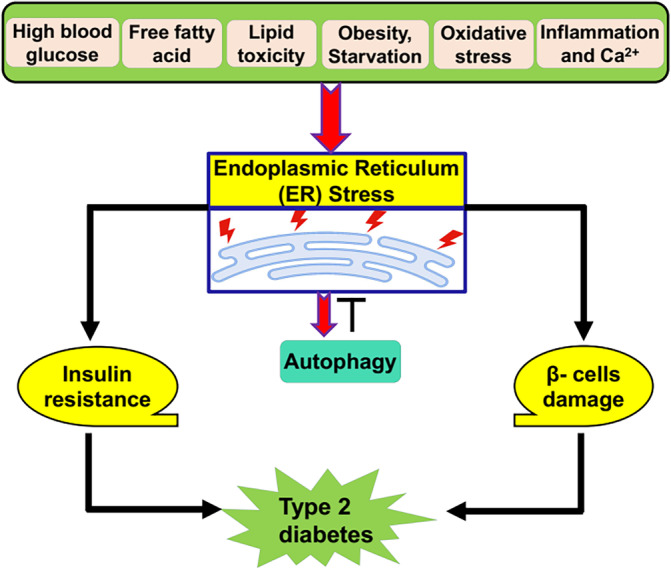
ER stress and autophagy in type 2 diabetes (T2D): High blood glucose and fatty acid, and associated obesity, oxidative stress, and inflammation induce the development of endoplasmic reticulum (ER) stress, which affect autophagy function, induce pancreatic β‐cell damage, and the development of insulin resistance. These changes contribute in the development of T2D.
2.1. Beneficial effects of autophagy in T2D
Basal autophagy preserves and protects pancreatic β‐cell function from the effect of oxidative stress. 25 Interestingly, dysregulated autophagy represents a key player in the pathophysiology of T2D and its complications. Basal autophagy promotes insulin signaling in both pancreatic β‐cells and peripheral tissues. 25 Thus, age‐mediated defective autophagy is implicated in the development of T2D and associated macrovascular and microvascular complications. 25
In a state of IR, hyperinsulinemia inhibits autophagy by activating mTOR in synergy with amino acids leading to the inhibition of autophagy‐related gene 1 (Atg1) which involved in the activation of autophagy. 26 , 27 , 28 , 29 Insulin‐mediated activation of protein kinase B also inhibit forkhead box O3 (FOXO3) which activates autophagy 30 (Figure 3).
FIGURE 3.
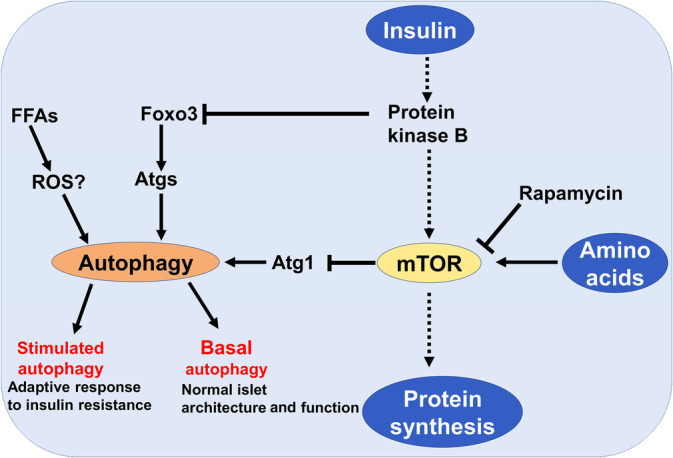
Insulin and autophagy in type 2 diabetes (T2D): Insulin through activation of protein kinase B inhibits autophagy either directly by inhibiting of forkhead box O3 (Foxo3) signaling or indirectly through activation of mammalian target of rapamycin (mTOR), which inhibit autophagy through downregulating of autophagy‐related gene 1 (Atg1). Amino acids and rapamycin activate and inhibit mTOR, respectively.
In T2D, different signaling pathways are dysregulated which affect expression of Beclin‐1. 31 For example, tumor growth factor beta (TGF‐β), nuclear factor kappa B (NF‐κB), and transcription factor FOXO3 activated Beclin‐1. Besides, reactive oxygen species (ROS) and advanced glycation end‐product (AGE) in response to the activated monocyte chemoattractant protein 1 (MCP‐1) induces Beclin‐1. However, suppression of Akt and mTOR by diabetes blocks the function of Beclin‐1. 31 Autophagy activation in T2D in response to different signaling pathways may be a compensatory pathway to overcome inflammatory and oxidative disorders 30 (Figure 4).
FIGURE 4.
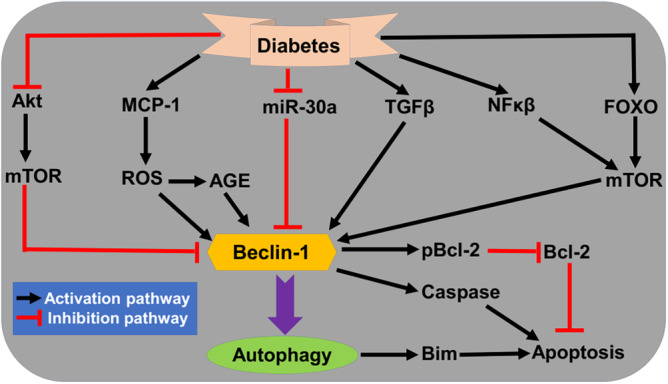
Activated autophagy in type 2 diabetes (T2D): Diabetes causes the activation and inhibition of many signaling pathways that affect the activation of autophagy through Beclin‐1‐dependent pathway. Metabolic alterations in diabetes activate monocyte chemoattractant protein 1 (MCP‐1), tumor growth factor beta (TGF‐β), nuclear factor kappa B (NF‐κB), and Foxo. However, Akt and miR‐30a which inhibit autophagy or through mammalian target of rapamycin (mTOR) pathway are inhibited in diabetes. These changes alter the functional activity of autophagy in T2D. miR‐30a, microRNA 30a; Akt, protein kinase B.
During IR development, autophagy is increased in the pancreatic β‐cells as a compensatory mechanism to overcome oxidative and inflammatory disorders. 32 Genetic deletion of Atg7 in mice triggers degeneration of pancreatic β cell, inhibition of insulin secretion, induction of abnormal glucose intolerance, and development of diabetes. 33 Loss of autophagy promotes the accumulation of ubiquitin‐containing proteins and proteins expressing LC3‐binding protein p62 which is required for delivery of aggregated proteins to autophagosomes. 34 , 35 , 36 In addition, loss of autophagy induce the accumulation of distended ER and malformed mitochondria. 33 It has been shown that autophagy‐deficient mice experience hyperglycemia and hyperinsulinemia due to the development of mitochondrial dysfunction and ER stress. 37 Notably, Atg7 mutant mice showed augmented apoptosis and reduced proliferation of pancreatic β‐cells leading to significant decrease of β‐cell mass. 37 As well, glucose induced Ca2+ signaling which is necessary for insulin release is severely impaired in Atg7 mutant mice. 37 Defective autophagy promotes the development of ER stress and mitochondrial dysfunction leading to dysfunction of pancreatic β‐cells through defect in ATP formation and augmentation of oxidative stress. 38 Findings from clinical studies observed extensive accumulation of autophagy vacuoles and p62 in pancreatic β‐cells of T2D patients 39 , 40 suggesting defective autophagy flux in T2D. These findings indicated that basal and even constitutive autophagy is essential for homeostasis of pancreatic β‐cells. 41
High‐fat diet increases level of autophagosomes in both diabetic and non‐diabetic mice due to impairment the interaction between autophagosomes and lysosomes or failure of lysosomal proton pump. 42 When Atg−/− mice fed on high fat diet, their blood glucose tolerance were further deteriorated, indicating importance of autophagy in the regulation function of pancreatic β‐cells. 42 It has been illustrated that pancreatic β‐cell mass is reduced by increasing apoptosis in Atg7‐deficient mice. Besides, in vitro study demonstrated that coadministration of lysosomal inhibitors and fatty acids increase autophagy flux as evident by increasing of LC3‐II in mice. 42 These observations proposed that autophagy increases IR to protect pancreatic β‐cells against oxidative injury. In addition, IR‐induced autophagy is regarded as an adaptive response to prevent glucolipotoxicity. 42
In addition, obesity‐induced IR is mediated by leptin resistance which affects the expression of autophagy genes leading to the inhibition of autophagy flux and impairment of autophagy. 43 Goldstein et al. 43 illustrated that leptin improves autophagy and lysosomal related degradation of misfolded proteins in adipocytes.
Furthermore, autophagy plays a critical role in preventing the accumulation of islet amyloid polypeptide (IAPP) in only human pancreatic β‐cells, but not in rodents due to the absence of IAPP expression in rodents. 22 Progressive accumulation of IAPP in transgenic mice expressing human IAPP are susceptible to developing diabetes. 44 Autophagy increases cellular elimination of IAPP thereby attenuating failure of pancreatic β‐cells and the development of T2D. Therefore, activation of autophagy could be effective in preventing the development of neurodegenerative diseases including Alzheimer disease in T2D patients. 22
These findings indicated that activated autophagy in T2D has a protective effect to prevent further deterioration of pancreatic β‐cells.
