Abstract
The early coding region of JC virus (JCV) encodes several regulatory proteins including large T antigen (LT-Ag), small t antigen (Sm t-Ag) and T’ proteins because of the alternative splicing of the pre-mRNA. LT-Ag plays a critical role in cell transformation by targeting the key cell cycle regulatory proteins including p53 and pRb, however, the role of Sm t-Ag in this process remains elusive. Here, we investigated the effect of Sm t-Ag on the cell cycle progression and demonstrated that it facilitates S phase entry and exit when cells are released from G0/G1 growth arrest. Examination of the cell cycle stage specific expression profiles of the selected cyclins and cyclin-dependent kinases, including those active at the G1/S and G2/M transition state, demonstrated a higher level of early expression of these regulators such as cyclin B, cycling E, and Cdk2. In addition, analysis of the effect of Sm t-Ag on the growth promoting pathways including those active in the PI3K/Akt/mTOR axis showed substantially higher levels of the phosphorylated-Akt, -Gsk3-β and -S6K1 in Sm t-Ag-positive cells. Collectively, our results demonstrate that Sm t-Ag promotes cell cycle progression by activating the growth promoting pathways through which it may contribute to LT-Ag-mediated cell transformation.
Keywords: Polyomavirus, JC virus, BK virus, Transformation, Cell cycle, Papillomavirus, Cancer, Merkel cell carcinoma virus
1. Introduction
Neoplastic transformation of human cells results from the combination of multiple genetic and biochemical alterations. DNA tumor viruses have made a significant contribution to our understanding of some of those alterations [1,2], because they have been frequently used as tools to understand the altered pathways, leading to the development of transformed phenotype.
The polyomaviruses are all small DNA viruses (∼5 kb genome) and encode tumorigenic proteins, large and small t antigens. To date, 14 members were isolated from humans [3], several of which either induce human diseases or cause cancers. Among those, for example, the human JC virus (JCV) causes a fetal brain disease, known as progressive multifocal leukoencephalopathy in certain subset of immunocompromised individuals [[4], [5], [6]] while BK virus (BKV) creates a serious concern for the organ transplant patients and may lead to kidney transplant injury by infection, known as BK virus-associated nephropathy (BKVAN or BKN) [[7], [8], [9]]. In addition, the integrated form of BKPyV was also found to be associated with human tumors [10,11]. Besides these two viruses, only trichodysplasia, spinulosa-associated polyomavirus (TSPyV) [12,13] and merkel cell carcinoma-associated polyomavirus (MCPyV) were also reported to cause human diseases, such as trichodysplasia spinulosa [13] and merkel cell carcinoma [14,15] respectively.
The genome of these viruses has been shown to be oncogenic in different experimental animal models [[16], [17], [18], [19], [20], [21], [22], [23], [24]]. Previous data indicate that these viruses may also be associated with a variety of human tumors [1,[25], [26], [27], [28]]. Oncogenic potential of these viruses results from the tumorigenic activity of their early proteins, LT-Ag and Sm t-Ag. LT-Ag was found to primarily target and inactivate two key tumor suppressor proteins, retinoblastoma protein (pRb) and p53, through which it dysregulates cell cycle progression and contributes to the development of a transformed phenotype [[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]]. In addition, recent proteomic studies revealed that JCV LT-Ag targets a number of cellular proteins, protein complexes and organelles in cells, including, Smc 5/6 complex, protein phosphatase 4, E3-ubiquitin protein ligase, V-ATPase, ribosomal proteins and actin-myosin network proteins [42].
Unlike LT-Ag, the function of Sm t-Ag (t-Ag) in cellular transformation is largely unknown. However, functional studies with SV40 Sm t-Ag showed that it plays an important role in LT-Ag-mediated cell transformation [2,29,[43], [44], [45], [46]]. It is also believed that Sm t-Ag antagonizes LT-Ag-induced cellular apoptosis and thereby contributes to more efficient transformation of rat embryo fibroblasts [47]. In addition, transgenic animals created with a Sm t-Ag deletion mutant of SV40 genome developed tumors almost exclusively in highly mitotic tissues such as lymphoid organs [48,49] suggesting that Sm t-Ag may play a significant role in tumor induction in nonproliferative tissues. This tumorigenic potential of Sm t-Ag is linked to the inhibition of protein phosphatase 2A (PP2A), an abundant family of serine/threonine phosphatases [37,50,51] or Sm t-Ag diverts PP2A function towards specific substrates rather than inhibiting its function [52]. In addition, several growth promoting pathways were also found to be altered in the presence of SV40 Sm t-Ag [29,44,50,53] including activation of phosphotidylinositol (PI) 3-kinase [53], protein kinase Cζ (PKCζ) [53] and mitogen activated protein kinase [50]. Furthermore, expression of Sm t-Ag in cells was observed to be associated with phosphorylation state of Akt [54] and Shc [55]; and stimulation of AP-1 [56]. Similar to the JCV LT-Ag proteomic studies, JCV Sm t-Ag was also found to target a number of proteins, protein complexes and organelles in cells, including actin/myosin network, chromatin remodeling complexes, mitochondrial proteins, chaperon proteins, protein transport, heterogeneous ribonuclear proteins, proteins phosphatase 2 and ribosomal proteins [42]. Interestingly, cell transformation studies using MCPyV Sm-Ag showed a striking association between MCPyV Sm t-Ag and tumor induction and such studies even further revealed that MCPyV Sm-Ag alone is more aggressive with respect to cell transformation than that of MCPyV LT-Ag [[57], [58], [59], [60], [61], [62]].
JCV Sm t-Ag is a small protein consisting of 172 amino acids, 82 amino acids of which are shared with N-terminal sequences of LT-Ag. It shows 30 % and 18 % sequence homology to SV40 and BKV Sm t-Ags respectively. Amino acid alignment of all three proteins shows that most of the divergent sequences are localized towards the central portion of the proteins. Since recent data suggest that JCV is associated with a variety of human tumors and studies with SV40 Sm t-Ag demonstrate an important role for it in cell transformation, we attempted to analyze the effect of this protein cell proliferation pathways. Our results demonstrated that JCV Sm t-Ag positive cells enter and exit S phase of the cell cycle with a significantly higher rate than the control, and these findings may have implications in JCV-induced cell transformation.
2. Materials and methods
Cell lines. U-87MG, a human glioblastoma cell line, was grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10 % heat-inactivated fetal bovine serum (FBS) and antibiotics (penicillin/streptomycin, 100 μg/ml). The cells were maintained at 37 °C in a humidified atmosphere with 7 % CO2.
Plasmid constructs. An expression plasmid (pcDNA3 JCV HA-Sm t-Ag) for JCV Sm t-Ag was created by a PCR amplification method. The coding sequence of JCV Sm t-Ag was PCR-amplified and subcloned into an expression vector (pcDNA3, Invitrogen) at BamHI/EcoRI sites but 5′-end of the protein was tagged with a hemagglutinin (HA) tag. JCV full length genome was used as a template for PCR amplification with specific primers:
5′-primer. (5′-ACCTTCCAGGATCCATGGACAAAGTGCTGAATAGGGAG-3′)
3′-primer. (5′-ACCTCCAGAATTCTTAAAGCTTTAGATCCCTGTA -3′).
In addition, JCV Sm t-Ag full length and its deletion mutant were also cloned into a prokaryotic expression vector (pGEX2T) at BamHI/EcoRI site by a PCR amplification method using specific primers.
Forward primers were prepared according to the JCV Mad-1 strain numberin (Accession number: J02226.1).
JCV Sm t-Ag 5′-BamHI (5013-4990)
(5′-ACCTTCCAGGATCC ATGGACAAAGTGCTGAATAGGGAG-3′),
FP aa 82 (4745–4767) (5′-ACCTTCCAGGATCC GGTTGTGATTTTCCTAATTC-3′
Reverse primer:
JCV Sm t-Ag 3′-EcoRI (nt 4495–4515) (5′-ACCTCCAGAATTC TTAAAGCTTTAGATCCCTGTA -3′)
Resulting PCR fragments were digested with BamHI/EcoRI and subcloned into the BamHI/EcoRI sites of the pGEX2T vector.
Human cyclin E promoter reporter plasmid (hCyc E (−363 to +1007)-pBLCAT3) was also created by PCR amplification method. Human cyclin E promoter region (−363 to +1007) was PCR amplified by using the following primers:
5'-primer (5′-ACGACGTTAAAGCTTGCGCCAGCCACGCGGCTTTTTGCC–3′) and 3′-primer (5′-ACGACGTTACTCGAGGGATTCTGCAAAAACTAACATGAA-3′). Specific PCR product was digested with Hind III/XhoI restriction enzymes and subcloned into the Hind III/XhoI sites of pBLCAT3 vector. A hCyc E (−363 to +1007)-Luc plasmid (a kind gift from Dr. R. A. Weinberg) was used a template for PCR amplification.
Stable cell lines. U-87MG cells were grown in subconfluency (2.5x105 cells/100 mm culture dish) and were stably transfected either with an expression vector containing HA-tagged JCV Sm t-Ag cDNA (pcDNA3-HA JCV Sm t-Ag) or with an empty vector (pcDNA3-HA). Transfected cells were then grown in a selection media containing G418 drug (400 μg/ml) for two weeks and positive colonies were selected by western blotting and used for cell cycle analysis by flow cytometry.
Indirect immunofluorescence microscopy. Indirect immunofluorescence microscopy studies were conducted as previously described [63,64]. Briefly, U-87MG cells transfected with pcDNA3 JCV HA-Sm t-Ag expression vector were seeded at subconfluency on polylysine-coated glass chamber slides. After two days following transfection, cells were washed twice with phosphate buffered saline (PBS) and fixed with cold acetone. Fixed cells were blocked with 5 % bovine serum albumin in PBS for 2 h and incubated with an anti-HA mouse monoclonal antibody (ThermoFisher Scientific, catalog no. 26183) overnight. Cells were subsequently washed four times with PBS-0.01 % Tween 20 for 10-min intervals and incubated with an anti-mouse fluorescein isothiocyanate (FITC)-conjugated goat secondary antibody (ThermoFisher Scientific, catalog no. 31569) for 45 min. Samples were then stained with DAPI (ThermoFisher, 4',6-Diamidino-2-Phenylindole, Dihydrochloride, catalog no. D1306) and mounted using “ProLong® Gold Antifade” mounting medium (Life Technologies, catalog no. P36934). Slides were then examined under a fluorescence microscope (Leica, DMI-6000B, objective: HCX PL APD 40x/1.25 oil, employing LAS AF operating software) for visualization of Sm t-Ag protein.
Cell synchronization by serum starvation. Either control cells (U-87MG cell stably transfected with empty vector) or the cells stably expressing Sm t-Ag were arrested at the G0/G1 phase of the cell cycle stage by serum deprivation for 72 h. Synchronized cells were then released from the arrested state by rinsing twice with PBS and feeding the cells with complete growth medium [DMEM, supplemented with 10 % FBS and antibiotics (penicillin/streptomycin, 100 μg/ml)].
Cell proliferation and flow cytometry. For cell proliferation assay, either control (U-87MG-stably transfected with an empty vector, pcDNA3-HA) cells or the cells stably expressing Sm t-Ag were plated in 100 mm petri tissue culture dishes at a density of 1x105 cells/plate and maintained in complete growth media (DMEM). Cells were then trypsinized and collected at various times points; and counted to determine cell number for control versus experimental samples. For flow cytometry analysis, both control and Sm t-Ag expressing cells were synchronized by serum deprivation, harvested, fixed in 70 % ethanol, treated with RNAse A (15 μg/ml) at 370C for 30 min, washed with phosphate-buffered saline (PBS), stained with propidium iodide (50 μg/ml in PBS final) and were kept at 40C. The DNA content of the cells was then determined with a Becton Dickinson FACScan flow cytometer at the Wistar Institute Flow Cytometry Facility, Philadelphia, PA, USA.
GST affinity chromatography assay (GST-pull down). Expression and purification of GST and GST fusion proteins were previously described [65,66]. Interaction of Sm t-Ag with eEF1A was tested by GST-pull down assay as described previously [66,67]. Two micrograms of either GST or GST-Sm t-Ag immobilized on Sepharose beads were incubated with whole cell extracts (500 μg/0.5 ml) prepared from U-87MG cells for 2 h at 40C in lysis buffer [50 mM Tris-HCL (pH 7.4), 150 mM NaCl, and 0.5 % Nonidet P-40, supplemented with protease inhibitors (ThermoFisher Scientific, catalog no. A32963),]. Columns were then washed extensively with lysis buffer and bound proteins were resolved by SDS-10 % PAGE followed by Western blot analysis using an anti-eEF1A rabbit polyclonal antibody (Cell Signaling, catalog no. 3586).
