Abstract
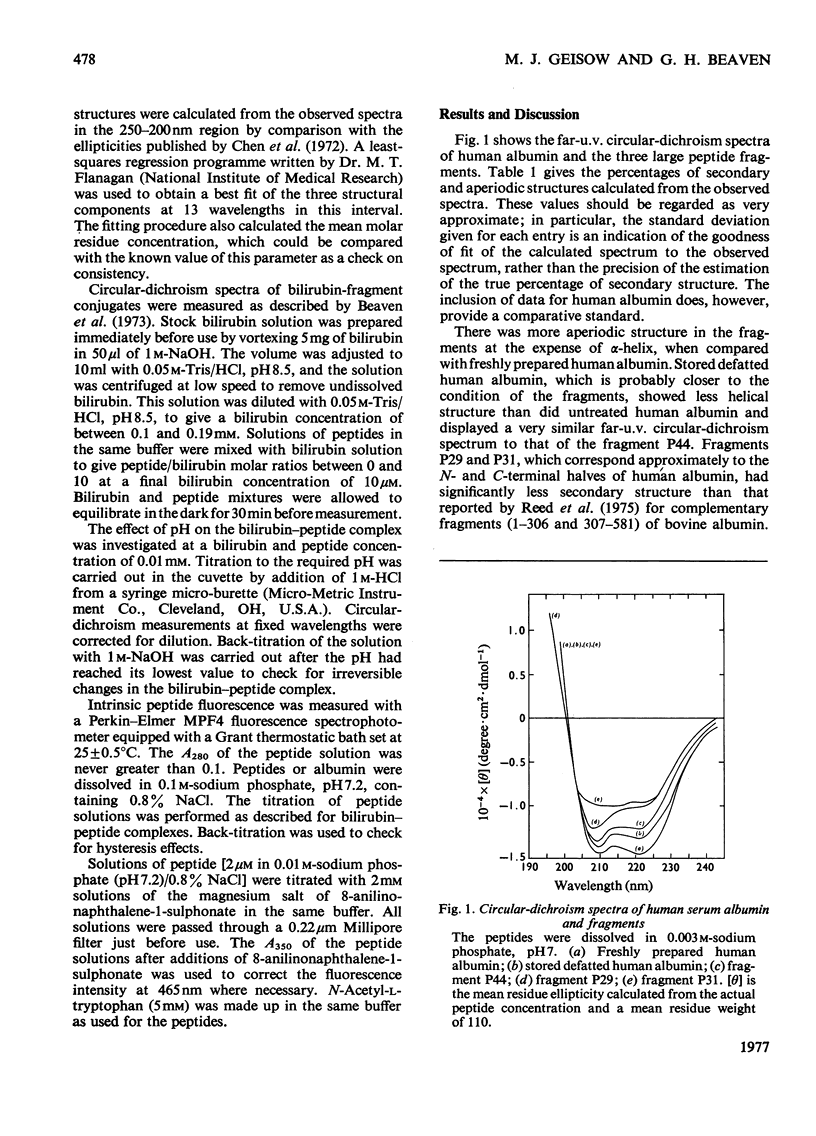
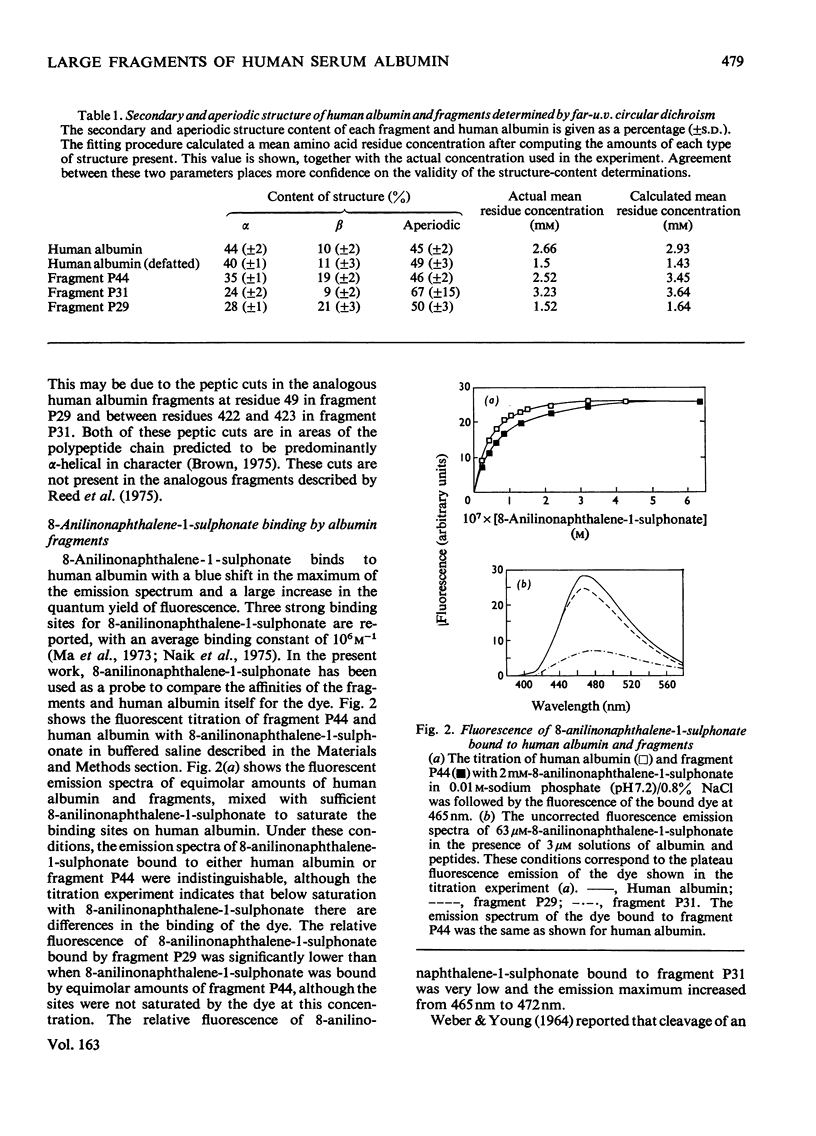