2.2. Detrimental effects of autophagy in T2D
On the other hand, different studies reported that autophagy plays a detrimental role in the pathogenesis of T2D. 45 , 46 , 47 The harmful effect of autophagy is related to the induction of ROS accumulation through Atg5‐dependent pathway and accelerating of pancreatic β‐cell deaths. Moreover, autophagy can induce cell deaths by inhibiting caspase pathway through induction of ROS formation, oxidation of lipid membrane and injury of plasma membrane. 47 It has been shown that knocking of Atg7 or Atg8 and use of autophagy inhibitors may attenuate ROS‐induced cell deaths. 47 Autophagic cell death occurs through the interaction of Atg5 with Fas‐associated protein with death domain (FADD). 45 Interferon gamma (INF‐γ)‐induced autophagic cell death is mediated by the expression of Atg5. 45 Furthermore, Atg5 promotes apoptotic stimuli in cancer cells both in vitro and in vivo. 46 Likewise, a Ca2+‐dependent nonlysosomal cysteine protease calpain, which is expressed ubiquitously, has ability to induce apoptosis through activation of Atg5. 46 Genetic variation in calpain‐like cysteine, calpain 10 is associated with pancreatic β‐cell deaths. 48 Of note, calpain pathway is augmented leading to platelet activation and thrombosis in T2D patients. 49 Therefore, exaggerated calpain pathway in T2D may associate with progressive pancreatic β‐cell deaths via activation of apoptosis and autophagic cell death.
Furthermore, mTOR a known inhibitor of autophagy plays an important in preventing oxidative stress‐induced pancreatic β‐cell deaths mediated by apoptosis and autophagy. Inhibition of apoptotic pathways by mTOR limits the development of IR and T2D due to pancreatic β‐cell deaths. 50 In addition, GLP‐1 agonists protect pancreatic β‐cells by activating mTOR signaling. 51 Findings from experimental studies highlighted that autophagy may induce progressive death of pancreatic β‐cells. In addition, induced autophagy promotes loss of hepatic and cardiac tissues in diabetic rats. 52
These findings proposed that autophagy plays a detrimental role on the pancreatic β‐cells leading to acceleration of cell deaths and development of T2D.
Taken together, autophagy has a double edge‐sword that could be beneficial or harmful in T2D. Therefore, use of autophagy modulators is logical in this regard to clarify the beneficial effects of autophagy activators or inhibitors in T2D.
3. AUTOPHAGY MODULATORS
Formation of autophagosomes is mediated by Atg1/PI3K, Atg8, and At5–Atg12 conjugation systems. 53 Beclin‐1 through interaction with class III PI3K initiates the formation of autophagosomes. 54 Notoriously, mTOR plays a central player in the regulation of autophagy signaling in response to starvation and hypoxia. 55 Moreover, calpain, PI3K, cAMP, and Ca2+ are also involved in the regulation of autophagy signaling. 56 In addition, histone deacetylase 6 (HDAC6) regulates the interaction between autophagosomes and lysosomes. 57 Therefore, targeting of these signaling mainly Beclin‐1, mTOR, calpain, PI3K, cAMP, HDAC6, and Ca2 by specific modulators could be an effective therapeutic strategy in the management of T2D.
3.1. Autophagy activators in T2D
It has been shown that activation of autophagy by caloric restriction promotes regeneration of pancreatic β‐cells. This effect mimic the effect produced by using mTORC1 inhibitors signifying that autophagy is an essential pathway for homeostasis of pancreatic β‐cells. 58 In addition, autophagy activators promote neurogenesis of pancreatic β‐cells and prevent their apoptosis. As well, autophagy augments peripheral insulin sensitivity mainly in the liver and skeletal muscles. 58 , 59 Remarkably, many antidiabetic drugs such as rosiglitazone, metformin and glucagon like peptide 1 (GLP‐1) prevent dysfunction of pancreatic β‐cells by inducing autophagy by increasing the expression of AMPK which induce autophagy by inhibiting mTORC1 or though activation of Vps34 a complex activated by Beclin‐1. 59 Therefore, autophagy activators are involved in the management of T2D.
3.1.1. mTOR inhibitors
Rapamycin which also termed sirolimus is a potent immunosuppressive and antifungal natural product. 60 Rapamycin interacts with immunophilin to form a complex which inhibits the kinase activity of mTOR leading to induction of autophagy. 60 In addition, ester of rapamycin which called temsirolimus has ability to induce autophagy. 61 Of note, mTOR inhibitors are primarily used as immunosuppressive agents in the management of various cancer types including renal cell carcinoma, breast cancer, and neuroendocrine tumors. 62 mTOR signaling plays a critical role in glucose metabolism and homeostasis. It has been reported that early use of mTOR inhibitors is associated with the development of new‐onset T2D. 63 , 64 , 65 , 66 mTOR inhibitors‐induced hyperglycemia is mediated by the development of IR and impairment of insulin secretion. 62 , 67 Fraenkel et al. 67 observed that mTOR inhibitor rapamycin attenuates adaptation of pancreatic β‐cells to the effect of hyperglycemia and contribute to the exacerbation of metabolic complications in T2D (Figure 5).
FIGURE 5.
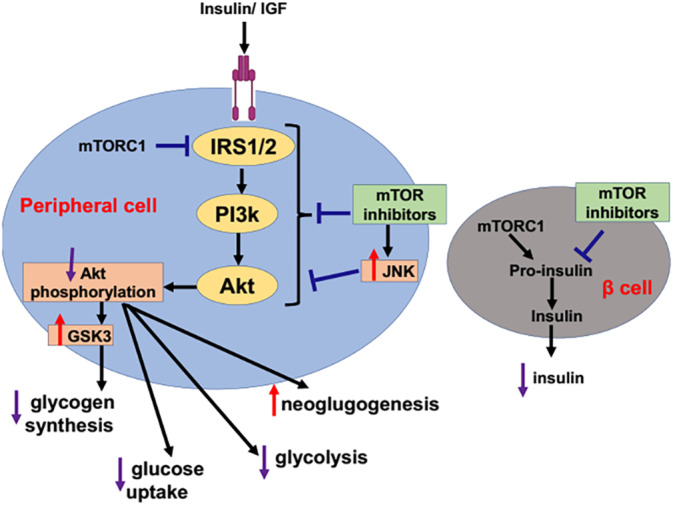
mTOR inhibitors and risk of type 2 diabetes (T2D): Mammalian target of rapamycin (mTOR) inhibitors reduce the conversion of proinsulin to insulin in the pancreatic β‐cells that increase risk of T2D development. In addition, mTOR inhibitors by increasing JNK signaling attenuate the activation of insulin receptor substrate 1/2 and P13k/Akt signaling activation leading to alteration of glucose metabolism. Decreasing Akt phosphorylation activates glycogen synthase kinase 3 (GSK3), which inhibits glycogen synthesis. Inhibition of Akt phosphorylation leads to activation of gluconeogenesis and inhibition of glycolysis and glucose uptake. IGF, Notice insulin‐like growth factor; IRS1/2, Insulin receptor substrates 1/2; mTOR, the mammalian target of rapamycin (mTOR); mTORC1, mammalian target of rapamycin complex1; PI3Ks, Phosphatidylinositol 3‐kinases; Akt, protein kinase B; GSK3, Glycogen synthase kinase‐3; JNK, Jun N‐terminal kinase.
Rapamycin can cause fulminant T2D in experimental animals by reducing mTOR signaling which is essential for the function of pancreatic β‐cells. 67 It has been shown that mTOR signaling triggers the activation of ribosomal S6 kinase 1 (S6K1) and 4 eukaryotic binding protein 1 (4EBP1). 68 , 69 , 70 Insulin activates mTOR signaling via PI3K/Akt pathway. In turn, mTOR/S6K1 signaling induces negative feedback inhibition of insulin sensitivity. 71 Thus, over‐activation of mTOR/S6K1 signaling is associated with impairment of insulin action. 72 Similarly, stimulation of mTOR/S6K1 signaling by amino acid induces IR which prevented by rapamycin. 73 Therefore, mTOR inhibitor rapamycin could be effective in the regulation of glucose metabolism and insulin sensitivity in mice. 74 An experimental study demonstrated that rapamycin improves hepatic insulin sensitivity and inhibit autophagy in rats with hepatic steatosis and IR. 75 A recent experimental study conducted by Reifsnyder et al 76 illustrated that co‐treatment with metformin and rapamycin improves insulin sensitivity in mice. Puzzling, rapamycin has a dual role it can induce IR and at the same time improves insulin sensitivity. Also, rapamycin can cause glucose intolerance without IR. This effect of rapamycin is called benevolent pseudo‐diabetes. 77 Conversely, rapamycin enhances life extension without diabetogenic effect in healthy subjects when used with caloric restriction and metformin. 77 , 78 Furthermore, insulin sensitizing agent metformin which used in the management of T2D has ability to blocks mTOR pathway independent of AMPK pathway. 79 Findings from in vitro and in vivo studies unveiled that metformin has anticancer effect via inhibition of mTOR pathway. 80 Interestingly, different studies revealed that metformin can induce autophagy through activation of AMPK and inhibition of mTOR pathway. 81 , 82 Furthermore, an isoquinoline alkaloid berberine has anti‐inflammatory effects by inducing autophagy through inhibition of mTOR pathway. 83 Pang et al. 84 illustrated that berberine is effective in the management of T2D by different mechanisms including inhibition of mTOR pathway and activation of autophagy pathway.