Immunoprecipitation and Western blotting. Whole cell lysates were prepared from control (U-87MG stably transfected with pcDNA3-HA empty vector) cells or the cells stably expressing HA-tagged Sm t-Ag in lysis buffer [20 mM Tris-HCl (pH 7.4), 1 mM EDTA, 150 mM NaCl, 1 % Triton X-100 and with complete protease inhibitor cocktail (Sigma, catalog no. 11836170001)]. Fifty micrograms of cell lysates were fractionated by SDS-12 % PAGE and transferred onto a nylon membrane (250 mA, 30 min). Blotted membrane was probed with a monoclonal antibody directed against HA tag of Sm t-Ag and was developed by an ECL kit (ThermoFisher Scientific, catalog no. A38555) according to the manufacture's recommendations. In parallel, 100 μg of whole cell lysates prepared from both control and Sm t-Ag-expressing cells were with an anti-HA antibody (2 μg) for 2 h at 40C. Antibody-protein complexes were precipitated with protein A Sepharose beads (20 μl of 50 % slurry), washed with lysis buffer and analyzed by Western blotting using a monoclonal antibody raised against HA tag. In addition, nuclear extracts were also prepared according to the previously described protocols to analyze the cell cycle stage specific cyclins and cyclin dependent kinases [68]. The rate of appearance of cyclin E, cyclin B and Cdk2 was analyzed by Western blotting too using Santa Cruz Biotechnology antibodies including anti-cyclin E (catalog no. Sc-2470, anti-cyclin B (Sc-166210, and anti-Cdk2 (Sc-6248).
Kinase assay. Whole cell extracts were prepared from both control cells (U-87MG transfected with pcDNA3-HA empty vector) and U-87MG cells stably expressing HA-tagged Sm t-Ag in lysis buffer [20 mM Tris-HCl (pH 7.4), 1 mM EDTA, 150 mM NaCl, 1 % Triton X-100 and a complete protease inhibitor cocktail]. Four hundred micrograms of protein lysate either from control cells or cells stably expressing HA-tagged Sm t-Ag were incubated with an immobilized anti-Cdk2 antibody (Santa Cruz Biotechnology, catalog no. Sc-6248) overnight at 40C. Immunoprecipitants were extensively washed in lysis buffer and assayed for kinase activity for 30 min at 300C in kinase buffer [20 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 5 μg histone H1, 5 μci of [γ32P] ATP, 1 mM DTT]. The reaction was stopped by the addition of SDS-PAGE sample buffer and analyzed by SDS-12 % PAGE followed by autoradiography.
Reporter gene assay. U-87MG cells (0.4 million cells/60-mm cultures dish) were transiently transfected with a reporter construct (hCycE-CAT) alone or in combination with an expression plasmid for JCV Sm t-Ag (pcDNA3 JCV HA-Sm t-Ag) by the calcium phosphate precipitation method [69,70]. Plasmid concentrations used in the transfections are indicated in the respective figure legend, but the total amount of DNA transfected into the cells was normalized using the respective empty vector. A plasmid (1 μg) expressing β-galactosidase was included in the transfection mixture as an internal control to account for transfection efficiencies for each transfectant. At 48h posttransfection, cells were lysed via three freeze-thaw cycles. Following the clearance of cell debris by centrifugation, chloramphenicol acetyltransferase (CAT) activity of supernatants was determined by using equal amount of protein for each sample in the assay reaction. Results were corrected according to β-galactosidase activity for each transfectant. Transfections were repeated three times in triplicate and variations for each data point were expressed as standard deviations of the means.
3. Results
3.1. Unique features of JCV small t antigen
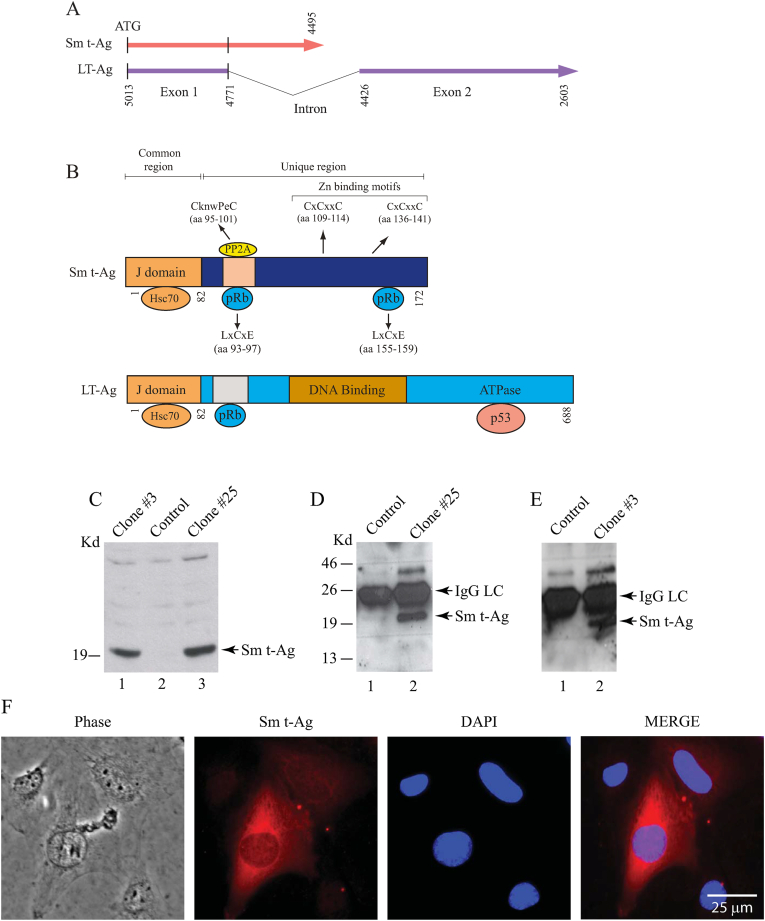
Sm t-Ag cDNA is produced as a result of alternative splicing of the early pre-mRNA transcripts (Fig. 1A). These transcripts produce a 172 amino acid long protein upon translation. Eighty-two amino acids of the N-terminus region of Sm t-Ag completely overlaps with that of LT-Ag and is designated as a common region for both proteins (Fig. 1B). At its unique region, Sm t-Ag contains a PP2A binding motif (CknwPeC, aa 95–101) which overlaps with one of two pRb binding regions (LxxCxE, aa 93–97). The second pRb binding region is located at the aa 155–159. Additionally, this unique region also contains two Zn binding motifs (CxCxxC) at amino acids 109–114 and 136–141 (Fig. 1B).
Fig. 1.
(A) Schematic representation of the JCV Sm-t-Ag splicing pattern. JCPyV early coding region produces alternatively spliced products [124,125]. LT- Ag and Sm t-Ag and T′ proteins result from such a splicing pattern [124,125]. (B) The common and unique region of Sm t-Ag. First 82 amino acid N-terminus region of Sm-Ag is common with LT-Ag sequences and this region is called the J-domain for both proteins. The unique region contains a PP2A binding motif, 2 Zn binding motifs and 2 pRb binding motif as indicated. (C) Analysis of the Sm t-Ag expression in stable U-87MG cells by Western blotting using whole cell extracts (WCE) and probing the blots with anti-HA antibody as described in materials and methods. (D and E) In parallel to panel C, WCEs were also analyzed by immunoprecipitation (IP) followed by Western blotting (WB) using an anti-HA antibody as described in the materials and methods. (F) Analysis of the Sm t-Ag distribution pattern in cells by immunocytochemistry (ICC). SVG-A cells were transiently transfected with a HA-tagged Sm t-Ag expression plasmid and at the 24h posttransfection cells were processed for ICC as described in materials and methods using anti-HA antibody.
3.2. Stable expression and subcellular distribution of Sm t-Ag
A number of reports indicate that JC virus may be associated with the development of human tumors [[71], [72], [73]]. In addition, functional studies on SV40 Sm t-Ag showed that it supports SV40 LT-Ag-mediated transformation [2,29,[43], [44], [45], [46]]. Experimental animal studies suggest that, in the absence of Sm t-Ag, tumors are induced primarily in lymphoid and other proliferative tissues [48,49,74]. These observations prompted us to investigate the possibility that JCV Sm t-Ag may also have a contributing role in cell cycle progression. For this purpose, U-87MG cells were stably transfected with an expression plasmid expressing HA-tagged Sm t-Ag, and clones were selected after two weeks posttransfection following the treatment with a selection drug (G418). Subsequently, expression of Sm t-Ag in stably transfected cells was analyzed by Western blotting (Fig. 1C) and transiently transfected (Fig. 1E) cells was analyzed by western blotting/IP-Western and immunocytochemistry respectively. As shown in Fig. 1C, whole cell extracts prepared from either U-87MG cells stably expressing Sm t-Ag (clones #3 and #25) (lanes 1 and 3), or control cells (lane 2) cells were analyzed by Western blotting and thereby Sm t-Ag expression was demonstrated in clones. In parallel, whole cell extracts were subjected of immunoprecipitation followed by SDS-10 % PAGE/Western blotting for control, clone #3 and clone #25 (Fig. 1D and E) either untransfected (lane 1) or transfected (lane 2) using an anti-HA antibody to detect Sm t-Ag expression in stably transfected clones.
Next, we analyzed the subcellular distribution of Sm t-Ag in U-87MG cells which were seeded on glass chambers and transfected with a Sm t-Ag expression plasmid. Cells were fixed at 48h posttransfection, and after incubation with primary (anti-HA monoclonal) and secondary (anti-mouse FITC-conjugated) antibodies, the cellular distribution of Sm t-Ag was examined by under a fluorescence microscope. As shown in Fig. 1F, Sm t-Ag is detected in the cytoplasmic and nuclear compartments of the cell. To ensure specificity of the anti-HA antibody, transfected cells were also reacted with pre-immune antisera and no apparent staining was observed (data not shown).
3.3. Sequence alignments for the human polyomaviruses
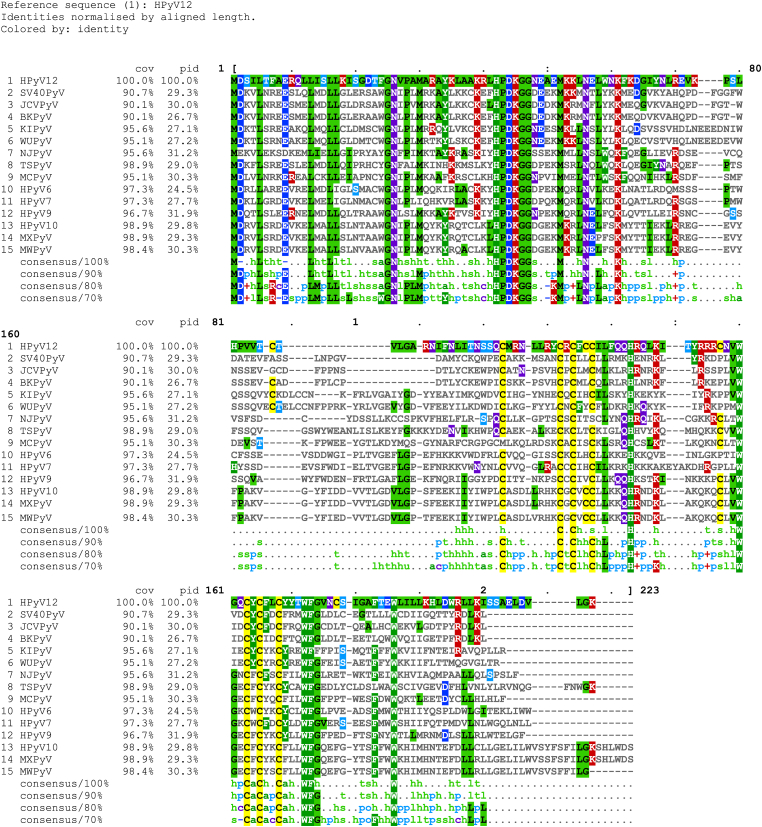
All fourteen human polyomavirus Sm t-Ag protein sequences were aligned using “Clustal Multiple Sequence Alignment Omega Website (https://www.ebi.ac.uk/jdispatcher/msa/clustalo) (Fig. 2A). Data showed that HPx10 and MxPyV have the longest Sm t-Ag sequences (206 aa each) and the JCPyV and BKPyV have the shortest ones (172 aa each). The common denominator for all these proteins is the following: The HPDKGG motif sequence and two different Zn binding motifs are all conserved among them. There are certain amino acids at certain positions that are also conserved among them (Fig. 2). However, only JCPyV and BKPyV Sm t-Ag poses the pRb binding motifs, BKPyV has one aa position 93–97 (LYCKE) and JCPyV has two, aa positions 93–97 (LYCKE) and 155–159 (LHCWE).
Fig. 2.
Sequence alignment analysis of the human polyomavirus Sm t-Ag sequences using “Clustal Multiple Sequence Alignment Omega Website” (https://www.ebi.ac.uk/jdispatcher/msa/clustalo).