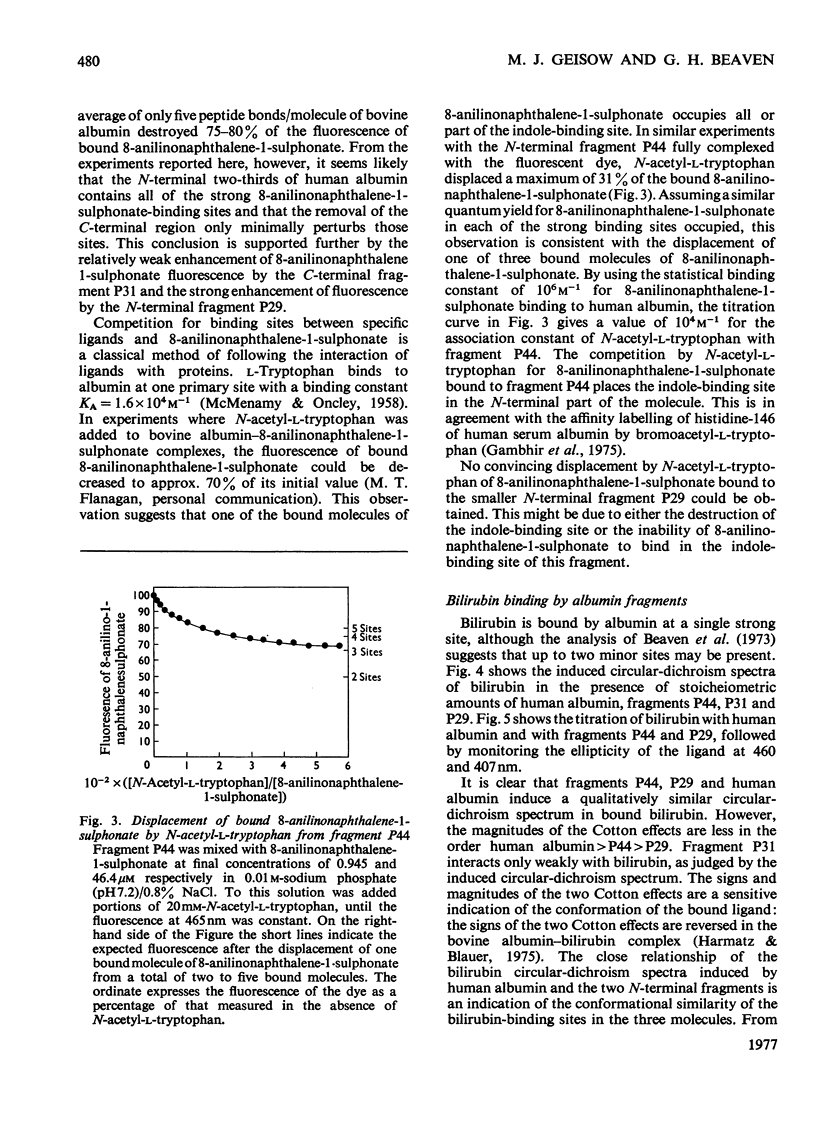