Therefore, mTOR inhibitors rapamycin, metformin, and berberine through induction of autophagy improve glucose homeostasis and prevent T2D‐induced complications.
3.1.2. ER stressing inducers
ER stress is regarded as a potent autophagy inducer. Notably, Sar1 and Rab1b are GTPase required for formation of autophagosomes. 85 ER stress promotes autophagy by inhibition of mTOR/Akt pathway. 85 ER stress inducers like thapsigargin, brefeldin, and tunicamycin enhance the expression of autophagic vesicles and Beclin‐1. 86
Thapsigargin is an inhibitor of ER Ca2+ ATPase (SERCA) extracted from Thapsia garganica. 87 Thapsigargin increases cytosolic Ca2+ by inhibiting Ca2+ pump into ER and sarcoplasmic reticulum. 88 Depletion of Ca2+ within ER and sarcoplasmic reticulum trigger the opening of plasma membrane Ca2+ channel. 89 This effect induces ER stress and expression of unfolded protein response (UPR) to overcome ER stress. 90 However, unresolved ER stress triggers apoptosis and cell death. Besides, Ca2+ store depletion prevents ferroptosis through phospholipids from ER. 91 Thapsigargin via induction of ER stress activates autophagy. 92 It has been shown that thapsigargin triggers apoptosis of pancreatic β‐cells. 93 Inhibition of SERCA reduces functional activity of pancreatic β‐cells and insulin release. 94
It has been shown that alteration of cytosolic Ca2+ is linked with development of T2D. 95 In vitro study demonstrated that lymphocytes from T2D patients had higher cytosolic Ca2+ and correlated with blood glucose as compared to controls. 95 Therefore, ER stress inducers may aggravate cytosolic Ca2+ by enhancing Ca2+ flux across the cell membrane. In addition, ER stress inducers reduce the expression of superoxide dismutase causing oxidative stress and exacerbation of T2D. 96 However, ER stress inducers promote the expression of UPR which has a protective effect against T2D. 90 , 94 Notoriously, augmentation of UPR by ER stress inducers is involved in the attenuation of pancreatic β‐cells death. 97 UPR conserves ER homeostasis and attenuates β‐cell failure. However, prolong ER stress reduces the protective effects of UPR leading to β‐cell death and reduced insulin secretion. 98 , 99 Therefore, ER stress inducers have a protective effect by increasing UPR and detrimental effect by inducing oxidative stress in T2D regardless of autophagy.
3.1.3. Inositol monophosphatase inhibitors
Inositol monophosphatase (IMPase) is an enzyme involved in the regulation of intracellular IP3 and free inositol. Different types of IMPase inhibitors include lithium, valproic acid, carbamazepine and L‐690330. 100 IMPase inhibitors induce autophagy in mTOR‐independent pathway and can be used in neurodegenerative diseases. 101 IMPase inhibitor lithium has a critical role in treating neurodegenerative diseases by enhancing autophagy. 102 It has been shown that lithium reduced T2D risk by inhibition of glycogen synthase kinase 3. 103 A retrospective study illustrated that long‐term use of lithium decreases T2D risk. 103 Of note, neuroprotective agents like lithium have cytoprotective effects against injury of pancreatic β‐cells. 104 Experimental study indicated that a microdose of lithium protects pancreatic β‐cells. 105 In addition, antiepileptic carbamazepine enhances survival of pancreatic β‐cells. 106 Preclinical study observed that carbamazepine slows the progression of pancreatic β‐cell injury by reducing inflammation in mice. 106 Furthermore, antiepileptic valproic acid has the ability to decrease IR, gluconeogenesis, and fat deposition by inhibiting IMPase and induce autophagy. 107 The underlying mechanism for the beneficial effect of valproic acid on glucose homeostasis and T2D is related to the modulation of HDAC, insulin signaling, glucagon secretion, and expression of FOXO1. 107 Of note, HDAC signaling which augments IR is increased in T2D patients. 108 Therefore, use of HDAC inhibitors like valproic acid could be effective in attenuating the development of IR and T2D. 108 In addition, valproic acid, carbamazepine, and lithium have been to induce autophagy. 109 , 110 , 111 Therefore, IMPase inhibitors seem to be effective in T2D by inducing autophagy.
3.1.4. Trehalose
Trehalose is a nonreducing sugar consisting of two glucose unites fused by alpha bond named α‐D‐glucopyranosyl. 112 Trehalose is derived from rye ergot that reduces protein denaturation and protect cell membrane integrity. 113 Trehalose via induction of autophagy increases the elimination of α‐synuclein and can be used in the management of neurodegenerative diseases. 114 Trehalose is not synthesized inside human body; it is commonly used as food stabilizer. 115 Ingested trehalose is metabolized by trehalases; therefore, trehalose is regarded as landmark of carbohydrate metabolite, and high blood trehalose level is associated with risk for the development of T2D. 115 It has been shown that trehalose can reduce the development of T2D by antioxidant effect in experimental animals. 116 Korolenko et al., 117 observed that trehalose induces myocardial and hepatic autophagy in diabetic mice. A placebo controlled clinical trial on 34 subjects with high body mass index showed that daily intake of trehalose 10 g per day for 12 weeks reduced IR and improved blood glucose homeostasis. 118 This finding suggests that prolonged intake of trehalose attenuates the development of IR and metabolic syndrome in high‐risk patients. A case control study involved 69 pairs of diabetic retinopathies and matched T2D patients showed that treatment with trehalose decreased risk of diabetic retinopathy. 119 In addition, trehalose prevents IR and postprandial insulin burst. 120 These observations indicated that trehalose reduces T2D risk by inducing autophagy and antioxidant effects.
3.1.5. PI3K inhibitors
PI3K pathway is intricate in different cellular functions including cell growth, differentiation, proliferation, and inhibition of autophagy. 121 , 122 , 123 , 124 , 125 Therefore, PI3K inhibitors like ceramide increase the expression of Beclin‐1 and improve autophagy function. 126 Sphingosine‐1‐phosphate, like ceramide, promotes autophagy in many cancer cell lines. 127 Ceramide regulates various cellular signaling including apoptosis and cell‐cycle arrest. Ceramide is phosphorylated to ceramide‐1 phosphate which has opposite effect and can induce inflammatory reactions via stimulation of cytosolic phospholipase A2 and consequent release of prostaglandins. 128 , 129 , 130 , 131 High ceramide is associated with the development of IR. 132 Of note, ceramide inhibits insulin action by attenuating the expression of Akt/protein kinase B. 133 Therefore, progressive tissue accumulation of ceramide participates in the development and progression of IR. 133 , 134 , 135 , 136 Thus, inhibition of ceramide prevents IR and the development of T2D induced by glucocorticoid therapy. 137 Ceramide has a diabetogenic effect through induction inflammation and apoptosis of pancreatic β‐cells. 138 Furthermore, increasing tissue ceramide promotes the development and progression of T2D‐related complications (137; 138). Therefore, ceramide seems to be harmful in T2D despite induction of autophagy.
3.1.6. Calpain inhibitors
Calpain is a Ca2+‐dependent, nonlysosomal cysteine protease expressed ubiquitously involved in cell‐cycle progression and cell mobility. 139 Calpain has ability to inhibit autophagy due to high cytosolic Ca2+ activates calpain. 140 Therefore, calpain inhibitor calpastatin could be effective to induce autophagy. 141 Variation in the expression of calpain‐10 gene in the pancreatic β‐cells increases risk of T2D. Caplain regulates the function of pancreatic β‐cells and improves insulin secretion. Therefore, calpastatin inhibits the functional activity of pancreatic β‐cells and may exacerbate T2D. 142 Zhu et al. 143 revealed that caplain over‐expression and calpastatin down‐regulation mediates the development of Alzheimer disease (AD) in diabetic mice. This finding suggests that chronic hyperglycemia‐induced AD via inhibition of calpastatin‐mediated autophagy. Interestingly, calpastatin‐mediated autophagy prevents cardiac fibrosis in murine model of T2D. 144 Other calpain inhibitors including peptidyl epoxide, ketoamide inhibitors, and aldehyde were shown to be effective against various metabolic diseases including T2D. 145 Therefore, autophagy induction by calpastatin could be an effective novel therapeutic strategy in preventing misfolded protein‐induced pancreatic β‐cell dysfunction. 146
3.1.7. Inositol triphosphate inhibitors
Inositol triphosphate (IP3) is cytosolic signaling pathway involved in the regulation of insulin secretion and inhibition of autophagy. 147 Expression of IP3 receptor in T2D patients is reduced leading to abnormal autophagy function. 148 IP3 receptors mediate insulin release in response to glucose administration. 149 IP3 receptor antagonist xestospondin inhibits insulin release in response to glucose. 149 Of note, xestospondin is regarded as mTOR‐independent autophagy activator. 150 Therefore, IP3 receptor antagonists improve autophagy but at the same time deteriorate insulin secretion and increase T2D risk.