3.4. Sm t-Ag promotes S phase entry
Cell division is a highly regulated process controlled by a number of cell cycle stage specific regulators. Under normal circumstances, cells undergo cell cycle progression which culminates in a successful mitotic division. When such a system is perturbed, cells respond to this new situation either by cell cycle arrest to repair the damage or if the damage is extensive, the affected cells undergo apoptosis.
One of the interesting characteristics of viruses is their ability to use the host machinery for their own use. Viral infection imposes tremendous pressure on an infected cell and mostly ends up with the destruction of cell integrity. Upon entering into the host cells, DNA viruses absolutely depend on the S phase of the cell cycle to replicate their DNA. To achieve this, viruses use their own encoded proteins to activate cell cycle progression by influencing the activity of specific cell cycle regulators. However, sometimes gene expression profiles of certain host cells may restrict the lytic infection cycle but allow the expression of certain viral proteins. In such cases, those viral proteins, when constitutively expressed, may lead to unscheduled mitotic division, genetic instability, and eventually cell transformation. Apparently, viral induced tumors develop as a result of such genomic changes in the absence of lytic infection of cells.
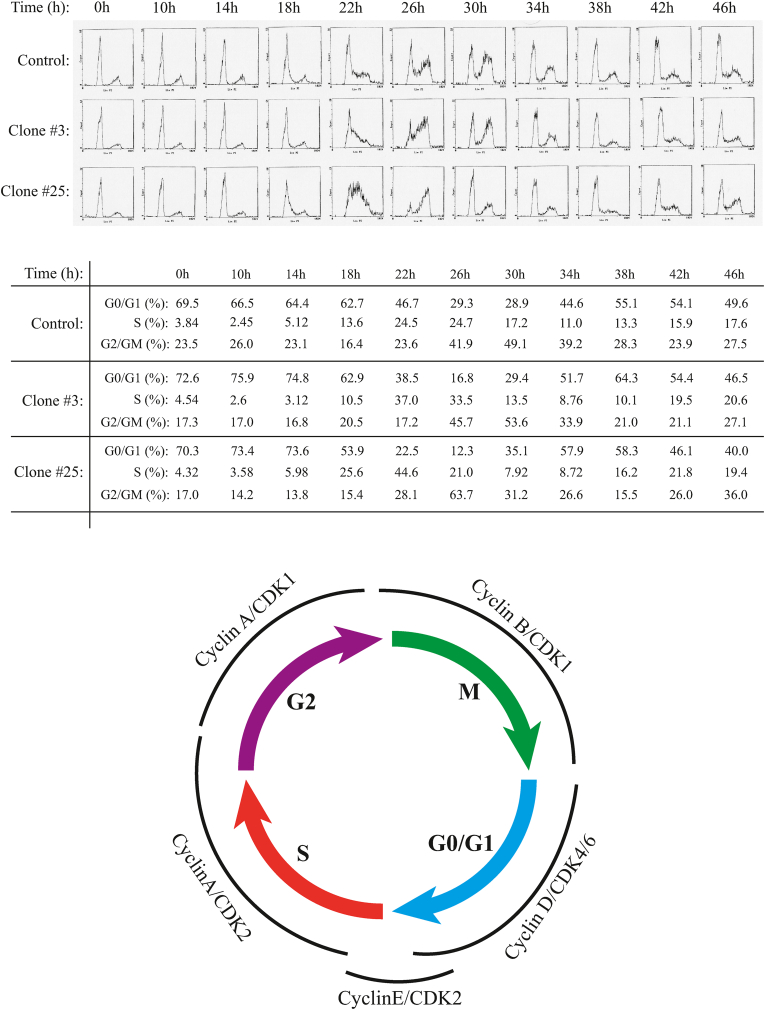
Since recent reports indicate the association of polyomavirus Sm t-Ags with certain human tumors [59,60], we wanted to determine whether JCV Sm t-Ag plays a role in cell cycle progression in the absence of other viral proteins. For this purpose, U-87MG cells were stably transfected with an expression plasmid expressing Sm t-Ag, and stable clones were selected as described in materials and methods. The stable clones (clone #3 and clone #25) and control clone were synchronized at G0/G1 phase of the cell cycle by serum deprivation, released by the addition of complete media, collected at the indicated time points and analyzed by flow cytometry to determine their differential cell cycle profiles. Comparison of the cell cycle profiles of the clones (clone #25 and clone #3) with that of control clearly indicate that cells stably expressing Sm t-Ag enter and exit from S phase earlier than control cells (Fig. 3A). The difference between clones and control is the most prominent when comparing the time points corresponding to S and G/M phases of cell cycle (22h through 34h). In addition, to show whether the observed effect is not cell type specific or species dependent, we stably expressed Sm t-Ag in NIH-3T3 cells, a mouse fibroblast cell line, and obtained a similar effect on cell cycle progression (data not shown) as observed for U-87MG cells suggesting that Sm t-Ag may show a common effect on different cell lines from different species, which is to promote S phase entry.
Fig. 3.
Sm t-Ag promotes cell cycle progression. (A) U-87MG cells stably expressing Sm t-Ag (clone #3 and #25) and control clone cells were arrested at G0/G1 phase of the cell cycle by serum deprivation and released by the addition of complete growth media containing 10 % fetal bovine serum. The cells were collected at the indicated time points (hour, h), fixed in 70 % ethanol, stained with propidium iodide and analyzed by a flow cytometer instrument for their differential cell cycle profiles. (B) Illustration of the cell cycle stages and the cell cycle-dependent cyclins and cyclin-dependent kinase expression profiles.
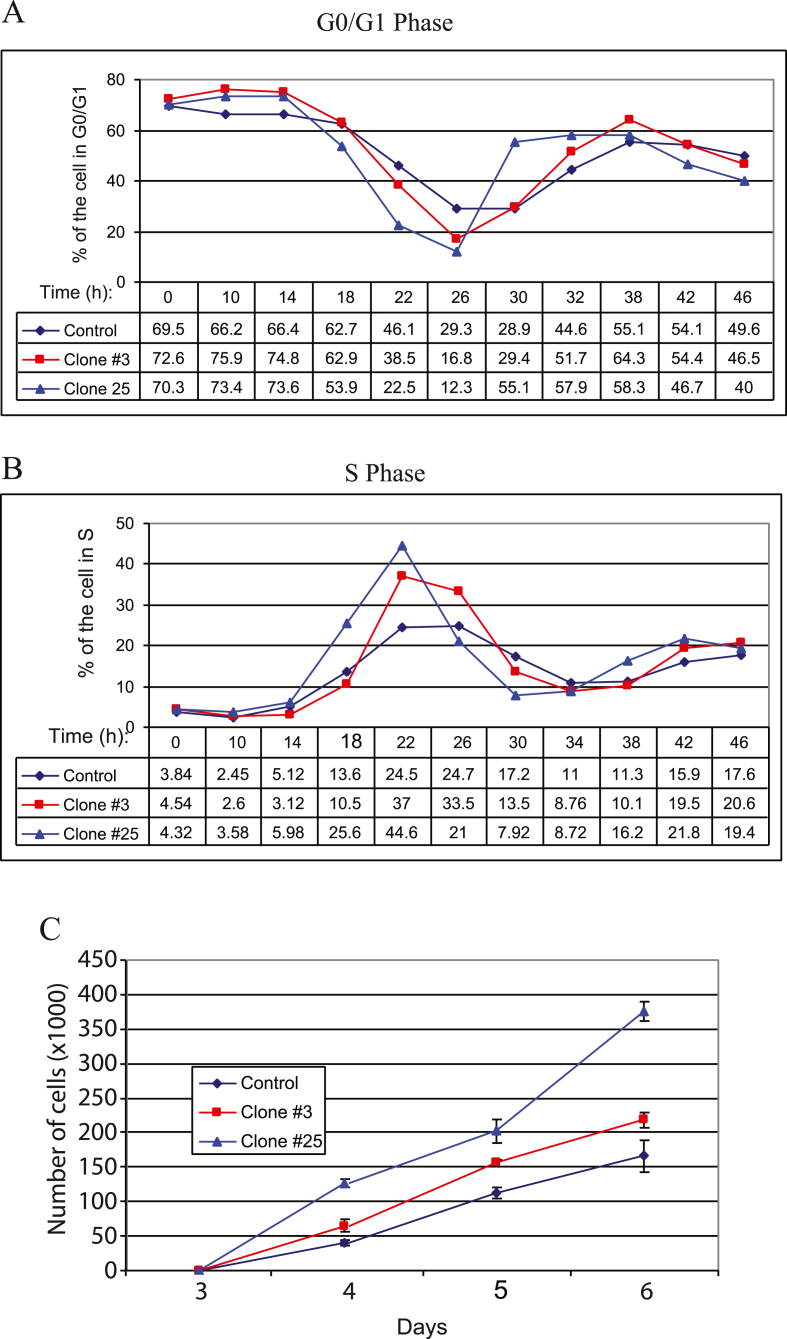
Alternative to the analysis of the cell cycle progression profiles of the Sm t-Ag positive clones using the flow cytometry traces as shown in Fig. 3A, we also analyzed these parameters graphically (Fig. 4AB) by focusing on percent of the cell numbers at G0/G1 and S phases of the cells. As shown in Fig. 4A and B, Sm t-Ag positive clones exit G0/G1 phase of the cell cycle earlier than control cells (Fig. 4A) and thus enters the S phase much earlier than control cells (Fig. 4B), which is consistent with our findings from Fig. 3A. Fig. 3B illustrates the cell cycle stages and timely expression of the cyclins and cyclin-dependent kinases in a more or less cell cycle stage-specific manner.
Fig. 4.
Graphical analysis of the cell cycle progression profiles of the Sm t-Ag positive clones and their growth properties. (A) Graphical analysis of the cell cycle progression of Sm t-Ag positive cells for G0/G1 phase specific parameters for 46 h. (B) Graphical analysis of the cell cycle progression profiles of Sm t-Ag positive clones for S phase specific parameters. (C) Graphical representation of the growth properties of the Sm t-Ag positive clones compared to control.
We also analyzed the growth properties of Sm t-Ag positive clones versus control. For this purpose, cells synchronized at G0/G1 phase of the cell cycle were released in complete media, and an equal number of cells for each clone were seeded on the plates in triplicate. At indicated time points, the cell number for each clone was determined and presented as a function of time on a graph. As shown in Fig. 4C, Sm t-Ag positive clones proliferate faster than the control cells, further confirming our data from cell cycle distribution profiles of the cells (Fig. 3A).
3.5. Cell cycle stage specific regulators are expressed relatively earlier in Sm t-Ag positive cells than controls
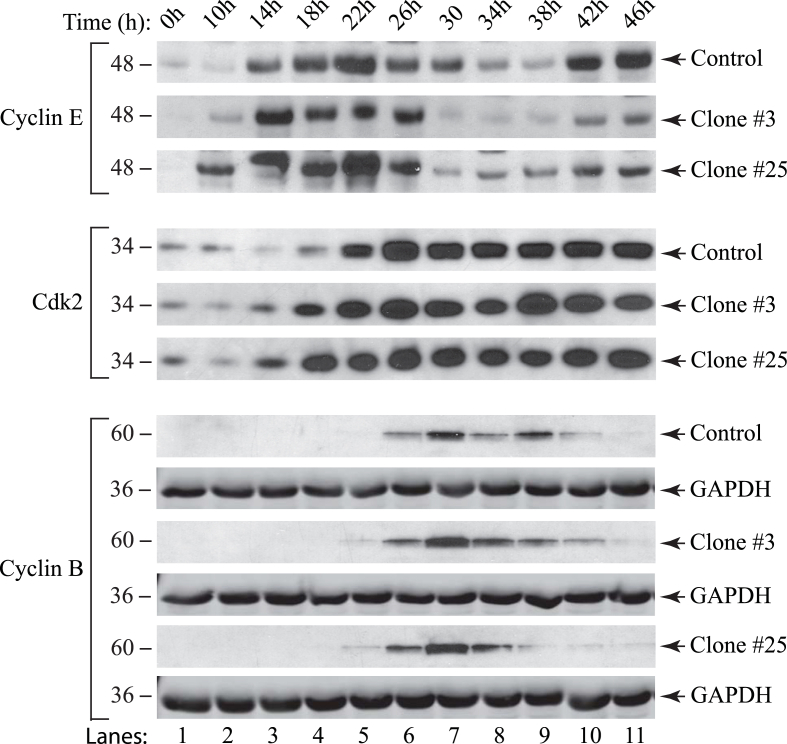
Our findings from cell cycle progression analysis demonstrate that Sm t-Ag positive cells enter the S phase noticeably earlier than the corresponding controls. As such, we wanted to examine the effect of Sm t-Ag on the expression profiles of the selected cyclins and cyclin-dependent kinases that are specific for G1/S and G2/M transitions states including cyclin B, cyclin E and Cdk2. For this purpose, nuclear extracts prepared from both control clone and clones (clone #3 and #25), which were arrested at G0/G1 phase by serum deprivation and released from the block by the addition of complete media, were analyzed by Western blotting. As seen in Fig. 5, the Sm t-Ag positive clones entered and exited from S phase of the cell cycle with a more facilitated rate than the control clone which was characterized by a relatively earlier significant expression levels of cyclin B, E and Cdk2 for the clones when the cells were released from G0/G1 arrest. These observations are consistent with our findings from the analysis of cell cycle profiles of clones from Fig. 3A. Also note that Fig. 3B illustrates cell cycle stages and cell cycle-dependent regulation of the cyclins and cyclin-dependent kinases.
