Abstract
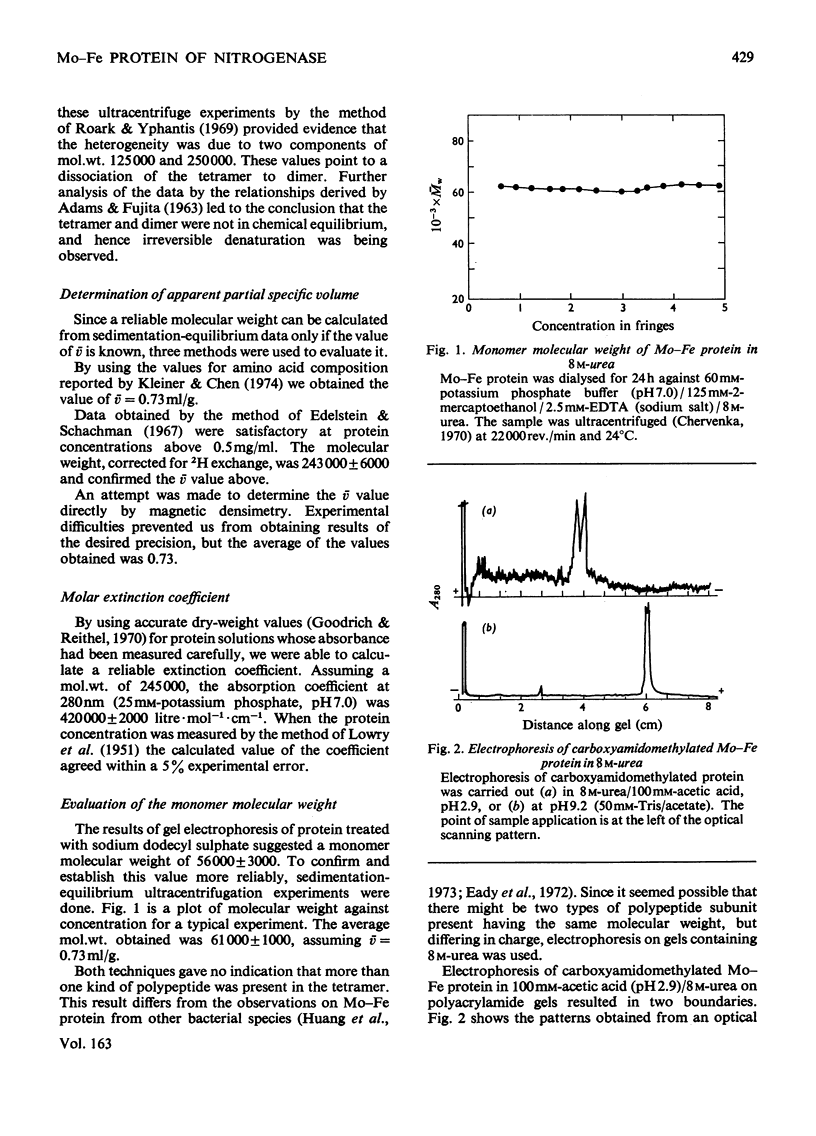
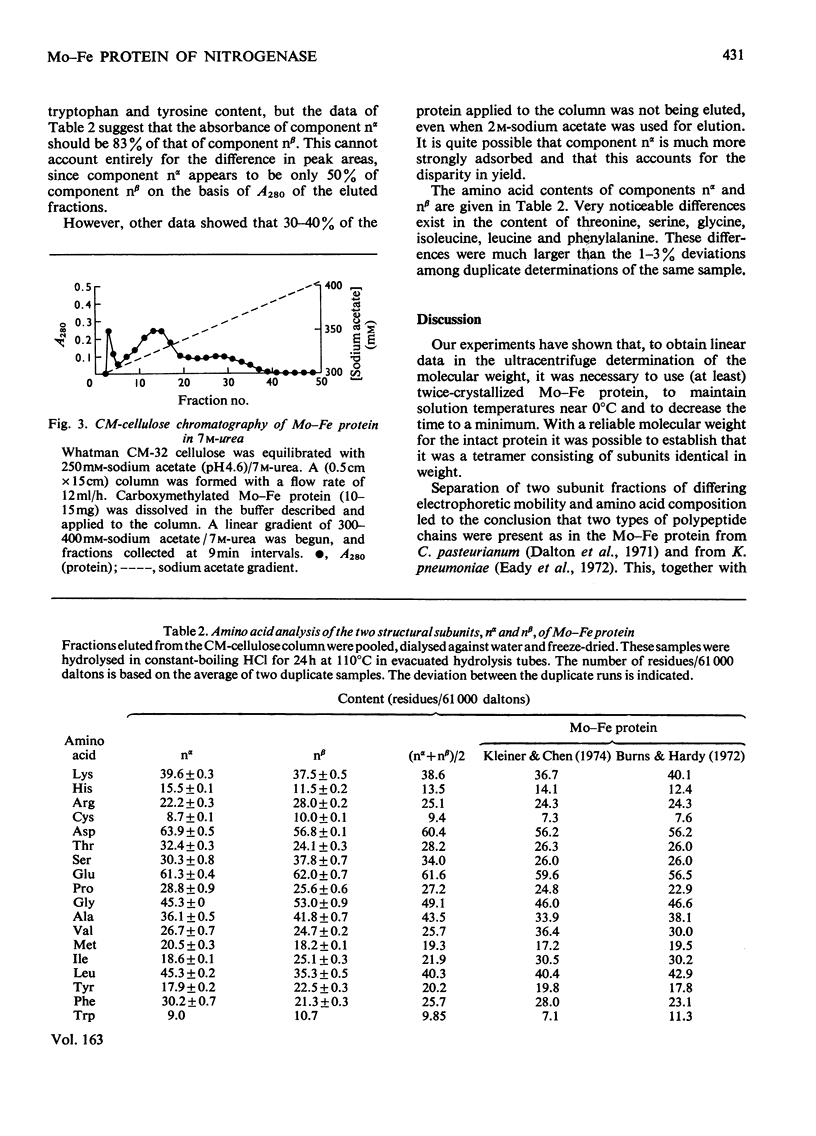
The weight-average molecular weight of the Mo-Fe protein isolated from Azotobacter vinelandii has been determined by sedimentation-equilibrium techniques. In buffer, the value is 245000+/-5000; in 8M-urea, the value is 61000+/-1000. The protein was separated into two components by chromatography on CM-cellulose in 7M-urea, pH 4.5. These components have similar molecular weights but were shown to differ in charge, amino acid content and arginine-containing peptides. It is proposed that the tetramer has the subunit composition (nalpha2nbeta2).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bulen W. A., LeComte J. R. Nitrogenase complex and its components. Methods Enzymol. 1972;24:456–470. doi: 10.1016/0076-6879(72)24091-5. [DOI] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. C., Hardy R. W. Purification of nitrogenase and crystallization of its Mo-Fe protein. Methods Enzymol. 1972;24:480–496. doi: 10.1016/0076-6879(72)24094-0. [DOI] [PubMed] [Google Scholar]
- Burns R. C., Holsten R. D., Hardy R. W. Isolation by crystallization of the Mo-Fe protein of Azotobacter nitrogenase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):90–99. doi: 10.1016/0006-291x(70)90762-x. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Chervenka C. H. Long-column meniscus depletion sedimentation equilibrium technique for the analytical ultracentrifuge. Anal Biochem. 1970 Mar;34:24–29. doi: 10.1016/0003-2697(70)90082-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dalton H., Morris J. A., Ward M. A., Mortenson L. E. Purification and some properties of molybdoferredoxin, a component of nitrogenase from Clostridium pasteurianum. Biochemistry. 1971 May 25;10(11):2066–2072. doi: 10.1021/bi00787a016. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein S. J., Schachman H. K. The simultaneous determination of partial specific volumes and molecular weights with microgram quantities. J Biol Chem. 1967 Jan 25;242(2):306–311. [PubMed] [Google Scholar]