3.1.8. Ca2+ channel blockers
Ca2+ is an essential intracellular second messenger regulating various cellular processes including autophagy. High intracellular Ca2+ inhibits autophagy in hepatocytes through inhibition the interaction between autophagosomes and lysosomes. 151 Therefore, Ca2+ channel blockers (CCBs) like verapamil can activate autophagy by inducing the interaction between autophagosomes and lysosomes. 152 CCBs through induction of autophagy prevent the accumulation of lipid droplets and protein inclusions with subsequent inhibition of inflammation and IR. 152 Herein, CCBs may reduce the metabolic complications in obesity by enhancing autophagy and decreasing the development of IR. It has been reported that CCBs reduce defective autophagy‐induced IR in obesity. 153 Furthermore, CCBs improve diabetic outcomes and prevent the progression of cognitive deficits and depression. 154 Remarkably, CCBs inhibit the activation of aldose reductase pathway which transforms glucose to sorbitol leading to the development of diabetic complications. 155 Among CCBs, cinnarizine is the most potent inhibitor of aldose reductase pathway. 155 In addition, CCB nifedipine improves glucose homeostasis and lipid profile in T2D patients. 156 Collectively, CCBs via induction of autophagy may improve glucose homeostasis and reduce diabetic complications.
3.2. Autophagy inhibitors in T2D
It has been illustrated that autophagy inhibitors could be effective against the development of IR and T2D by inhibiting pancreatic β‐cell deaths. Autophagy triggers apoptosis by inhibiting caspase pathway and induction of ROS formation, oxidation of lipid membrane, and injury of plasma membrane. 47 It has been shown that uses of autophagy inhibitors may attenuate ROS‐induced cell deaths. 47 An updated experimental study revealed that autophagy inhibitors prevent retinal inflammation in diabetic mice. 148 Autophagy inhibitors can prevent hyperglycemia‐induced exaggeration of autophagy, which is harmful rather than beneficial. Of note, low autophagy is required for normal pancreatic β‐cell function; however, exaggerated autophagy triggers autophagic‐programmed cell death and apoptosis. 47 Therefore, autophagy inhibitors can mitigate IR‐induced autophagy over‐activity and associated inflammatory and oxidative stress disorders.
3.2.1. Lysosomal alkalizer
Lysosomal lumen alkalizers like chloroquine and hydroxychloroquine are anti‐malarial and anti‐inflammatory agents, which act by impairing of lysosomal function and inhibiting of autophagy. 157 Chloroquine‐induced autophagy inhibition is mediated by inhibiting the interaction between autophagosomes and lysosomes rather than affecting the acidity or degradative capacity. 158 A previous clinical trial observed that hydroxychloroquine was effective in the management of T2D refractory to the effect of sulfonylureas. 159 In comparison with pioglitazone, hydroxychloroquine was effective in the management of T2D through modulation of lipid metabolism and glucose homeostasis. 160 A systematic review and meta‐analysis of 11 randomized controlled clinical trials showed that there was no strong clinical evidence to recommend use of hydroxychloroquine in the management of T2D. 161 These findings proposed that hydroxychloroquine may have some beneficial effect in the management of T2D, despite of the inhibitory effect on the autophagy function.
Moreover, lys01 is a dimeric form of two chloroquine moieties that is 10 time more potent autophagy inhibitor compared to hydroxychloroquine. 162 A water‐soluble form of lys01 is known as lys05 has higher ability to deacidifying the lysosomes and inhibition of autophagy. 163 However, the effects of lys01 and lys05 were not evaluated on insulin sensitivity and pancreatic β‐cell functions.
3.2.2. Cycloheximide
Cycloheximide is a protein synthesis inhibitor produced by Streptomyces griseus. Cycloheximide is widely used in various biomedical research regarding autophagy function. Because of the toxic adverse effects of cycloheximide, including teratogenesis and DNA damage, it used in research only. 164 It has a potent inhibitor effect on the autophagy function by inhibiting the formation of autolysosomes. 164 , 165 Cycloheximide inhibits starvation‐induced autophagy through activation of mTOR signaling. 164 Previous preclinical studies exposed that cycloheximide promotes the expression of insulin‐like growth factors in hepatoma cell lines. 166 , 167 In addition, cycloheximide reduces glucose transporters in adipocytes. 168 Therefore, cycloheximide has detrimental effect on glucose homeostasis and insulin signaling.
3.2.3. Bafilomycins
Bafilomycins are macrolide antibiotics produced by Streptomycetes. Bafilomycins have a wide‐range biological activities including antifungal, antiparasitic, antitumor, and immunosuppressant. 169 Bafilomycin A1 inhibits autophagy leading to mitochondrial dysfunction and cell death, particularly, bafilomycin A1 block vacuolar type H‐ATPase (V‐ATPase) enzyme, which is responsible for acidification of lysosomes and other intracellular organelles. 170 It has been shown that V‐ATPase inhibits insulin release, and the use of bafilomycin A1 for 1 week attenuates renal gluconeogenesis and improves glucose homeostasis in rats with T2D. 171 However, transplacental exposure to bafilomycin A1 inhibits pancreatic organogenesis and accelerates diabetes in mice. 172 Moreover, the expression of V‐ATPase is downregulated in T2D patients. 173 Therefore, bafilomycin A1 has a detrimental effect on the T2D outcomes by inhibiting autophagy.
Different sites of actions are affected by autophagy activators and inhibitors (Figure 6).
FIGURE 6.
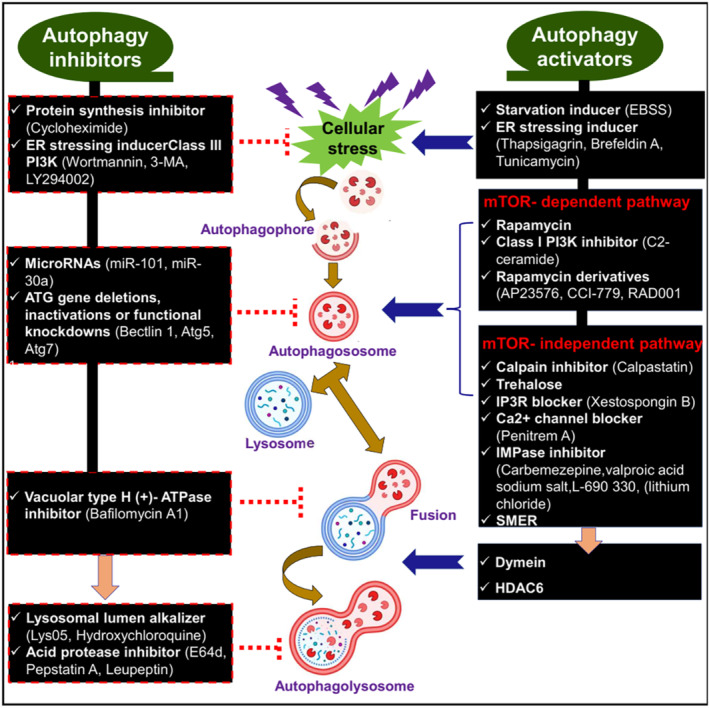
Action of autophagy activators and inhibitors: Autophagy activators inhibit cellular stress and induce autophagosome formation and fusion of autophagosome with the lysosomes; the reverse is done by autophagy inhibitors.
Taken together, both autophagy activators and inhibitors have conflicting effects regardless of autophagy function.
4. CONCLUSIONS AND PERSPECTIVES
T2D is a chronic metabolic disorder associated with dysfunction autophagy pathway Basal autophagy under normal physiological conditions seems to be protective against the development of pancreatic β‐cell dysfunction. During the development of IR and overt T2D with the development of chronic hyperglycemia and associated ER stress, autophagy is activated as a compensatory pathway against oxidative stress and inflammatory to preserve homeostasis of pancreatic β cell. However, in late T2D autophagy is over‐activated due to unresolved oxidative stress leading to the induction of pancreatic β‐cell apoptosis. Therefore, autophagy plays a double‐edged sword role in T2D seeming protective in the early stage and detrimental in the late stage. Therefore, autophagy activators improve defective autophagy in early T2D preventing further deteriorations. However, autophagy inhibitors seem to produce a protective effect in late T2D to prevent exaggerated autophagy which implicated in the induction of pancreatic β‐cell apoptosis. Most autophagy activators seem to have protective effects; however, the majority of autophagy inhibitors have detrimental effects. Collectively, autophagy activators improve pancreatic β‐cells and reduce IR in T2D. In this claim, repurposing of natural products that have potential effects on the autophagy process as adjuvant treatment with antidiabetic agents could be promising as a novel therapeutic strategy. Preclinical and clinical studies are warranted in this concern.
AUTHOR CONTRIBUTIONS
A.T.Z., H.M.A.‐k., and A.I.A.‐G. collected the related research literature and papers and drafted the manuscript. A.A., M.P., A.A.‐F., Y.H.A.E., M.H.Z., and G.E.‐S.B. participated in the design, editing, and revising the manuscript draft. G.E.‐S.B. contributed to conceptualization. All authors have approved and read the final manuscript.
FUNDING INFORMATION
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the University of Witten‐Herdecke Germany.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The authors would like to thank the University of Petra for their invaluable support throughout the research process. Their insights and expertise were instrumental in shaping the direction of this research. Open Access funding enabled and organized by Projekt DEAL.
Zaidalkilani AT, Al‐kuraishy HM, Fahad EH, et al. Autophagy modulators in type 2 diabetes: A new perspective. Journal of Diabetes. 2024;16(12):e70010. doi: 10.1111/1753-0407.70010
Contributor Information
Yaser Hosny Ali Elewa, Email: y-elewa@vetmed.hokudai.ac.jp.