Fig. 5.
Analysis of cell cycle regulators (cyclin B, cyclin E and Cdk2) in Sm t-Ag positive cells by western blotting. A control clone and Sm t-Ag positive clones (clones (#3 and #25) of U-87MG cells were synchronized at G0/G1 state of the cell cycle by serum deprivation. Nuclear extracts were prepared at indicated time points as previously described [68] and analyzed for G1/S phase transition state specific cyclin E and cyclin-dependent kinase Cdk2 by western blotting. Extracts were also analyzed for G2/M phase specific cyclin B. GAPDH levels were analyzed in the western blots as a loading control using anti-GAPDH antibody (Santa Cruz Biotechnology, catalog no. sc355062) for each cell line.
3.6. Sm t-Ag positive clones exhibit higher levels of cyclin E/Cdk2 associated kinase activity
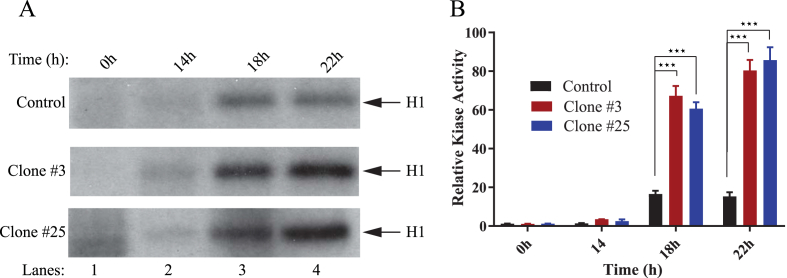
Results from the analysis of cell cycle regulators specific for G1/S phase transition state demonstrated that Sm t-Ag positive clones exhibit higher levels of cyclin E at 18h and 22h post release from serum deprivation compared to control. To provide further evidence to these findings, we evaluated the level of kinase activity associated with cyclin E/Cdk2 complex by H1 kinase assay in clones versus control cells. It was expected that if Sm t-Ag is associated with a facilitated S phase entry, the level of cyclin E/Cdk2 complex-associated kinase activity might be higher in the clones than the control. As such, in parallel to examining the cell cycle profiles of clones described in Fig. 5, cell extracts were also prepared from both the control and clones (clone #3 and #25), and equal amounts of protein was immunoprecipitated using an anti-Cdk2 antibody. Immunoprecipitants were subsequently subjected to an in vitro kinase assay as described in materials and methods. Our results consistently showed that the level of cyclin E/Cdk2-associated kinase activity of the extracts prepared from the Sm t-Ag positive cells was significantly higher than that of the control at 18h and 22h (Fig. 6A and B), where data was analyzed by using two-way ANOVA followed by Tukey's multiple comparison test. As expected, this difference is most substantial in the samples prepared at the 18h and 22h time points, which is also consistent with our results obtained from Fig. 5 where we observed that the level of Cdk2 for both clones (clone #3 and #25) is noticeably higher than that of the control for the same time points.
Fig. 6.
Higher level of cyclin E/Cdk2 associated kinase activity was observed in Sm t-Ag positive cells. (A) The clones stably expressing Sm t-Ag (clone #3 and clone #25) and control cells were synchronized at G0/G1 stage of the cell cycle by serum deprivation and released by the addition of the media containing complete growth factors. Whole cell extracts were prepared at the indicated time points. Subsequently, Cdk2 was immunoprecipitated with an anti-Cdk2 antibody and immunoprecipitants were analyzed for their specific kinase activity utilizing histone 1 (H1) as a substrate as described in materials and methods. (B) Experiments were repeated more than three times, and a representative data is shown here. In addition, data was evaluated with the GraphPad Prism 7 program for statistical analysis and making a graph. Analysis of the data by using two-way ANOVA followed by Tukey's multiple comparison test showed that the means for the control and the experimental data for the clones at 18h and 22h are statistically significant at p < 0.05. The three stars on the figure represent the statistical significance between the control and clones at 18h and 22h.
3.7. Sm t-Ag stimulates cyclin E promoter activity
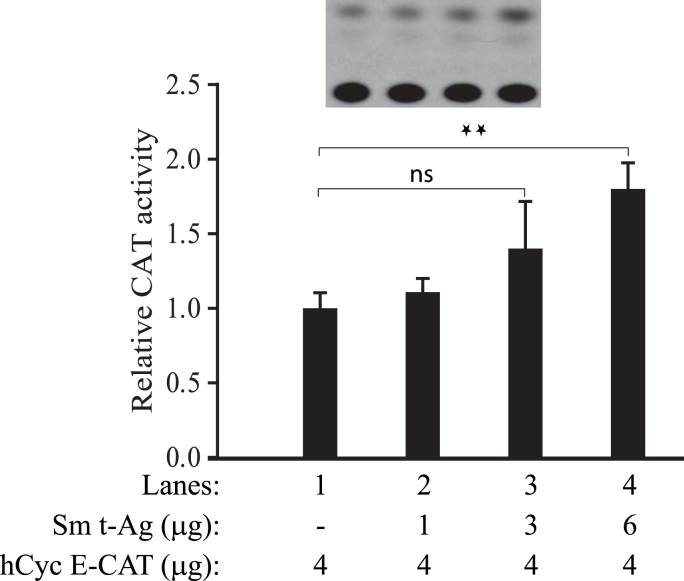
Analysis of cell cycle stage-dependent expression of cyclin E in Sm t-Ag positive clones showed a relatively earlier appearance of this protein at 10h and 14h post release compared to the control (Fig. 5), suggesting that Sm t-Ag may directly influence the transcriptional activity of cyclin E promoter at G1/S phase transition. To investigate this possibility, we performed reporter gene assays. U-87MG cells were transiently transfected alone with a human cyclin E reporter construct or co-transfected with an expression plasmid for Sm t-Ag, and reporter activity of cyclin E promoter was determined at 48h posttransfection. As shown in Fig. 7, the reporter activity of cyclin E promoter in the presence of Sm t-Ag was gradually but modestly increased as the concentration of expression plasmid for Sm t-Ag in transfection mixture was increased (compare lane 1 with lanes 2–4) which is consistent with our findings from Fig. 5, where we observed that cyclin E levels are relatively higher in cells stably expressing Sm t-Ag compared to the control. Also, the statistical analysis of data by using two-way ANOVA followed by Tukey's multiple comparison test showed statistically significant differences at p < 0.05 between control and data line 4 but not with two other experimental data points, which is also consistent with our findings from Fig. 5.
Fig. 7.
Sm t-Ag upregulates the human cyclin E promoter activity. (A) U-87MG cells were transiently co-transfected with a human cyclin E reporter construct (hCycE-CAT) and with an expression plasmid for Sm t-Ag (CMV-JCV HA-Sm t-Ag) as indicated. The amount of DNA in the transfection mixture was normalized by using the empty vector alone. At 24h posttransfection, whole cell lysates were prepared and reporter activity of the cyclin E promoter in the absence and presence of Sm t-Ag was determined as described in materials and methods. Analysis of the data by using two-way ANOVA followed by Tukey's multiple comparison test showed that the means for control and experimental lane 4 are statistically significant at p < 0.05 (illustrated by two-star signs) but not with the other two experimental data points. The statistical significance is illustrated by two small stars on the figure. “ns” stands for “not significant”.
3.8. Sm t-Ag interacts with the translation elongation factor, eEF1A
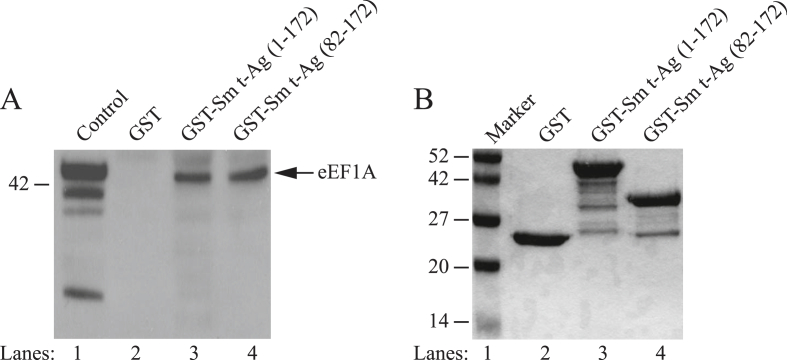
Some viral proteins directly target the translation machinery through which viruses would be able to manipulate the cellular environment more efficiently. The prime example to such viral proteins is the HIV Nef [75] and Gag proteins [76]. We also reason that, since Sm t-Ag facilitates the cell cycle progression, it may also target the translation machinery during this process to contribute to the facilitated cell cycle progression. To determine whether JCV Sm t-Ag might also interact with cellular eEF1A protein, we performed a protein-protein interaction assay (GST pull-down). For this purpose, Sm t-Ag full length (1–172) and its deletion mutant (81–172) fused to GST and expressed in bacteria along with GST alone, purified, immobilized on glutathione-Sepharose beads and incubated with whole-cell extracts prepared from U-87MG cells. After washing extensively with incubation buffer, bound proteins were resolved by SDS-10 % PAGE and analyzed by Western blotting using an eEF1A antibody. As demonstrated in Fig. 8A, eEF1A was retained on the Sepharose column containing GST-Sm t-Ag full length (lane 3) and its deletion mutant (lane 4) and an interaction of this type between eEF1A and GST alone was not observed (lane 2), indicating the specificity of association between Sm t-Ag and eEF1A. This finding suggests that the unique region of Sm t-Ag contain sequences, which interact with eEF1A. Fig. 8B illustrates the analysis of the bacterially expressed and purified GST-Sm t-Ag full length (1–172) and its deletion mutant (GST-Sm t-Ag (82–172) by SDS-10 % PAGE followed by Coomassie blue staining.
Fig. 8.
Sm t-Ag interacts with cellular translation elongation factor, eEF1A. (A) Interaction of Sm t-Ag with eEF1A was demonstrated employing a GST-pull down assay as describe in materials and methods. (B) SDS-10 % PAGE analysis of the bacterially produced GST, GST-Sm t-Ag full length (aa 1–172) and GST Sm t-Ag mutant (aa 82–172) after purification followed by Coomassie blue staining.