- Goodrich R., Reithel F. J. Macromolecule dry weight determination with a vacuum balance. Anal Biochem. 1970 Apr;34(2):538–543. doi: 10.1016/0003-2697(70)90139-9. [DOI] [PubMed] [Google Scholar]
- Goodrich R., Swinehart D. F., Kelly M. J., Reithel F. J. A transistor-controlled magnetic densitometer designed for small sample volumes. Anal Biochem. 1969 Apr 4;28(1):25–34. doi: 10.1016/0003-2697(69)90153-5. [DOI] [PubMed] [Google Scholar]
- Huang T. C., Zumft W. G., Mortenson L. E. Structure of the molybdoferredoxin complex from Clostridium pasteurianum and isolation of its subunits. J Bacteriol. 1973 Feb;113(2):884–890. doi: 10.1128/jb.113.2.884-890.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D., Chen C. H. Physical and chemical properties of the nitrogenase proteins form Azotobacter vinelandii. Arch Mikrobiol. 1974 Jun 7;98(1):93–100. doi: 10.1007/BF00425272. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Roark D. E., Yphantis D. A. Studies of self-associating systems by equilibrium ultracentrifugation. Ann N Y Acad Sci. 1969 Nov 7;164(1):245–278. doi: 10.1111/j.1749-6632.1969.tb14043.x. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Nitrogenase. IV. Simple method of purification to homogeneity of nitrogenase components from Azotobacter vinelandii. Biochim Biophys Acta. 1973 May 30;305(2):445–454. doi: 10.1016/0005-2728(73)90190-4. [DOI] [PubMed] [Google Scholar]
- Swisher R. H., Landt M., Reithel F. J. Molecular weights of nitrogenase components from Azotobacter vinelandii. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1476–1482. doi: 10.1016/0006-291x(75)90525-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamada S., Itano H. Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta. 1966 Dec 28;130(2):538–540. doi: 10.1016/0304-4165(66)90256-x. [DOI] [PubMed] [Google Scholar]