Marios Papadakis, Email: drmariospapadakis@gmail.com.
Gaber El‐Saber Batiha, Email: gaberbatiha@gmail.com.
REFERENCES
- 1. Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11:45‐63. [PMC free article] [PubMed] [Google Scholar]
- 2. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ajuwon AM, Insel K. Health literacy, illness perception, depression, and self‐management among African Americans with type 2 diabetes. J Am Assoc Nurse Pract. 2022;34:1066‐1074. [DOI] [PubMed] [Google Scholar]
- 4. Johansen MY, Karstoft K, MacDonald CS, et al. Effects of an intensive lifestyle intervention on the underlying mechanisms of improved glycaemic control in individuals with type 2 diabetes: a secondary analysis of a randomised clinical trial. Diabetologia. 2020;63:2410‐2422. [DOI] [PubMed] [Google Scholar]
- 5. Tremblay J, Hamet P. Environmental and genetic contributions to diabetes. Metabolism. 2019;100S:153952. [DOI] [PubMed] [Google Scholar]
- 6. Vilas‐Boas EA, Almeida DC, Roma LP, Ortis F, Carpinelli AR. Lipotoxicity and β‐cell failure in type 2 diabetes: oxidative stress linked to NADPH oxidase and ER stress. Cells. 2021;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Y, Xiang H, Xiong W, et al. Glucolipotoxicity induces endothelial cell dysfunction by activating autophagy and inhibiting autophagic flow. Diab Vasc Dis Res. 2022;19:14791641221102513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO J. 2021;40:e108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitada M, Koya D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021;17:647‐661. [DOI] [PubMed] [Google Scholar]
- 10. Wang J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy. 2008;4:947‐948. [DOI] [PubMed] [Google Scholar]
- 11. Al‐Kuraishy HM, Sulaiman GM, Jabir MS, et al. Defective autophagy and autophagy activators in myasthenia gravis: a rare entity and unusual scenario. Autophagy. 2024;20:1473‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jawad MH, Jabir MS, Ozturk K, et al. Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen‐activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF‐α and combined with doxorubicin. Nanotechnol Rev. 2023;12:12. [Google Scholar]
- 13. Yan X, Zhou R, Ma Z. Autophagy‐cell survival and death. Adv Exp Med Biol. 2019;1206:667‐696. [DOI] [PubMed] [Google Scholar]
- 14. Yao D, GangYi Y, QiNan W. Autophagic dysfunction of β cell dysfunction in type 2 diabetes, a double‐edged sword. Genes Dis. 2021;8:438‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martino L, Masini M, Novelli M, et al. Palmitate activates autophagy in INS‐1E β‐cells and in isolated rat and human pancreatic islets. PLoS One. 2012;7:e36188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ardestani A, Lupse B, Kido Y, Leibowitz G, Maedler K. mTORC1 signaling: a double‐edged sword in diabetic β cells. Cell Metab. 2018;27:314‐331. [DOI] [PubMed] [Google Scholar]
- 17. Bugliani M, Mossuto S, Grano F, et al. Modulation of autophagy influences the function and survival of human pancreatic Beta cells under endoplasmic reticulum stress conditions and in type 2 diabetes. Front Endocrinol (Lausanne). 2019;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al‐Kuraishy HM, Al‐Gareeb AI, Shams HA, Al‐Mamorri F. Endothelial dysfunction and inflammatory biomarkers as a response factor of concurrent coenzyme Q10 add‐on metformin in patients with type 2 diabetes mellitus. J Lab Physicians. 2019;11:317‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al‐Kuraishy HM, Sami OM, Hussain NR, Al‐Gareeb AI. Metformin and/or vildagliptin mitigate type II diabetes mellitus induced‐oxidative stress: the intriguing effect. J Adv Pharm Technol Res. 2020;11:142‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al‐Nami MS, Al‐Kuraishy HM, Al‐Gareeb AI, Al‐Mamoori F. Metabolic profile and prolactin serum levels in men with type 2 diabetes mellitus: old‐new rubric. Int J Crit Illn Inj Sci. 2019;9:120‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rasheed HA, Al‐Kuraishy HM, Al‐Gareeb AI, Hussien NR, Al‐Nami MS. Effects of diabetic pharmacotherapy on prolactin hormone in patients with type 2 diabetes mellitus: bane or boon. J Adv Pharm Technol Res. 2019;10:163‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alrouji M, Al‐Kuraishy HM, Al‐Gareeb AI, et al. The potential role of human islet amyloid polypeptide in type 2 diabetes mellitus and Alzheimer's diseases. Diabetol Metab Syndr. 2023;15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdul‐Hadi MH, Naji MT, Shams HA, et al. Oxidative stress injury and glucolipotoxicity in type 2 diabetes mellitus: the potential role of metformin and sitagliptin. Biomed Biotechnol Res J (BBRJ). 2020;4:166‐172. [Google Scholar]
- 24. Liu H, Cao MM, Wang Y, et al. Endoplasmic reticulum stress is involved in the connection between inflammation and autophagy in type 2 diabetes. Gen Comp Endocrinol. 2015;210:124‐129. [DOI] [PubMed] [Google Scholar]
- 25. Meijer AJ, Codogno P. Autophagy: a sweet process in diabetes. Cell Metab. 2008;8:275‐276. [DOI] [PubMed] [Google Scholar]
- 26. Babalghith AO, Al‐Kuraishy HM, Al‐Gareeb AI, et al. The potential role of growth differentiation factor 15 in COVID‐19: a corollary subjective effect or not? Diagnostics (Basel). 2022;12:2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El‐Saber Batiha G, Al‐Gareeb AI, Saad HM, Al‐Kuraishy HM. COVID‐19 and corticosteroids: a narrative review. Inflammopharmacology. 2022;30:1189‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al‐Kuraishy HM, Al‐Gareeb AI, Qusti S, Alshammari EM, Atanu FO, Batiha GE. Arginine vasopressin and pathophysiology of COVID‐19: an innovative perspective. Biomed Pharmacother. 2021;143:112193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al‐Kuraishy HM, Al‐Gareeb AI. Effects of rosuvastatin on metabolic profile: versatility of dose‐dependent effect. J Adv Pharm Technol Res. 2019;10:33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mammucari C, Milan G, Romanello V, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458‐471. [DOI] [PubMed] [Google Scholar]
- 31. Munasinghe PE, Riu F, Dixit P, et al. Type‐2 diabetes increases autophagy in the human heart through promotion of Beclin‐1 mediated pathway. Int J Cardiol. 2016;202:13‐20. [DOI] [PubMed] [Google Scholar]
- 32. Kaniuk NA, Kiraly M, Bates H, Vranic M, Volchuk A, Brumell JH. Ubiquitinated‐protein aggregates form in pancreatic beta‐cells during diabetes‐induced oxidative stress and are regulated by autophagy. Diabetes. 2007;56:930‐939. [DOI] [PubMed] [Google Scholar]
- 33. Jung HS, Chung KW, Kim JW, et al. Loss of autophagy diminishes pancreatic β cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318‐324. [DOI] [PubMed] [Google Scholar]
- 34. Al‐Kuraishy HM, Jabir MS, Al‐Gareeb AI, Saad HM, Batiha GE, Klionsky DJ. The beneficial role of autophagy in multiple sclerosis: yes or no? Autophagy. 2024;20:259‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ali NH, Al‐kuraishy HM, Al‐Gareeb AI, et al. Batiha GE‐S: autophagy and autophagy signaling in epilepsy: possible role of autophagy activator. Mol Med. 2023;29:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jabir MS, Mohammed MKA, Albukhaty S, et al. Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS‐mediated apoptosis and autophagy in liver cancer cells. Nanotechnol Rev. 2023;12:12. [Google Scholar]
- 37. Hur KY, Jung HS, Lee MS. Role of autophagy in β‐cell function and mass. Diabetes Obes Metab. 2010;12(Suppl 2):20‐26. [DOI] [PubMed] [Google Scholar]
- 38. Liang J, Shao SH, Xu ZX, et al. The energy sensing LKB1‐AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218‐224. [DOI] [PubMed] [Google Scholar]
- 39. Masini M, Bugliani M, Lupi R, et al. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52:1083‐1086. [DOI] [PubMed] [Google Scholar]
- 40. Abe H, Uchida T, Hara A, et al. Exendin‐4 improves β‐cell function in autophagy‐deficient β‐cells. Endocrinology. 2013;154:4512‐4524. [DOI] [PubMed] [Google Scholar]
- 41. Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19‐40. [DOI] [PubMed] [Google Scholar]
- 42. Ebato C, Uchida T, Arakawa M, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high‐fat diet. Cell Metab. 2008;8:325‐332. [DOI] [PubMed] [Google Scholar]
- 43. Goldstein N, Haim Y, Mattar P, et al. Leptin stimulates autophagy/lysosome‐related degradation of long‐lived proteins in adipocytes. Adipocyte. 2019;8:51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wirth F, Heitz FD, Seeger C, et al. A human antibody against pathologic IAPP aggregates protects beta cells in type 2 diabetes models. Nat Commun. 2023;14:6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pyo JO, Jang MH, Kwon YK, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280:20722‐20729. [DOI] [PubMed] [Google Scholar]