3.9. Activation of PI3K/Akt/mTOR signaling pathway by Sm t-Ag
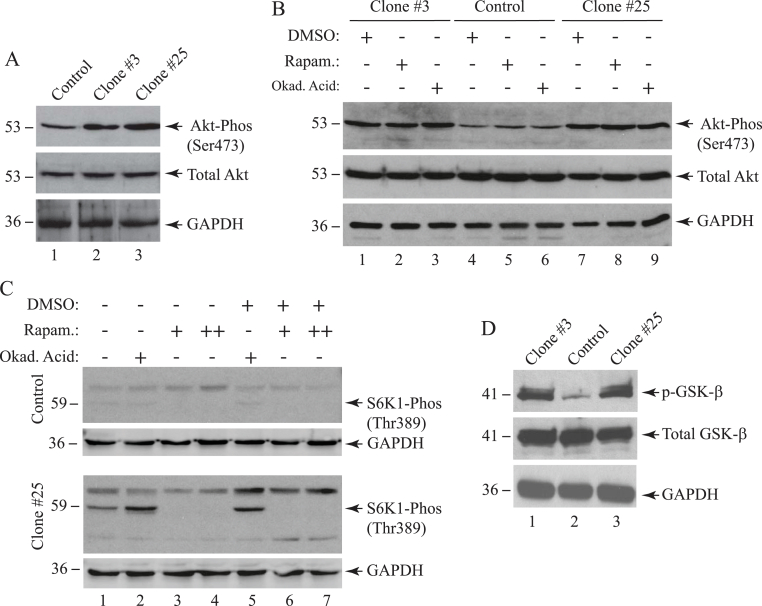
Two of the main functions of Akt at the G1-S phase cell cycle transition is to promote the degradation of cyclin D [77] through the inhibition of its phosphorylation and to inhibit the glycogen synthase kinase 3 (GSK-3) activity by directly phosphorylating it. During this process, Akt also indirectly stimulates mTOR and, consequently, activates one of the mTOR downstream effector kinases, named the ribosomal protein S6K1 kinase, to further stimulate the protein synthesis and thus cell growth [78,79]. Given the importance of Akt in G1/S cell cycle phase transition, we reason that Sm t-Ag most likely directly or indirectly actives the PI3K/Akt,/mTOR pathway to promote this transition. To provide experimental evidence to this assumption, whole cell extract from Sm t-Ag positive clones as well as control cells were analyzed by Western blotting. As shown in Fig. 9A, phosphorylated Akt levels for Sm t-Ag positive clone #3 and #25 increased significantly (∼2-fold, lane 2, and ∼3-fold, lane 3, respectively) but the total Akt levels remained relatively unchanged, clearly suggesting that Sm t-Ag stimulates the activation of the Akt pathway. Next, we also analyzed the phosphorylated Akt and total Akt levels in Sm t-Ag positive cells treated with rapamycin and okadaic acid (Fig. 9B). Rapamycin is prominent inhibitor of mTOR pathway and inhibits mTORC1 and mTORC2 at low [80] and high concentrations [[81], [82], [83]] respectively. Okadaic acid, on the other hand, inhibits protein phosphatase PP1 and PP2A. Treatment of both Sm t-Ag positive and control cells with these two chemicals did not alter the levels of phosphorylated Akt levels (compare lanes 2,3, and 8, 9 with control lanes 5 and 6 respectively) suggesting that Akt activation appears to be independent of PP1, PP2A and mTOR inhibition. Under the same treatment conditions, we also analyzed the effect of rapamycin and okadaic acid on the phosphorylation levels of the downstream effector molecules of mTOR pathway such as p70 SP6K1. SP6 kinase 1 (SP6K1) phosphorylates the S6 ribosomal protein. The phosphorylation of SP6 at Thr389 is correlated with mTOR pathway activation to stimulate protein synthesis. In the absence of the treatment of the cells with both chemicals, we observed a significant level of phosphorylation at this residue in Sm t-Ag positive cells (∼3-fold increase) (clone #25) (compare control lane 1 with that of clone #25). Treatment of the cells with okadaic acid increased phosphorylation levels even further (∼5-fold) (compare control lane 2 with that of for clone #25) suggesting a stabilization of the phosphorylation in the presence of okadaic acid. As expected, rapamycin inhibited this phosphorylation (lanes 3, 4, 6 and 7), suggesting that Sm t-Ag also activates the mTOR pathway downstream of Akt. Another interesting downstream effector molecule of the activated Akt is the glycogen synthase 3 beta (GSK3-β). It is also a Ser/Thr kinase and regulates a wide range of biological activities, including cell transport, cell signaling, glycogen metabolism etc [84]. The phosphorylation of this kinase by Akt inactivates its function [85] but leads to the degradation of cyclin D and thus stimulates cell cycle progression. We also compared the phosphorylated GSK3-β level in Sm t-Ag positive cells with that of control cells by Western blot analysis (Fig. 9D) and observed that phosphorylated GSK3-β levels in Sm t-Ag positive cells also significantly increased by Sm t-Ag expression (compare lane 2 with lanes 1 and 3), suggesting the inhibition of GSK3-β by Akt in Sm t-Ag positive cells.
Fig. 9.
Analysis of the activated Akt, GSK3-β and S6K1 levels by Western blotting. (A) Analysis of phosphorylated Akt levels in Sm t-Ag positive clones. Whole cell extracts prepared from the control and Sm t-Ag positive cells (U-87MG) and 40 μg of protein was analyzed by Western blotting using specific antibodies that detect either total Akt (Cell Signaling, catalog no. #9272) or phosphorylated Akt (Ser473) (Cell Signaling, catalog no. #9271). (B) Treatment of Sm t-Ag cells with mTOR inhibitor, rapamycin, and protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) inhibitor, okadaic acid and analysis of the extract prepared from these cells by Western blotting. Sm t-Ag positive clones (clones #3 and #25) and control cells were treated with either rapamycin (Cell Signaling, catalog no. #9904, 10 nM final) for 48h or okadaic acid (Cell Signaling, catalog no. 5934, 1 μM final) for 3h and whole cell extract were analyzed for the phosphorylated and total Akt levels using specific antibodies that detect the total Akt (Cell Signaling, catalog no. #9272) or phosphorylated Akt (Ser473) (Cell Signaling, catalog no. #9271). (C) Analysis of the phosphorylated S6K1(Thr389) levels in cells treated with either rapamycin or okadaic acid by Western blotting. Sm t-Ag positive (clone #25) and control clone cells were treated with either rapamycin or okadaic acid with the same conditions described for panel B and whole cell extracts were prepared and analyzed by Western blotting (40 μg/lane) using an anti-S6K1 antibody that detects the phosphorylated S6K1 (Thr389) (Cell Signaling, catalog no. #9206S). Rapamycin concentration for + was 10 nM and for ++ 20 nM for cellular treatments. (D) Analysis of total and phosphorylated GSK3-β levels in Sm t-Ag positive clones, (#3 and #25) and control clone cells by Western blotting using anti-GSK3-β antibodies that detects total (Cell Signaling, catalog no. #9315) and phosphorylated GSK3-βb (Ser9) (Cell Signaling, catalog no. #9322) antibodies. GAPDH levels were analyzed in the Western blots as a loading control using anti-GAPDH antibody (Santa Cruz Biotechnology, catalog no. sc355062).
4. Discussion
Detection of polyomavirus DNA sequences in a variety of human tumors suggests a possibility that certain types of human tumors may be induced by this group of viruses [1,[25], [26], [27], [28]]. It should be noted here that the oncogenic potential of these viruses has already been previously shown in experimental animals [[17], [18], [19], [20], [21], [22], [23], [24]], and even recent reports demonstrated the integration of BKPyV and MCPyV DNA into the human genome and such integration appears to trigger the development of human tumors [10,11,14,15] JCV is the only family member that was shown to induce tumors in nonhuman primates [16,18]. As such, understanding the mechanisms of the tumorigenic activity of these viruses will help us to trace the molecular pathways that lead to cell transformation through which we may gain an opportunity to effectively target such pathways by therapeutic approaches.
It is well documented that LT-Ag of polyomaviruses plays a critical role in cell transformation, and Sm t-Ag of SV40 also plays an important role in this process in vivo and in vitro. In this respect, Sm t-Ag was found to enhance cell transformation induced by SV40 LT-Ag in several nonpermissive murine cell lines in tissue culture [2,29,[43], [44], [45], [46]]. It appears that, in the absence of Sm t-Ag, SV40-mediated tumorigenesis is mostly restricted to actively dividing tissues (e.g. lymphoid tissue) suggesting an active role for Sm t-Ag in such processes. Although the mechanism by which Sm t-Ag exerts its biological activity in this process is largely unknown, its interaction with PP2A, a serine-threonine phosphatase, and inhibition of its phosphatase activity is thought to contribute to the protein's ability to enhance cellular transformation. Previous reports also indicate, however, that Sm t-Ag diverts PP2A activity towards specific substrates rather than inhibiting its function [52] Recent proteomic studies with JCV Sm t-Ag showed that it interacts with a wide range of cellular targets and such a targeting conceivably contributes to cell transformation [42]. In addition, recent reports indicate that the overexpression of SV40 Sm t-Ag in mammalian cells appears to activate growth factor stimulated signaling pathways, including activation of phosphatidylinositol (PI) 3-kinase [53], protein kinase Cζ (PKCζ) [53], mitogen activated protein kinase [50], and phosphorylation of Akt [54]. Furthermore, it was also found that SV40 Sm t-Ag stimulates AP-1 [56] and facilitates Shc phosphorylation [55]. Whether JCV Sm t-Ag also affects similar growth promoting pathways is unknown and requires further investigation.
As mentioned above, JCV genome is detected in a variety of human tumors, but to date there is no report describing the biological activity of JCV Sm t-Ag in such processes. In analyzing the function of this protein, we demonstrated that it facilitates cell cycle progression which may have implications in JCV-induced cell transformation. Our results consistently demonstrated that Sm t-Ag positive clones enter and exit S phase relatively faster than the control (Figs. 3A, 4AB and 5). Our results from the analysis of the levels of the selected cell cycle stage specific regulators including cyclin B, cyclin E and Cdk2 by Western blotting and kinase assays support such an observation. In addition, a direct analysis of the effect of Sm t-Ag on cyclin E promoter by reporter gene assays further showed that JCV Sm t-Ag could activate this promoter (Fig. 7) in glial cells.
Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway play a pivotal role in multiple interconnected cellular signaling mechanism involved in cell survival, growth, division, apoptosis suppression and angiogenesis [86,87]. Akt is a Ser/Thr kinase and partially activated upon its translocation into the cell membrane and by binding to the second messenger lipid molecules such as phosphatidylinositol (3,4,5)-triphosphate, and its phosphorylation at Thr308 residue by the phosphoinositide-dependent kinase 1 (PDK1). Its full activation, however, occurs when its Ser473 residue is phosphorylated by various kinases including mTORC2, DNA-dependent protein kinase (DNA-PK), and phosphoinositide-dependent kinase 2 (PDK2) [[88], [89], [90]] or integrin-linked kinase [91]. Akt could also be activated independently of PI3K mode of activation process by other Ser/Thr or Tyr kinases in response to DNA damage, growth factors and inflammation, including those but not limited to, Ca2+/calmodulin-dependent protein kinase kinase (CaMKK), and increased Ca2+ concentration [90,92] of the cells. Once activated, Akt translocate from plasma membrane to cytosol and nucleus to phosphorylate a wide range of substrates at a specific consensus sequences (RxRxx(Ser/Thr)-Hyd, where Hyd is a hydrophobic residue). Akt plays an important role in G1-S phase cell cycle transition by inhibiting the phosphorylation of cyclin D and thus leading to its degradation [77] and by phosphorylating glycogen synthase kinase 3 (GSK-3) and therefore inhibiting its activity. Akt also indirectly stimulates mTOR and, consequently, activates one of the mTOR downstream effector kinases, S6K1, to stimulate the protein synthesis and therefore cell growth [78,79]. We have investigated whether Sm t-Ag directly or indirectly actives the PI3K/Akt/mTOR pathway to promote this transition. Indeed, our results clearly demonstrated that in the presence of JCV Sm-Ag, Akt was found to be phosphorylated at its Ser473, an accepted indication of Akt activation (Fig. 9A). One of the downstream effector molecules of Akt, GSK3-β was also found be phosphorylated thereby its function is inhibited by Akt. (Fig. 9C). S6K1, is a downstream effector kinase of mTOR, was also detected to be phosphorylated at its Thr389 (Fig. 9D). S6K1 plays a pivotal role in protein synthesis pathway and therefore in cell growth. In summary, these findings support our conclusion that JCV Sm t-Ag activates growth promoting pathways.
Association of JCV with human tumors was first reported by Richardson [93], who first described progressive multifocal leukoencephalopathy (PML). JCV was incidentally detected in an oligodendroglioma from a patient with concomitant occurrences of chronic lymphatic leukemia and PML. Following this report, concomitant occurrences of PML with different human tumors was also described in several more cases including astrocytomas [[94], [95], [96]]. In addition to these cases, JCV genome has also been detected in human brain tumors in the absence of PML lesions including xantoastrocytoma [97], oligoastrocytoma [98], ependymomas, pilocytic astrocytomas, oligodendrogliomas, and astrocytomas [99,100]. JCV early genome was also found to be present in tumors that are not originated from neural tissues such as colorectal cancers [72,73,101,102]. The genome of the other two polyomaviruses, BKV and SV40, were also detected in different types of tumors and the presence of viral DNA appears to show certain preference to specific tissues. BKV was found to exhibit a specific oncogenic tropism for the ependymal tissue, endocrine pancreas, and osteosarcomas in rodents [[103], [104], [105], [106], [107]], and its genome was also detected in a number of related tumors in humans [10,11,20,108]. SV40 appears to be mostly associated with malignant mesotheliomas [[109], [110], [111]] and sometimes with brain tumors including glioblastomas, gliomas, gliosarcomas, medullablastomas, meningiomas, pituitary adenomas and oligodendromas [[112], [113], [114], [115], [116], [117]]. As mentioned previously, MCPyV Sm-Ag alone is more aggressive with respect to cell transformation than that of MCPyV LT-Ag [[57], [58], [59], [60], [61], [62]]. As a result, understanding of the unique contributions of each Sm t-Ags to the LT-Ag functions will tremendously advance our knowledge with respect to the virus-induced cell transformation.
Many regulatory viral and cellular proteins are known to undergo posttranslational modifications in several ways including phosphorylation, glycosylation and myristylation. As with other modifications, phosphorylation has been shown to play a critical role in many aspects of cell function, such as differentiation, proliferation, signal transduction, cell cycle progression, gene transcription. A prominent example of regulation by phosphorylation is observed in the behavior of stress inducible proteins. For instance, NF-κB family of proteins is retained in cytoplasm in their inactive form by their inhibitor, IκB, which masks the nuclear localization signal (NLS) of NF-κB. Upon exposure of the cells to extracellular stimuli such as viral infection, UV light, etc., IκB is phosphorylated at its Ser-33 and -36 residues by activated IκB kinase complex [118], dissociates from NF-κB and undergoes degradation by ubiquitin-mediated proteolysis [119]. NF-κB in turn translocates into the nucleus and transactivates many NF-κB responsive genes [120].