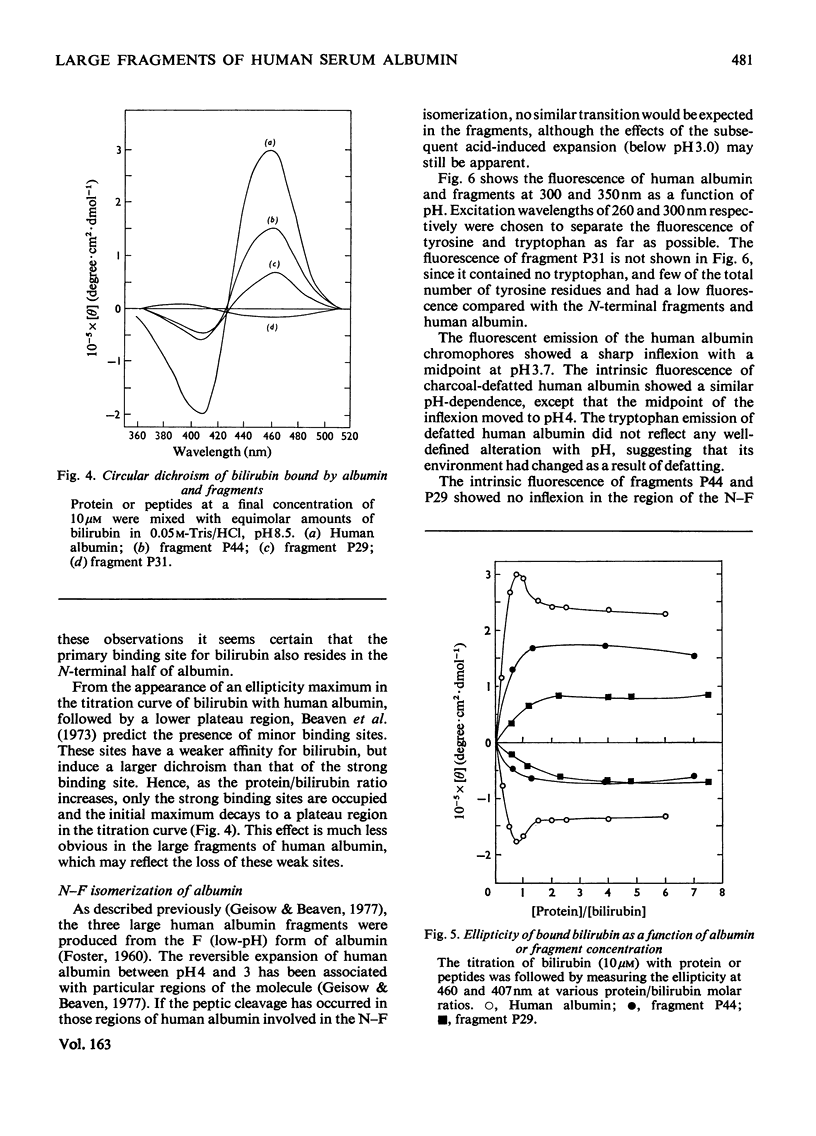
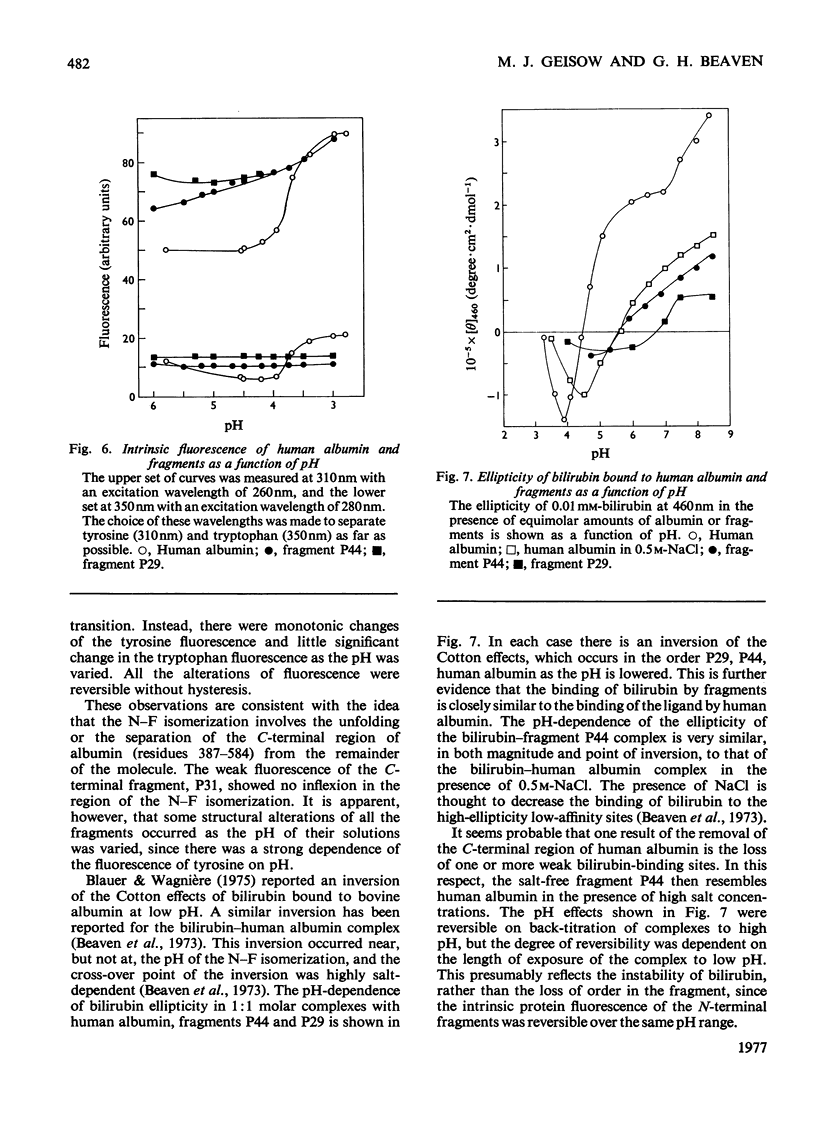