- 46. Yousefi S, Perozzo R, Schmid I, et al. Calpain‐mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124‐1132. [DOI] [PubMed] [Google Scholar]
- 47. Yu L, Wan F, Dutta S, et al. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci U S A. 2006;103:4952‐4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Horikawa Y, Oda N, Cox NJ, et al. Genetic variation in the gene encoding calpain‐10 is associated with type 2 diabetes mellitus. Nat Genet. 2000;26:163‐175. [DOI] [PubMed] [Google Scholar]
- 49. Giannella A, Ceolotto G, Radu CM, et al. PAR‐4/Ca(2+)‐calpain pathway activation stimulates platelet‐derived microparticles in hyperglycemic type 2 diabetes. Cardiovasc Diabetol. 2021;20:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maiese K. mTOR: driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J Diabetes. 2015;6:217‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carlessi R, Chen Y, Rowlands J, et al. Bittencourt PIHd, Newsholme P: GLP‐1 receptor signalling promotes β‐cell glucose metabolism via mTOR‐dependent HIF‐1α activation. Sci Rep. 2017;7:2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee JH, Lee JH, Jin M, et al. Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp Mol Med. 2014;46:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ding X, Jiang X, Tian R, et al. RAB2 regulates the formation of autophagosome and autolysosome in mammalian cells. Autophagy. 2019;15:1774‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hill SM, Wrobel L, Ashkenazi A, et al. VCP/p97 regulates Beclin‐1‐dependent autophagy initiation. Nat Chem Biol. 2021;17:448‐455. [DOI] [PubMed] [Google Scholar]
- 55. Li B, Yao X, Luo Y, Niu L, Lin L, Li Y. Inhibition of autophagy attenuated intestinal injury after intestinal I/R via mTOR signaling. J Surg Res. 2019;243:363‐370. [DOI] [PubMed] [Google Scholar]
- 56. Amemiya Y, Maki M, Shibata H, Takahara T. New insights into the regulation of mTOR signaling via Ca(2+)‐binding proteins. Int J Mol Sci. 2023;24:3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zheng Z, Zhou Y, Ye L, et al. Histone deacetylase 6 inhibition restores autophagic flux to promote functional recovery after spinal cord injury. Exp Neurol. 2020;324:113138. [DOI] [PubMed] [Google Scholar]
- 58. Dinicolantonio J, McCarty M. Autophagy‐induced degradation of Notch1, achieved through intermittent fasting, may promote beta cell neogenesis: implications for reversal of type 2 diabetes. Open Heart. 2019;6:e001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marzoog B, Vlasova T. Beta‐cell autophagy under the scope of hypoglycemic drugs; possible mechanism as a novel therapeutic target. Obes Metab. 2022;18:465‐470. [Google Scholar]
- 60. Selvarani R, Mohammed S, Richardson A. Effect of rapamycin on aging and age‐related diseases—past and future. GeroScience. 2021;43:1135‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kaley TJ, Panageas KS, Pentsova EI, et al. Phase I clinical trial of temsirolimus and perifosine for recurrent glioblastoma. Ann Clin Transl Neurol. 2020;7:429‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vergès B, Cariou B. mTOR inhibitors and diabetes. Diabetes Res Clin Pract. 2015;110:101‐108. [DOI] [PubMed] [Google Scholar]
- 63. Hussien NR, Al‐Naimi MS, Rasheed HA, Al‐Kuraishy HM, Al‐Gareeb AI. Sulfonylurea and neuroprotection: the bright side of the moon. J Adv Pharm Technol Res. 2018;9:120‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Al‐Kuraishy HM, Al‐Gareeb AI. Effect of orlistat alone or in combination with Garcinia cambogia on visceral adiposity index in obese patients. J Intercult Ethnopharmacol. 2016;5:408‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Al‐Kuraishy HM, Al‐Gareeb AI, Qusty N, Alexiou A, Batiha GE. Impact of sitagliptin on non‐diabetic Covid‐19 patients. Curr Mol Pharmacol. 2022;15:683‐692. [DOI] [PubMed] [Google Scholar]
- 66. Al‐Kuraishy HM, Al‐Gareeb AI, Al‐Niemi MS, et al. The prospective effect of allopurinol on the oxidative stress index and endothelial dysfunction in Covid‐19. Inflammation. 2022;45:1651‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fraenkel M, Ketzinel‐Gilad M, Ariav Y, et al. mTOR inhibition by rapamycin prevents beta‐cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57:945‐957. [DOI] [PubMed] [Google Scholar]
- 68. Al‐Kuraishy HM, Al‐Gareeb AI, Alkazmi L, Alexiou A, Batiha GE. Levamisole therapy in COVID‐19. Viral Immunol. 2021;34:722‐725. [DOI] [PubMed] [Google Scholar]
- 69. Al‐Thomali AW, Al‐Kuraishy HM, Al‐Gareeb AI, et al. Role of neuropilin 1 in COVID‐19 patients with acute ischemic stroke. Biomedicine. 2022;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alorabi M, Cavalu S, Al‐Kuraishy HM, et al. Pentoxifylline and berberine mitigate diclofenac‐induced acute nephrotoxicity in male rats via modulation of inflammation and oxidative stress. Biomed Pharmacother. 2022;152:113225. [DOI] [PubMed] [Google Scholar]
- 71. Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393‐402. [DOI] [PubMed] [Google Scholar]
- 72. Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity‐linked insulin resistance. Endocrinology. 2005;146:1473‐1481. [DOI] [PubMed] [Google Scholar]
- 73. Tremblay F, Krebs M, Dombrowski L, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54:2674‐2684. [DOI] [PubMed] [Google Scholar]
- 74. Reifsnyder PC, Flurkey K, Te A, Harrison DE. Rapamycin treatment benefits glucose metabolism in mouse models of type 2 diabetes. Aging (Albany NY). 2016;8:3120‐3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhou W, Ye S. Rapamycin improves insulin resistance and hepatic steatosis in type 2 diabetes rats through activation of autophagy. Cell Biol Int. 2018;42:1282‐1291. [DOI] [PubMed] [Google Scholar]
- 76. Reifsnyder PC, Flurkey K, Doty R, Calcutt NA, Koza RA, Harrison DE. Rapamycin/metformin co‐treatment normalizes insulin sensitivity and reduces complications of metabolic syndrome in type 2 diabetic mice. Aging Cell. 2022;21:e13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Blagosklonny MV. Fasting and rapamycin: diabetes versus benevolent glucose intolerance. Cell Death Dis. 2019;10:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Al‐Buhadily AK, Al‐Uqabi RU, Al‐Gareeb AI. Evaluation of protective effect of metformin in rats with experimental osteoarthritis. Mustansiriya Med J. 2023;22:50‐53. [Google Scholar]
- 79. Ben Sahra I, Regazzetti C, Robert G, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell‐cycle arrest through REDD1. Cancer Res. 2011;71:4366‐4372. [DOI] [PubMed] [Google Scholar]
- 80. Amin S, Lux A, O'Callaghan F. The journey of metformin from glycaemic control to mTOR inhibition and the suppression of tumour growth. Br J Clin Pharmacol. 2019;85:37‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lu G, Wu Z, Shang J, Xie Z, Chen C, Zhang C. The effects of metformin on autophagy. Biomed Pharmacother. 2021;137:111286. [DOI] [PubMed] [Google Scholar]
- 82. Xu X, Sun Y, Cen X, et al. Metformin activates chaperone‐mediated autophagy and improves disease pathologies in an Alzheimer disease mouse model. Protein Cell. 2021;12:769‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fan X, Wang J, Hou J, et al. Berberine alleviates ox‐LDL induced inflammatory factors by up‐regulation of autophagy via AMPK/mTOR signaling pathway. J Transl Med. 2015;13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pang B, Zhao LH, Zhou Q, et al. Application of berberine on treating type 2 diabetes mellitus. Int J Endocrinol. 2015;2015:905749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zoppino FC, Militello RD, Slavin I, Alvarez C, Colombo MI. Autophagosome formation depends on the small GTPase Rab1 and functional ER exit sites. Traffic. 2010;11:1246‐1261. [DOI] [PubMed] [Google Scholar]
- 86. Petrovski G, Das S, Juhasz B, Kertesz A, Tosaki A, Das DK. Cardioprotection by endoplasmic reticulum stress‐induced autophagy. Antioxid Redox Signal. 2011;14:2191‐2200. [DOI] [PubMed] [Google Scholar]
- 87. Andersen TB, López CQ, Manczak T, Martinez K, Simonsen HT. Thapsigargin—from Thapsia L. to mipsagargin. Molecules. 2015;20:6113‐6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pick T, Gamayun I, Tinschert R, Cavalié A. Kinetics of the thapsigargin‐induced Ca(2+) mobilisation: a quantitative analysis in the HEK‐293 cell line. Front Physiol. 2023;14:1127545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lemos FO, Bultynck G, Parys JB. A comprehensive overview of the complex world of the endo‐and sarcoplasmic reticulum Ca2+−leak channels. Biochim Biophys Acta Mol Cell Res. 2021;1868:119020. [DOI] [PubMed] [Google Scholar]