Likewise, glycosylation and myristylation also play important roles in various functions of regulatory proteins such as stabilizing protein-protein interactions and enhancing the association of the modified protein with cellular membranes. A potential glycosylation site of JCV Sm t-Ag (aa 78–81 (NSSE)) exhibits substantial divergence from those of both SV40 and BKV. Potential myristylation sites present within the JCV Sm t-Ag also show significant sequence divergence than those of SV40. Such differences may contribute highly to the unique biological functions of JCV Sm t-Ag. With respect to functional relevance of these two posttranslational modifications, it has been shown that glycosylation inhibits the ability of Sp1 to bind to a TATA-binding protein-associated factor II 110 and decreases Sp1's transcriptional activity on certain promoters [121]. With respect to myristylation, it has been observed that the attachment of the lipid moiety results in an increase in the hydrophobicity of a protein which triggers membrane and/or protein-protein association, the importance of which is shown by the functional activity of oncoprotein p59fyn where it was observed that myristylation and palmitoylation of the src family member p59fyn affect its subcellular distribution [122,123].
Computer-aided motif search analysis of JCV Sm t-Ag indicates that it contains several potential posttranslational modification sites that involve phosphorylation, myristylation and glycosylation. Potential phosphorylation sites of Sm t-Ag are targets for serine-threonine and tyrosine kinases and are scattered throughout the protein sequence. According to the computer predictions, Ser-10 and –129, Thr-165 and Tyr-167 have high probabilities of being phosphorylated by kinases. Some of these phosphorylation sites present within JCV Sm t-Ag are unique in that they do not share a common phosphorylation site with its counterparts from BKV and SV40 Sm t-Ags. For instance, while Ser-129 is unique to JCV Sm t-Ag in its position, Tyr-167 is common with SV40 Sm t-Ag but differs with BKV Sm t-Ag. These differential phosphorylation sites may differentially influence the biological activity of these Sm t-Ags. The functional relevance of all these possible posttranslational modification sites is subject to further investigation.
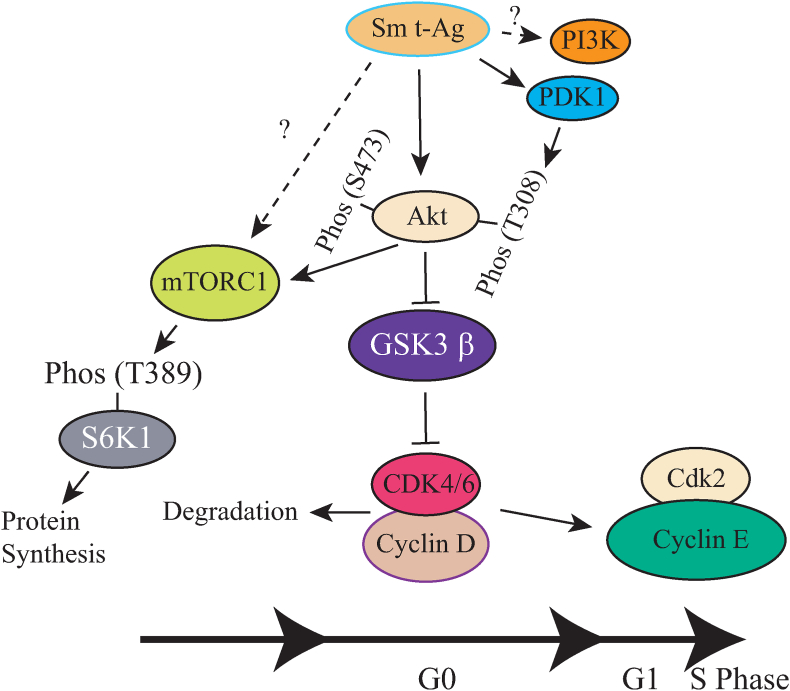
Based on our findings, we propose a model system where Sm t-Ag activates specific signal transduction pathways to promote cell cycle progression. As shown in Fig. 10, Sm t-Ag activates the PI3K/Akt/mTOR growth promoting pathways primarily by mediating the phosphorylation of Akt at T308 and S473 residues. Akt then directly or indirectly stimulates its downstream effector molecules such as mTORC1, leading to phosphorylation of S6K1 at Thr389 residue which then promotes the protein synthesis and thus cell growth. In the meantime, Sm t-Ag mediates the phosphorylation of another downstream effector molecule, GSK3-β and inhibits its function, which then appears to cause the degradation of cdk4/6/cyclin D complexes while leading to an increased expression of cdk2/cyclin E levels, and thus S phase entry.
Fig. 10.
Model for activation of the cell proliferation pathways by Sm t-Ag expression. Sm t-Ag activates the PI3K/Akt/mTOR growth promoting pathways by mediating the phosphorylation of Akt at T308 and S473 residues. Akt then stimulates its downstream effector molecules such as mTORC1, which then phosphorylates S6K1 at Thr389 residue and activates it. The phosphorylated S6K1 activates the protein synthesis pathways and thus cell growth. In the meantime, Akt directly phosphorylates GSK3-β and inhibits its function which then causes the degradation of cdk4/6/cyclin D complexes while leading to an increased expression of cdk2/cyclin E levels. Finally, cells enter S phase.
We have just begun to analyze the functional activity of JCV Sm t-Ag with respect to its role in cell cycle progression. We hope that such investigations at the molecular level will help us understand better the process of JCV-induced diseases and perhaps JCV-induced cell transformation.
CRediT authorship contribution statement
Renato Biffi: Writing – review & editing, Data curation. Stefanie W. Benoit: Writing – review & editing, Data curation. Ilker K. Sariyer: Writing – review & editing, Data curation. Mahmut Safak: Writing – original draft, Validation, Supervision, Project administration, Methodology, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
Authors declare no conflict of interest.
Acknowledgements
We would like to thank past and present members of the Center for Neurovirology and Gene Editing for their insightful discussion and sharing of ideas and reagents. This work was made possible by grants awarded by NIH (R01NS043108) and by the Temple University Bridge Award, an internal grant provided by the Office of the Vice President for Research (162136) to M. Safak.
Data availability
Data will be made available on request.
References
- 1.J.S. Butel. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis 21 (200) 405-426. [DOI] [PubMed]
- 2.Chen W., Hahn W.C. SV40 early region oncoproteins and human cell transformation. Histol. Histopathol. 2003;18:541–550. doi: 10.14670/HH-18.541. [DOI] [PubMed] [Google Scholar]
- 3.Saribas A.S., Coric P., Bouaziz S., Safak M. Expression of novel proteins by polyomaviruses and recent advances in the structural and functional features of agnoprotein of JC virus, BK virus, and simian virus 40. J. Cell. Physiol. 2019;234:8295–8315. doi: 10.1002/jcp.27715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Major E.O., Ault G.S. Progressive multifocal leukoencephalopathy: clinical and laboratory observations on a viral induced demyelinating disease in the immunodeficient patient. Curr. Opin. Neurol. 1995:184–190. [PubMed] [Google Scholar]
- 5.Frisque R.J., White F.A. In: Molecular Neurovirology, Pathogenesis of Viral CNS Infections. Roose R.P., editor. Humana Press Inc.; Totowa, NJ: 1992. The molecular biology of JC virus, causative agent of progressive multifocal leukoencophalopathy, p 25-158. [Google Scholar]
- 6.Katz D.A., Berger J.R., Hamilton B., Major E.O., Post M.J. Progressive multifocal leukoencephalopathy complicating Wiskott-Aldrich syndrome. Report of a case and review of the literature of progressive multifocal leukoencephalopathy with other inherited immunodeficiency states. Arch. Neurol. 1994;51:422–426. doi: 10.1001/archneur.1994.00540160128016. [DOI] [PubMed] [Google Scholar]
- 7.Wali R.K., Drachenberg C., Hirsch H.H., Papadimitriou J., Nahar A., Mohanlal V., Brisco M.A., Bartlett S.T., Weir M.R., Ramos E. BK virus-associated nephropathy in renal allograft recipients: rescue therapy by sirolimus-based immunosuppression. Transplantation. 2004;78:1069–1073. doi: 10.1097/01.tp.0000142127.84497.50. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch H.H. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am. J. Transplant. 2002;2:25–30. doi: 10.1034/j.1600-6143.2002.020106.x. [DOI] [PubMed] [Google Scholar]
- 9.Mayr M., Nickeleit V., Hirsch H.H., Dickenmann M., Mihatsch M.J., Polyomavirus J. Steiger. BK nephropathy in a kidney transplant recipient: critical issues of diagnosis and management. Am. J. Kidney Dis. 2001;38:E13. doi: 10.1053/ajkd.2001.26917. [DOI] [PubMed] [Google Scholar]
- 10.Kenan D.J., Mieczkowski P.A., Burger-Calderon R., Singh H.K., Nickeleit V. The oncogenic potential of BK-polyomavirus is linked to viral integration into the human genome. J. Pathol. 2015;237:379–389. doi: 10.1002/path.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenan D.J., Mieczkowski P.A., Latulippe E., Cote I., Singh H.K., Nickeleit V. BK polyomavirus genomic integration and large T antigen expression: evolving paradigms in human oncogenesis. Am. J. Transplant. 2017;17:1674–1680. doi: 10.1111/ajt.14191. [DOI] [PubMed] [Google Scholar]
- 12.van der Meijden E., Bialasiewicz S., Rockett R.J., Tozer S.J., Sloots T.P., Feltkamp M.C. Different serologic behavior of MCPyV, TSPyV, HPyV6, HPyV7 and HPyV9 polyomaviruses found on the skin. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Meijden E., Janssens R.W., Lauber C., Bouwes Bavinck J.N., Gorbalenya A.E., Feltkamp M.C. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng H., Shuda M., Chang Y., Moore P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spurgeon M.E., Cheng J., Bronson R.T., Lambert P.F., DeCaprio J.A. Tumorigenic activity of merkel cell polyomavirus T antigens expressed in the stratified epithelium of mice. Cancer Res. 2015;75:1068–1079. doi: 10.1158/0008-5472.CAN-14-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.London W.T., Houff S.A., Madden D.L., Fuccillo D.A., Gravell M., Wallen W.C., Palmer A.E., Sever J.L., Padgett B.L., Walker D.L., ZuRhein G.M., Ohashi T. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus) Science. 1978;201:1246–1249. doi: 10.1126/science.211583. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda M., Jona M., Yasui K., Nagashima K. Genetic characterization of JC virus Tokyo-1 strain, a variant oncogenic in rodents. Virus Res. 1987;7:159–168. doi: 10.1016/0168-1702(87)90077-3. [DOI] [PubMed] [Google Scholar]
- 18.London W.T., Houff S.A., McKeever P.E., Wallen W.C., Sever J.L., Padgett B.L., Walker D.L. Viral-induced astrocytomas in squirrel monkeys. Prog. Clin. Biol. Res. 1983;105:227–237. [PubMed] [Google Scholar]
- 19.Franks R.R., Rencic A., Gordon J., Zoltick P.W., Curtis M., Knobler R.L., Khalili K. Formation of undifferentiated mesenteric tumors in transgenic mice expressing human neurotropic polymavirus early protein. Oncogene. 1996;12:2573–2578. [PubMed] [Google Scholar]
- 20.Corallini A., Pagnani M., Viadana P., Camellin P., Caputo A., Reschiglian P., Rossi S., Altavilla G., Selvatici R., Barbanti-Brodano G. Induction of malignant subcutaneous sarcomas in hamsters by a recombinant DNA containing BK virus early region and the activated human c-Harvey-ras oncogene. Cancer Res. 1987;47:6671–6677. [PubMed] [Google Scholar]
- 21.Diamandopoulos G.T. Induction of lymphocytic leukemia, lymphosarcoma, reticulum cell sarcoma, and osteogenic sarcoma in the Syrian golden hamster by oncogenic DNA simian virus 40. J. Natl. Cancer Inst. 1973;50:1347–1365. doi: 10.1093/jnci/50.5.1347. [DOI] [PubMed] [Google Scholar]
- 22.Noss G., Stauch G. Oncogenic activity of the BK type of human papova virus in inbred rat strains. Arch. Virol. 1984;81:41–51. doi: 10.1007/BF01309295. [DOI] [PubMed] [Google Scholar]
- 23.Allison A.C., Chesterman F.C., Baron S. Induction of tumors in adult hamsters with simian virus 40. J. Natl. Cancer Inst. 1967;38:567–572. [PubMed] [Google Scholar]
- 24.Gordon J., Del Valle L., Otte J., Khalili K. Pituitary neoplasia induced by expression of human neurotropic polyomavirus, JCV, early genome in transgenic mice. Oncogene. 2000;19:4840–4846. doi: 10.1038/sj.onc.1203849. [DOI] [PubMed] [Google Scholar]
- 25.Vilchez R.A., Kozinetz C.A., Arrington A.S., Madden C.R., Butel J.S. Simian virus 40 in human cancers. Am. J. Med. 2003;114:675–684. doi: 10.1016/s0002-9343(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 26.Khalili K., Del Valle L., Otte J., Weaver M., Gordon J. Human neurotropic polyomavirus, JCV, and its role in carcinogenesis. Oncogene. 2003;22:5181–5191. doi: 10.1038/sj.onc.1206559. [DOI] [PubMed] [Google Scholar]
- 27.Weiss R., Giordano A., Furth P., DeCaprio J., Pipas J., Ozer H., Strickler H., Procopio A., Garcea R., Carbone M. SV40 as an oncogenic virus and possible human pathogen. Dev. Biol. Stand. 1998;94:355–360. 369-360. [PubMed] [Google Scholar]
- 28.Tognon M., Corallini A., Martini F., Negrini M., Barbanti-Brodano G. Oncogenic transformation by BK virus and association with human tumors. Oncogene. 2003;22:5192. doi: 10.1038/sj.onc.1206550. 360. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J.J., Gjoerup O.V., Subramanian R.R., Cheng Y., Chen W., Roberts T.M., Hahn W.C. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell. 2003;3:483–495. doi: 10.1016/s1535-6108(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 30.Hahn W.C. Immortalization and transformation of human cells. Mol. Cell. 2002;13:351–361. [PubMed] [Google Scholar]
- 31.Gonin S., Diaz-Latoud C., Richard M.J., Ursini M.V., Imbo A., Manero F., Arrigo A.P. p53/T-antigen complex disruption in T-antigen transformed NIH3T3 fibroblasts exposed to oxidative stress: correlation with the appearance of a Fas/APO-1/CD95 dependent, caspase independent, necrotic pathway. Oncogene. 1999;18:8011–8023. doi: 10.1038/sj.onc.1203319. [DOI] [PubMed] [Google Scholar]
- 32.Ray S., Anderson M.E., Tegtmeyer P. Differential interaction of temperature-sensitive simian virus 40 T antigens with tumor suppressors pRb and p53. J. Virol. 1996;70:7224–7227. doi: 10.1128/jvi.70.10.7224-7227.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang S., Wang N.P., Tseng B.Y., Lee W.H., Lee E.H. Two distinct and frequently mutated regions of retinoblastoma protein are required for binding to SV40 T antigen. EMBO J. 1990;9:1815–1822. doi: 10.1002/j.1460-2075.1990.tb08306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dyson N., Buchkovich K., Whyte P., Harlow E. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell. 1989;58:249–255. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- 35.Dyson N., Buchkovich K., Whyte P., Harlow E. Cellular proteins that are targetted by DNA tumor viruses for transformation. Princess Takamatsu Symp. 1989;20:191–198. [PubMed] [Google Scholar]
- 36.Vassetzky Y.S., Tchang F., Fanning E., Mechali M. T-antigen interactions with chromatin and p53 during the cell cycle in extracts from xenopus eggs. J. Cell. Biochem. 1999;75:288–299. [PubMed] [Google Scholar]
- 37.Ali S.H., DeCaprio J.A. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin. Cancer Biol. 2001;11:15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- 38.Staib C., Pesch J., Gerwig R., Gerber J.K., Brehm U., Stangl A., Grummt F. p53 inhibits JC virus DNA replication in vivo and interacts with JC virus large T-antigen. Virology. 1996;219:237–246. doi: 10.1006/viro.1996.0241. [DOI] [PubMed] [Google Scholar]
- 39.Schmieg F.I., Simmons D.T. Intracellular location and kinetics of complex formation between simian virus 40 T antigen and cellular protein p53. J. Virol. 1984;52:350–355. doi: 10.1128/jvi.52.2.350-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmieg F.I., Simmons D.T. Characterization of the in vitro interaction between SV40 T antigen and p53: mapping the p53 binding site. Virology. 1988;164:132–140. doi: 10.1016/0042-6822(88)90628-9. [DOI] [PubMed] [Google Scholar]
- 41.Reich N.C., Levine A.J. Specific interaction of the SV40 T antigen-cellular p53 protein complex with SV40 DNA. Virology. 1982;117:286–290. doi: 10.1016/0042-6822(82)90531-1. [DOI] [PubMed] [Google Scholar]
- 42.Saribas S., Safak M. A comprehensive proteomics analysis of the JC virus (JCV) large and small tumor antigen interacting proteins: large T primarily targets the host protein complexes with V-ATPase and ubiquitin ligase activities while small t mostly associates with those having phosphatase and chromatin-remodeling functions. Viruses. 2020;12:1192. doi: 10.3390/v12101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porras A., Gaillard S., Rundell K. The simian virus 40 small-t and large-T antigens jointly regulate cell cycle reentry in human fibroblasts. J. Virol. 1999;73:3102–3107. doi: 10.1128/jvi.73.4.3102-3107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nunbhakdi-Craig V., Craig L., Machleidt T., Sontag E. Simian virus 40 small tumor antigen induces deregulation of the actin cytoskeleton and tight junctions in kidney epithelial cells. J. Virol. 2003;77:2807–2818. doi: 10.1128/JVI.77.5.2807-2818.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaillard S., Fahrbach K.M., Parkati R., Rundell K. Overexpression of simian virus 40 small-T antigen blocks centrosome function and mitotic progression in human fibroblasts. J. Virol. 2001;75:9799–9807. doi: 10.1128/JVI.75.20.9799-9807.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porras A., Bennett J., Howe A., Tokos K., Bouck N., Henglein B., Sathyamangalam S., Thimmapaya B., Rundell K. A novel simian virus 40 early-region domain mediates transactivation of the cyclin A promoter by small-t antigen and is required for transformation in small-t antigen-dependent assays. J. Virol. 1996;70:6902–6908. doi: 10.1128/jvi.70.10.6902-6908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolzau T., Hansen R.S., Zahra D., Reddel R.R., Braithwaite A.W. Inhibition of SV40 large T antigen induced apoptosis by small T antigen. Oncogene. 1999;18:5598–6908. doi: 10.1038/sj.onc.1202942. [DOI] [PubMed] [Google Scholar]
- 48.Choi Y.W., Lee I.C., Ross S.R. Requirement for the simian virus 40 small tumor antigen in tumorigenesis in transgenic mice. Mol. Cell Biol. 1988;8:3382–3390. doi: 10.1128/mcb.8.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carbone M., Lewis A.M., Jr., Matthews B.J., Levine A.S., Dixon K. Characterization of hamster tumors induced by simian virus 40 small t deletion mutants as true histiocytic lymphomas. Cancer Res. 1989;49:1565–1571. [PubMed] [Google Scholar]
- 50.Sontag E., Fedorov S., Kamibayashi C., Robbins D., Cobb M., Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 51.Rundell K., Parakati R. The role of the SV40 ST antigen in cell growth promotion and transformation. Semin. Cancer Biol. 2001;11:5–13. doi: 10.1006/scbi.2000.0341. [DOI] [PubMed] [Google Scholar]
- 52.Yang C.S., Vitto M.J., Busby S.A., Garcia B.A., Kesler C.T., Gioeli D., Shabanowitz J., Hunt D.F., Rundell K., Brautigan D.L., Paschal B.M. Simian virus 40 small t antigen mediates conformation-dependent transfer of protein phosphatase 2A onto the androgen receptor. Mol. Cell Biol. 2005;25:1298–1308. doi: 10.1128/MCB.25.4.1298-1308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sontag E., Sontag J.M., Garcia A. Protein phosphatase 2A is a critical regulator of protein kinase C zeta signaling targeted by SV40 small t to promote cell growth and NF-kappaB activation. EMBO J. 1997;16:5662–5671. doi: 10.1093/emboj/16.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan H., Veldman T., Rundell K., Schlegel R. Simian virus 40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J. Virol. 2002;76:10685–10691. doi: 10.1128/JVI.76.21.10685-10691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ugi S., Imamura T., Ricketts W., Olefsky J.M. Protein phosphatase 2A forms a molecular complex with Shc and regulates Shc tyrosine phosphorylation and downstream mitogenic signaling. Mol. Cell Biol. 2002;22:2375–2387. doi: 10.1128/MCB.22.7.2375-2387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frost J.A., Alberts A.S., Sontag E., Guan K., Mumby M.C., Feramisco J.R. Simian virus 40 small t antigen cooperates with mitogen-activated kinases to stimulate AP-1 activity. Mol. Cell Biol. 1994;14:6244–6252. doi: 10.1128/mcb.14.9.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwun H.J., Guastafierro A., Shuda M., Meinke G., Bohm A., Moore P.S., Chang Y. The minimum replication origin of merkel cell polyomavirus has a unique large T-antigen loading architecture and requires small T-antigen expression for optimal replication. J. Virol. 2009;83:12118–12128. doi: 10.1128/JVI.01336-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shuda M., Kwun H.J., Feng H., Chang Y., Moore P.S. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J. Clin. Invest. 2011;121:3623–3634. doi: 10.1172/JCI46323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwun H.J., Shuda M., Feng H., Camacho C.J., Moore P.S., Chang Y. Merkel cell polyomavirus small T antigen controls viral replication and oncoprotein expression by targeting the cellular ubiquitin ligase SCFFbw7. Cell Host Microbe. 2013;14:125–135. doi: 10.1016/j.chom.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shuda M., Chang Y., Moore P.S. Merkel cell polyomavirus-positive Merkel cell carcinoma requires viral small T-antigen for cell proliferation. J. Invest. Dermatol. 2014;134:1479–1481. doi: 10.1038/jid.2013.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shuda M., Guastafierro A., Geng X., Shuda Y., Ostrowski S.M., Lukianov S., Jenkins F.J., Honda K., Maricich S.M., Moore P.S., Chang Y. Merkel cell polyomavirus small T antigen induces cancer and embryonic merkel cell proliferation in a transgenic mouse model. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwun H.J., Wendzicki J.A., Shuda Y., Moore P.S., Chang Y. Merkel cell polyomavirus small T antigen induces genome instability by E3 ubiquitin ligase targeting. Oncogene. 2017;36:6838. doi: 10.1038/onc.2017.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sadowska B., Barrucco R., Khalili K., Safak M. Regulation of human polyomavirus JC virus gene transcription by AP-1 in glial cells. J. Virol. 2003;77:665–672. doi: 10.1128/JVI.77.1.665-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ansari S.A., Safak M., Del Valle L., Enam S., Amini S., Khalili K. Cell cycle regulation of NF-kappa b-binding activity in cells from human glioblastomas. Exp. Cell Res. 2001;265:221–233. doi: 10.1006/excr.2001.5168. [DOI] [PubMed] [Google Scholar]
- 65.Safak M., Gallia G.L., Ansari S.A., Khalili K. Physical and functional interaction between the Y-box binding protein YB-1 and human polyomavirus JC virus large T antigen. J. Virol. 1999;73:10146–10157. doi: 10.1128/jvi.73.12.10146-10157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Safak M., Sadowska B., Barrucco R., Khalili K. Functional interaction between JC virus late regulatory agnoprotein and cellular Y-box binding transcription factor, YB-1. J. Virol. 2002;76:3828–3838. doi: 10.1128/JVI.76.8.3828-3838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Safak M., Barrucco R., Darbinyan A., Okada Y., Nagashima K., Khalili K. Interaction of JC virus agno protein with T antigen modulates transcription and replication of the viral genome in glial cells. J. Virol. 2001;75:1476–1486. doi: 10.1128/JVI.75.3.1476-1486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carey M.F., Peterson C.L., Smale S.T. Dignam and Roeder nuclear extract preparation. Cold Spring Harb Protoc. 2009;12 doi: 10.1101/pdb.prot5330. pdb.prot5330. [DOI] [PubMed] [Google Scholar]
- 69.Safak M., Gallia G.L., Khalili K. Reciprocal interaction between two cellular proteins, Puralpha and YB-1, modulates transcriptional activity of JCVCY in glial cells. Mol. Cell Biol. 1999;19:2712–2723. doi: 10.1128/mcb.19.4.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graham F.L., van der Eb A.J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 71.Del Valle L., Baehring J., Lorenzana C., Giordano A., Khalili K., Croul S. Expression of a human polyomavirus oncoprotein and tumour suppressor proteins in medulloblastomas. Mol. Pathol. 2001;54:331–337. doi: 10.1136/mp.54.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Enam S., Del Valle L., Lara C., Gan D.D., Ortiz-Hidalgo C., Palazzo J.P., Khalili K. Association of human polyomavirus JCV with colon cancer: evidence for interaction of viral T-antigen and beta-catenin. Cancer Res. 2002;62:7093–7101. [PubMed] [Google Scholar]