- 90. Ren J, Bi Y, Sowers JR, Hetz C, Zhang Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol. 2021;18:499‐521. [DOI] [PubMed] [Google Scholar]
- 91. Gleitze S, Ramírez OA, Vega‐Vásquez I, et al. Ryanodine receptor mediated calcium release contributes to Ferroptosis induced in primary hippocampal neurons by GPX4 inhibition. Antioxidants. 2023;12:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lindner P, Christensen SB, Nissen P, Møller JV, Engedal N. Cell death induced by the ER stressor thapsigargin involves death receptor 5, a non‐autophagic function of MAP1LC3B, and distinct contributions from unfolded protein response components. Cell Commun Signal. 2020;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li J, Shirakawa J, Togashi Y, Terauchi Y. 2130‐P: Imeglimin modulated ER stress to prevent ß‐cell apoptosis induced by high glucose or thapsigargin. Diabetes. 2019;68 (Suppl 1). [Google Scholar]
- 94. Zarain‐Herzberg A, García‐Rivas G, Estrada‐Avilés R. Regulation of SERCA pumps expression in diabetes. Cell Calcium. 2014;56:302‐310. [DOI] [PubMed] [Google Scholar]
- 95. Balasubramanyam M, Balaji RA, Subashini B, Mohan V. Evidence for mechanistic alterations of Ca2+ homeostasis in type 2 diabetes mellitus. Int J Exp Diabetes Res. 2001;1:275‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kamiya T, Hara H, Adachi T. Effect of endoplasmic reticulum (ER) stress inducer thapsigargin on the expression of extracellular‐superoxide dismutase in mouse 3T3‐L1 adipocytes. J Clin Biochem Nutr. 2013;52:101‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pandey VK, Mathur A, Kakkar P. Emerging role of unfolded protein response (UPR) mediated proteotoxic apoptosis in diabetes. Life Sci. 2019;216:246‐258. [DOI] [PubMed] [Google Scholar]
- 98. Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta‐cell failure and diabetes. Endocr Rev. 2008;29:317‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Iwawaki T, Oikawa D. The role of the unfolded protein response in diabetes mellitus. Semin Immunopathol. 2013;35:333‐350. [DOI] [PubMed] [Google Scholar]
- 100. Harrison PJ, Cipriani A, Harmer CJ, et al. Innovative approaches to bipolar disorder and its treatment. Ann N Y Acad Sci. 2016;1366:76‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Forlenza OV, De‐Paula VJ, Diniz BS. Neuroprotective effects of lithium: implications for the treatment of Alzheimer's disease and related neurodegenerative disorders. ACS Chem Nerosci. 2014;5:443‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Puglisi‐Allegra S, Lazzeri G, Busceti CL, Giorgi FS, Biagioni F, Fornai F. Lithium engages autophagy for neuroprotection and neuroplasticity: translational evidence for therapy. Neurosci Biobehav Rev. 2023;148:105148. [DOI] [PubMed] [Google Scholar]
- 103. Rohde C, Köhler‐Forsberg O, Nierenberg AA, Østergaard SD. Pharmacological treatment of bipolar disorder and risk of diabetes mellitus: a nationwide study of 30,451 patients. Bipolar Disord. 2023;25:323‐334. [DOI] [PubMed] [Google Scholar]
- 104. Ostrovskaya RU, Ivanov SV. Neuroprotective substances: are they able to protect the pancreatic beta‐cells too? Endocr Metab Immune Disord Drug Targets. 2022;22:834‐841. [DOI] [PubMed] [Google Scholar]
- 105. Zhang J, Anshul F, Malhotra DK, Jaume J, Dworkin LD, Gong R. Microdose lithium protects against pancreatic islet destruction and renal impairment in Streptozotocin‐elicited diabetes. Antioxidants (Basel). 2021;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lee JTC, Shanina I, Chu YN, Horwitz MS, Johnson JD. Carbamazepine, a beta‐cell protecting drug, reduces type 1 diabetes incidence in NOD mice. Sci Rep. 2018;8:4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Khan S, Kumar S, Jena G. Valproic acid reduces insulin‐resistance, fat deposition and FOXO1‐mediated gluconeogenesis in type‐2 diabetic rat. Biochimie. 2016;125:42‐52. [DOI] [PubMed] [Google Scholar]
- 108. Sharma S, Taliyan R. Histone deacetylase inhibitors: future therapeutics for insulin resistance and type 2 diabetes. Pharmacol Res. 2016;113:320‐326. [DOI] [PubMed] [Google Scholar]
- 109. Xia Q, Zheng Y, Jiang W, et al. Valproic acid induces autophagy by suppressing the Akt/mTOR pathway in human prostate cancer cells. Oncol Lett. 2016;12:1826‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Puls F, Goldschmidt I, Bantel H, et al. Autophagy‐enhancing drug carbamazepine diminishes hepatocellular death in fibrinogen storage disease. J Hepatol. 2013;59:626‐630. [DOI] [PubMed] [Google Scholar]
- 111. Sarkar S, Floto RA, Berger Z, et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fichtner F, Lunn JE. The role of Trehalose 6‐phosphate (Tre6P) in plant metabolism and development. Annu Rev Plant Biol. 2021;72:737‐760. [DOI] [PubMed] [Google Scholar]
- 113. Kupnik K, Primožič M, Knez Ž, Leitgeb M. Chapter 9—Trehalose. In: Gupta VK, ed. Valorization of Biomass to Bioproducts. Elsevier; 2023:163‐207. [Google Scholar]
- 114. Khalifeh M, Barreto GE, Sahebkar A. Therapeutic potential of trehalose in neurodegenerative diseases: the knowns and unknowns. Neural Regen Res. 2021;16:2026‐2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rebholz CM, Yu B, Zheng Z, et al. Serum metabolomic profile of incident diabetes. Diabetologia. 2018;61:1046‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Radbakhsh S, Ganjali S, Moallem SA, Guest PC, Sahebkar A. Antioxidant effects of Trehalose in an experimental model of type 2 diabetes. Adv Exp Med Biol. 2021;1328:473‐480. [DOI] [PubMed] [Google Scholar]
- 117. Korolenko TA, Ovsyukova MV, Bgatova NP, et al. Trehalose activates hepatic and myocardial autophagy and has anti‐inflammatory effects in db/db diabetic mice. Life (Basel). 2022;12:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mizote A, Yamada M, Yoshizane C, et al. Daily intake of Trehalose is effective in the prevention of lifestyle‐related diseases in individuals with risk factors for metabolic syndrome. J Nutr Sci Vitaminol (Tokyo). 2016;62:380‐387. [DOI] [PubMed] [Google Scholar]
- 119. Guo C, Xu Y, Ma Y, et al. Individual and joint effects of trehalose and glutamate on diabetic retinopathy: a propensity score‐matched case‐control study. Endocr. Connect. 2022;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sokołowska E, Sadowska A, Sawicka D, Kotulska‐Bąblińska I, Car H. A head‐to‐head comparison review of biological and toxicological studies of isomaltulose, d‐tagatose, and trehalose on glycemic control. Crit Rev Food Sci Nutr. 2022;62:5679‐5704. [DOI] [PubMed] [Google Scholar]
- 121. Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170:605‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mostafa‐Hedeab G, Al‐Kuraishy HM, Al‐Gareeb AI, Jeandet P, Saad HM, Batiha GE. A raising dawn of pentoxifylline in management of inflammatory disorders in Covid‐19. Inflammopharmacology. 2022;30:799‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hasan Khudhair D, Al‐Gareeb AI, Al‐Kuraishy HM, et al. Combination of vitamin C and curcumin safeguards against methotrexate‐induced acute liver injury in mice by synergistic antioxidant effects. Front Med (Lausanne). 2022;9:866343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Al‐Kuraishy HM, Al‐Gareeb AI. From SARS‐CoV to nCoV‐2019: ruction and argument. Arch Clin Infect Dis. 2020;15:e102624. [Google Scholar]
- 125. Alsubaie N, Al‐Kuraishy HM, Al‐Gareeb AI, et al. Statins use in Alzheimer disease: bane or boon from frantic search and narrative review. Brain Sci. 2022;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang M, Yang L, Chen Z, et al. Geniposide ameliorates chronic unpredictable mild stress induced depression‐like behavior through inhibition of ceramide‐PP2A signaling via the PI3K/Akt/GSK3β axis. Psychopharmacology (Berl). 2021;238:2789‐2800. [DOI] [PubMed] [Google Scholar]
- 127. Bedia C, Levade T, Codogno P. Regulation of autophagy by sphingolipids. Anticancer Agents Med Chem. 2011;11:844‐853. [DOI] [PubMed] [Google Scholar]
- 128. Arana L, Gangoiti P, Ouro A, Trueba M, Gómez‐Muñoz A. Ceramide and ceramide 1‐phosphate in health and disease. Lipids Health Dis. 2010;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sindhu RK, Verma R, Salgotra T, et al. Impacting the remedial potential of Nano delivery‐based flavonoids for breast cancer treatment. Molecules. 2021;26:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Al‐Kuraishy HM, Al‐Gareeb AI, Al‐Nami MS. Irbesartan attenuates gentamicin‐induced nephrotoxicity in rats through modulation of oxidative stress and endogenous antioxidant capacity. Int J Prev Med. 2020;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Al‐Kuraishy HM, Al‐Gareeb AI, Hussien NR, Al‐Naimi MS, Rasheed HA. Statins an oft‐prescribed drug is implicated in peripheral neuropathy: the time to know more. J Pak Med Assoc. 2019;69(Suppl 3):S108‐s112. [PubMed] [Google Scholar]
- 132. Chavez JA, Summers SA. A ceramide‐centric view of insulin resistance. Cell Metab. 2012;15:585‐594. [DOI] [PubMed] [Google Scholar]
- 133. Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608‐36615. [DOI] [PubMed] [Google Scholar]
- 134. Al‐Kuraishy HM, Al‐Gareeb AI, Butnariu M, Batiha GE. The crucial role of prolactin‐lactogenic hormone in Covid‐19. Mol Cell Biochem. 2022;477:1381‐1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Al‐Kuraishy HM, Al‐Gareeb AI. Potential effects of pomegranate on lipid peroxidation and pro‐inflammatory changes in daunorubicin‐induced cardiotoxicity in rats. Int J Prev Med. 2016;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Batiha GE, Al‐Gareeb DAI, Qusti S, et al. WITHDRAWN: common NLRP3 inflammasome inhibitors and Covid‐19: divide and conquer. Sci Afr. 2021;18:e01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid‐, saturated‐fat‐, and obesity‐induced insulin resistance. Cell Metab. 2007;5:167‐179. [DOI] [PubMed] [Google Scholar]
- 138. Yaribeygi H, Bo S, Ruscica M, Sahebkar A. Ceramides and diabetes mellitus: an update on the potential molecular relationships. Diabet Med. 2020;37:11‐19. [DOI] [PubMed] [Google Scholar]
- 139. Baudry M. Calpain‐1 and Calpain‐2 in the brain: Dr. Jekill and Mr Hyde? Curr Neuropharmacol. 2019;17:823‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wang Y, Liu Y, Bi X, Baudry M. Calpain‐1 and Calpain‐2 in the brain: new evidence for a critical role of Calpain‐2 in neuronal death. Cells. 2020;9:2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Nian H, Ma B. Calpain–calpastatin system and cancer progression. Biol Rev. 2021;96:961‐975. [DOI] [PubMed] [Google Scholar]
- 142. Johnson JD, Otani K, Bell GI, Polonsky KS. Impaired insulin secretion in transgenic mice over‐expressing calpastatin in pancreatic β‐cells. Islets. 2009;1:242‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhu L, Gong L, Yang T, Xiao X. Calpastatin mediates development of Alzheimer's disease in diabetes. J Alzheimers Dis. 2019;68:1051‐1059. [DOI] [PubMed] [Google Scholar]
- 144. Singh M. Calpastatin Over‐Expression Reduces Cardiac Fibrosis in a Murine Model of Type 2 Diabetes. Master's Thesis. Western University; 2011. [Google Scholar]
- 145. Carragher NO. Calpain inhibition: a therapeutic strategy targeting multiple disease states. Curr Pharm des. 2006;12:615‐638. [DOI] [PubMed] [Google Scholar]
- 146. Furterer AE, Gurlo T, Wang Q, et al. Type 2 diabetes is a beta cell protein misfolding disease. Social Science Research Network. 2020. [Google Scholar]
- 147. Jayanarayanan S, Anju TR, Smijin S, Paulose CS. Vitamin D3 supplementation increases insulin level by regulating altered IP3 and AMPA receptor expression in the pancreatic islets of streptozotocin‐induced diabetic rat. J Nutr Biochem. 2015;26:1041‐1049. [DOI] [PubMed] [Google Scholar]
- 148. Zhang Z, Tian J, Liao Q, Wang Y. The analysis of expression of CCK and IP3 receptors in gallstones patients with type 2 diabetes mellitus. Hepatogastroenterology. 2014;61:2173‐2176. [PubMed] [Google Scholar]
- 149. Suzuki T, Imai J, Yamada T, et al. Interleukin‐6 enhances glucose‐stimulated insulin secretion from pancreatic beta‐cells: potential involvement of the PLC‐IP3‐dependent pathway. Diabetes. 2011;60:537‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Criollo A, Maiuri MC, Tasdemir E, et al. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14:1029‐1039. [DOI] [PubMed] [Google Scholar]
- 151. Lewis AJ, Billiar TR, Rosengart MR. Biology and metabolism of sepsis: innate immunity, bioenergetics, and autophagy. Surg Infect (Larchmt). 2016;17:286‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Park HW, Park H, Semple IA, et al. Pharmacological correction of obesity‐induced autophagy arrest using calcium channel blockers. Nat Commun. 2014;5:4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Park HW, Lee JH. Calcium channel blockers as potential therapeutics for obesity‐associated autophagy defects and fatty liver pathologies. Autophagy. 2014;10:2385‐2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bergantin LB. Debating the “bidirectional link” between diabetes and depression through the Ca2+/cAMP signalling: off‐label effects of Ca2+ channel blockers. Pharmacol Res. 2019;141:298‐302. [DOI] [PubMed] [Google Scholar]
- 155. Türkeş C, Demir Y, Beydemir Ş. Anti‐diabetic properties of Calcium Channel blockers: inhibition effects on aldose reductase enzyme activity. Appl Biochem Biotechnol. 2019;189:318‐329. [DOI] [PubMed] [Google Scholar]
- 156. Emara E, Abdel‐Sater K. Beneficial effects of calcium channel blocker “nifedipine” on abnormalities of platelets and lipid metabolism in patients with type II diabetes mellitus. Diabetes Metab. 2011;2:131. [Google Scholar]
- 157. Dunmore BJ, Drake KM, Upton PD, Toshner MR, Aldred MA, Morrell NW. The lysosomal inhibitor, chloroquine, increases cell surface BMPR‐II levels and restores BMP9 signalling in endothelial cells harbouring BMPR‐II mutations. Hum Mol Genet. 2013;22:3667‐3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Mauthe M, Orhon I, Rocchi C, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome‐lysosome fusion. Autophagy. 2018;14:1435‐1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Gerstein HC, Thorpe KE, Taylor DW, Haynes RB. The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas—a randomized trial. Diabetes Res Clin Pract. 2002;55:209‐219. [DOI] [PubMed] [Google Scholar]
- 160. Pareek A, Chandurkar N, Thomas N, et al. Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: a double blind, randomized comparison with pioglitazone. Curr Med Res Opin. 2014;30:1257‐1266. [DOI] [PubMed] [Google Scholar]
- 161. Dutta D, Jindal R, Mehta D, Kumar M, Sharma M. Efficacy and safety of hydroxychloroquine for managing glycemia in type‐2 diabetes: a systematic review and meta‐analysis. J Postgrad Med. 2022;68:85‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Amaravadi RK, Winkler JD. Lys05: a new lysosomal autophagy inhibitor. Autophagy. 2012;8:1383‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. McAfee Q, Zhang Z, Samanta A, et al. Autophagy inhibitor Lys05 has single‐agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc Natl Acad Sci U S A. 2012;109:8253‐8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Watanabe‐Asano T, Kuma A, Mizushima N. Cycloheximide inhibits starvation‐induced autophagy through mTORC1 activation. Biochem Biophys Res Commun. 2014;445:334‐339. [DOI] [PubMed] [Google Scholar]
- 165. Li C, Chen L, Song M, et al. Ferulic acid protects cardiomyocytes from TNF‐α/cycloheximide‐induced apoptosis by regulating autophagy. Arch Pharm Res. 2020;43:863‐874. [DOI] [PubMed] [Google Scholar]
- 166. Angervo M, Leinonen P, Koistinen R, Julkunen M, Seppälä M. Tri‐iodothyronine and cycloheximide enhance insulin‐like growth factor‐binding protein‐1 gene expression in human hepatoma cells. J Mol Endocrinol. 1993;10:7‐13. [DOI] [PubMed] [Google Scholar]
- 167. Ooi GT, Brown DR, Suh DS, Tseng LY, Rechler MM. Cycloheximide stabilizes insulin‐like growth factor‐binding protein‐1 (IGFBP‐1) mRNA and inhibits IGFBP‐1 transcription in H4‐II‐E rat hepatoma cells. J Biol Chem. 1993;268:16664‐16672. [PubMed] [Google Scholar]
- 168. Matthaei S, Olefsky JM, Karnieli E. Cycloheximide decreases glucose transporters in rat adipocyte plasma membranes without affecting insulin‐stimulated glucose transport. Biochem J. 1988;251:491‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lee H, Koh JY. Roles for H(+) /K(+)‐ATPase and zinc transporter 3 in cAMP‐mediated lysosomal acidification in bafilomycin A1‐treated astrocytes. Glia. 2021;69:1110‐1125. [DOI] [PubMed] [Google Scholar]
- 170. Rong W, Wang J, Hassan A, Lee C‐H, Xie X‐S, Li X. Molecular basis of V‐ATPase inhibition by bafilomycin A1. Nat Commun. 2021;12:1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hirao J, Tojo A, Hatakeyama S, Satonaka H, Ishimitsu T. V‐ATPase blockade reduces renal gluconeogenesis and improves insulin secretion in type 2 diabetic rats. Hypertens Res. 2020;43:1079‐1088. [DOI] [PubMed] [Google Scholar]
- 172. Hettiarachchi KD, Zimmet PZ, Myers MA. Transplacental exposure to bafilomycin disrupts pancreatic islet organogenesis and accelerates diabetes onset in NOD mice. J Autoimmun. 2004;22:287‐296. [DOI] [PubMed] [Google Scholar]
- 173. Molina M. Evidence for the Down‐Regulation of ATP6V1H Gene Expression in Type 2 Diabetes. Honors Thesis. The University of Texas at Austin; 2012. [Google Scholar]