- 73.Laghi L., Randolph A.E., Chauhan D.P., Marra G., Major E.O., Neel J.V., Boland C.R. JC virus DNA is present in the mucosa of the human colon and in colorectal cancers. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7484–7489. doi: 10.1073/pnas.96.13.7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matthews B.J., Levine A.S., Dixon K. Deletion mutations in the small t antigen gene alter the tissue specificity of tumors induced by simian virus 40. J. Virol. 1987;61:1282–1285. doi: 10.1128/jvi.61.4.1282-1285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abbas W., Khan K.A., Kumar A., Tripathy M.K., Dichamp I., Keita M., Mahlknecht U., Rohr O., Herbein G. Blockade of BFA-mediated apoptosis in macrophages by the HIV-1 Nef protein. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cimarelli A., Luban J. Translation elongation factor 1-alpha interacts specifically with the human immunodeficiency virus type 1 Gag polyprotein. J. Virol. 1999;73:5388–5401. doi: 10.1128/jvi.73.7.5388-5401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alao J.P. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol. Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hay N., Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 79.Nicholson K.M., Anderson N.G. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 80.Sarbassov D.D., Ali S.M., Kim D.H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 81.Gaubitz C., Oliveira T.M., Prouteau M., Leitner A., Karuppasamy M., Konstantinidou G., Rispal D., Eltschinger S., Robinson G.C., Thore S., Aebersold R., Schaffitzel C., Loewith R. Molecular basis of the rapamycin insensitivity of target of rapamycin complex 2. Mol. Cell. 2015;58:977–988. doi: 10.1016/j.molcel.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 82.Yip C.K., Murata K., Walz T., Sabatini D.M., Kang S.A. Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol. Cell. 2010;38:768–774. doi: 10.1016/j.molcel.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mannick J.B., Lamming D.W. Targeting the biology of aging with mTOR inhibitors. Nat Aging. 2023;3:642–660. doi: 10.1038/s43587-023-00416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ali A., Hoeflich K.P., Woodgett J.R. Glycogen synthase kinase-3: properties, functions, and regulation. Chem. Rev. 2001;101:2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- 85.Zhou X., Wang H., Burg M.B., Ferraris J.D. Inhibitory phosphorylation of GSK-3beta by AKT, PKA, and PI3K contributes to high NaCl-induced activation of the transcription factor NFAT5 (TonEBP/OREBP) Am. J. Physiol. Ren. Physiol. 2013;304:F908–F917. doi: 10.1152/ajprenal.00591.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nitulescu G.M., Van De Venter M., Nitulescu G., Ungurianu A., Juzenas P., Peng Q., Olaru O.T., Gradinaru D., Tsatsakis A., Tsoukalas D., Spandidos D.A., Margina D. The Akt pathway in oncology therapy and beyond. Int. J. Oncol. 2018;53:2319–2331. doi: 10.3892/ijo.2018.4597. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Manning B.D., Cantley L.C. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hemmings B.A., Restuccia D.F. PI3K-PKB/Akt pathway. Cold Spring Harbor Perspect. Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ballesteros-Alvarez J., Andersen J.K. mTORC2: the other mTOR in autophagy regulation. Aging Cell. 2021;20 doi: 10.1111/acel.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vanhaesebroeck B., Alessi D.R. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 2000;346(Pt 3):561–576. [PMC free article] [PubMed] [Google Scholar]
- 91.Osaki M., Oshimura M., Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 92.Soderling T.R. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem. Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- 93.Richardson E.P. Progressive multifocal encephalopathy. N. Engl. J. Med. 1961;265:815–823. doi: 10.1056/NEJM196110262651701. [DOI] [PubMed] [Google Scholar]
- 94.Sima A.A.F.F., McLachlan S.D., D R. Multiple malignant astrocytomas in a patient with spontanous progressive multifocal leukoencephalopathy. Ann. Neurol. 1983;14:183–188. doi: 10.1002/ana.410140205. [DOI] [PubMed] [Google Scholar]
- 95.Casteigne P., Rondot P., Escourolle R., Ribadeau D.J.L., Cathala F., Hauw J.J. Progressive multifocal leukoencephalopathy and multiple gliomas. Rev Neuro Paris. 1974;9–10:379–392. [PubMed] [Google Scholar]
- 96.Shintaku M., Matsumoto R., Sawa H., Nagashima K. Infection with JC virus and possible dysplastic ganglion-like transformation of the cerebral cortical neurons in a case of progressive multifocal leukoencephalopathy. J. Neuropathol. Exp. Neurol. 2000;59:921–929. doi: 10.1093/jnen/59.10.921. [DOI] [PubMed] [Google Scholar]
- 97.Boldorini R., Caldarelli-Stefano R., Monga G., Zocchi M., Mediati M., Tosoni A., Ferrante P. PCR detection of JC virus DNA in the brain tissue of a 9-year-old child with pleomorphic xanthoastrocytoma. J. Neurovirol. 1998;4:242–245. doi: 10.3109/13550289809114524. [DOI] [PubMed] [Google Scholar]
- 98.Rencic A., Gordon J., Otte J., Curtis M., Kovatich A., Zoltick P., Khalili K., Andrews D. Detection of JC virus DNA sequence and expression of the viral oncoprotein, tumor antigen, in brain of immunocompetent patient with oligoastrocytoma. Proc. Natl. Acad. Sci. U. S. A. 1996;93:7352–7357. doi: 10.1073/pnas.93.14.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Del Valle L., Gordon J., Assimakopoulou M., Enam S., Geddes J.F., Varakis J.N., Katsetos C.D., Croul S., Khalili K. Detection of JC virus DNA sequences and expression of the viral regulatory protein T-antigen in tumors of the central nervous system. Cancer Res. 2001;61:4287–4293. [PubMed] [Google Scholar]
- 100.Del Valle L., Delbue S., Gordon J., Enam S., Croul S., Ferrante P., Khalili K. Expression of JC virus T-antigen in a patient with MS and glioblastoma multiforme. Neurology. 2002;58:895–900. doi: 10.1212/wnl.58.6.895. [DOI] [PubMed] [Google Scholar]
- 101.Ricciardiello L., Chang D.K., Laghi L., Goel A., Chang C.L., Boland C.R. Mad-1 is the exclusive JC virus strain present in the human colon, and its transcriptional control region has a deleted 98-base-pair sequence in colon cancer tissues. J. Virol. 2001;75:1996–2001. doi: 10.1128/JVI.75.4.1996-2001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ricciardiello L., Laghi L., Ramamirtham P., Chang C.L., Chang D.K., Randolph A.E., Boland C.R. JC virus DNA sequences are frequently present in the human upper and lower gastrointestinal tract. Gastroenterology. 2000;119:1228–1235. doi: 10.1053/gast.2000.19269. [DOI] [PubMed] [Google Scholar]
- 103.Corallini A., Altavilla G., Carra L., Grossi M.P., Federspil G., Caputo A., Negrini M., Barbanti-Brodano G. Oncogenity of BK virus for immunosuppressed hamsters. Arch. Virol. 1982;73:243–253. doi: 10.1007/BF01318078. [DOI] [PubMed] [Google Scholar]
- 104.Corallini A., Altavilla G., Cecchetti M.G., Fabris G., Grossi M.P., Balboni P.G., Lanza G., Barbanti-Brodano G. Ependymomas, malignant tumors of pancreatic islets, and osteosarcomas induced in hamsters by BK virus, a human papovavirus. J. Natl. Cancer Inst. 1978;61:875–883. [PubMed] [Google Scholar]
- 105.Corallini A., Barbanti-Brodano G., Bortoloni W., Nenci I., Cassai E., Tampieri M., Portolani M., Borgatti M. High incidence of ependymomas induced by BK virus, a human papovavirus: brief communication. J. Natl. Cancer Inst. 1977;59:1561–1564. doi: 10.1093/jnci/59.5.1561. [DOI] [PubMed] [Google Scholar]
- 106.Uchida S., Watanabe S., Aizawa T., Furuno A., Muto T. Polyoncogenicity and insulinoma-inducing ability of BK Virus, a human Papovavirus, in Syrian golden hamsters. J. Natl. Cancer Inst. 1979;63:119–126. [PubMed] [Google Scholar]
- 107.Chenciner N., Grossi M.P., Meneguzzi G., Corallini A., Manservigi R., Barbanti-Brodano G., Milanesi G. State of viral DNA in BK virus-transformed rabbit cells. Virology. 1980;103:138–148. doi: 10.1016/0042-6822(80)90132-4. [DOI] [PubMed] [Google Scholar]
- 108.Corallini A., Pagnani M., Viadana P., Silini E., Mottes M., Milanesi G., Gerna G., Vettor R., Trapella G., Silvani V., et al. Association of BK virus with human brain tumors and tumors of pancreatic islets. Int. J. Cancer. 1987;39:60–67. doi: 10.1002/ijc.2910390111. [DOI] [PubMed] [Google Scholar]
- 109.Testa J.R., Carbone M., Hirvonen A., Khalili K., Krynska B., Linnainmaa K., Pooley F.D., Rizzo P., Rusch V., Xiao G.H. A multi-institutional study confirms the presence and expression of simian virus 40 in human malignant mesotheliomas. Cancer Res. 1998;58:4505–4509. [PubMed] [Google Scholar]
- 110.Carbone M., Rizzo P., Grimley P.M., Procopio A., Mew D.J., Shridhar V., de Bartolomeis A., Esposito V., Giuliano M.T., Steinberg S.M., Levine A.S., Giordano A., Pass H.I. Simian virus-40 large-T antigen binds p53 in human mesotheliomas. Nat. Med. 1997;3:908–912. doi: 10.1038/nm0897-908. [DOI] [PubMed] [Google Scholar]
- 111.Bocchetta M., Di Resta I., Powers A., Fresco R., Tosolini A., Testa J.R., Pass H.I., Rizzo P., Carbone M. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10214–10219. doi: 10.1073/pnas.170207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bergsagel D.J., Finegold M.J., Butel J.S., Kupsky W.J., Garcea R.L. DNA sequences similar to those of simian virus 40 in ependymomas and choroid plexus tumors of childhood. N. Engl. J. Med. 1992;326:988–993. doi: 10.1056/NEJM199204093261504. [DOI] [PubMed] [Google Scholar]
- 113.Weiss A.F., Portmann R., Fischer H., Simon J., Zang K.D. Simian virus 40-related antigens in three human meningiomas with defined chromosome loss. Proc. Natl. Acad. Sci. U. S. A. 1975;72:609–613. doi: 10.1073/pnas.72.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tognon M., Casalone R., Martini F., De Mattei M., Granata P., Minelli E., Arcuri C., Collini P., Bocchini V. Large T antigen coding sequences of two DNA tumor viruses, BK and SV40, and nonrandom chromosome changes in two glioblastoma cell lines. Cancer Genet. Cytogenet. 1996;90:17–23. doi: 10.1016/0165-4608(96)00067-2. [DOI] [PubMed] [Google Scholar]
- 115.Martini F., Iaccheri L., Lazzarin L., Carinci P., Corallini A., Gerosa M., Iuzzolino P., Barbanti-Brodano G., Tognon M. SV40 early region and large T antigen in human brain tumors, peripheral blood cells, and sperm fluids from healthy individuals. Cancer Res. 1996;56:4820–4825. [PubMed] [Google Scholar]
- 116.Lednicky J.A., Garcea R.L., Bergsagel D.J., Butel J.S. Natural simian virus 40 strains are present in human choroid plexus and ependymoma tumors. Virology. 1995;212:710–717. doi: 10.1006/viro.1995.1529. [DOI] [PubMed] [Google Scholar]
- 117.P. Krieg, E. Amtmann, D. Jonas, H. Fischer, K. Zang, G. Sauer. Episomal simian virus 40 genomes in human brain tumors. Proc. Natl. Acad. Sci. U. S. A. 78:6446-6450. [DOI] [PMC free article] [PubMed]
- 118.Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J. Biol. Chem. 1999;274(1981):27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 119.Winston J.T., Strack P., Beer-Romero P., Chu C.Y., Elledge S.J., Harper J.W. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thanos D. NF-kappa B: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 121.Yang X., Su K., Roos M.D., Chang Q., Paterson A.J., Kudlow J.E. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6611–6616. doi: 10.1073/pnas.111099998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alland L., Peseckis S.M., Atherton R.E., Berthiaume L., Resh M.D. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J. Biol. Chem. 1994;269:16701–16705. [PubMed] [Google Scholar]
- 123.Resh M.D. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 124.Frisque R.J., Bream G.L., Cannella M.T. Human polyomavirus JC virus genome. J. Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Trowbridge P.W., Frisque R.J. Identification of three new JC virus proteins generated by alternative splicing of the early viral mRNA. J. Neurovirol. 1995;1:195–206. doi: 10.3109/13550289509113966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.