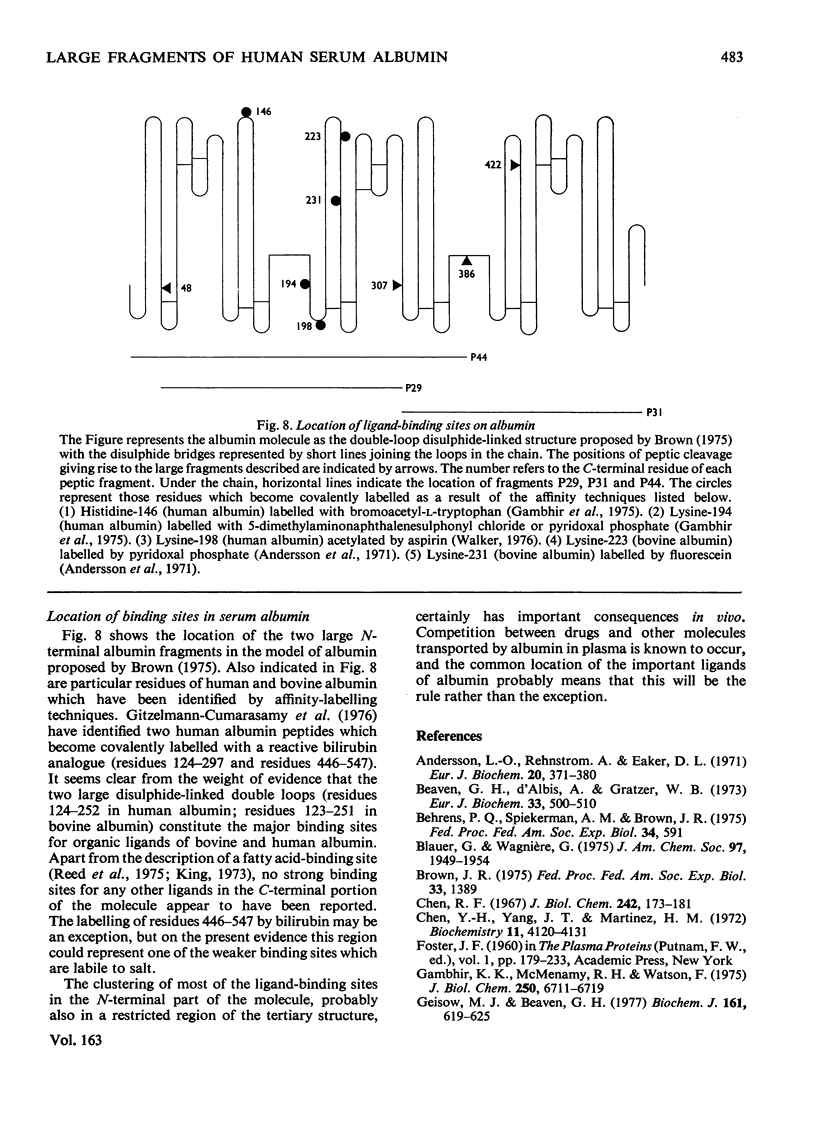
Three large fragments of human serum albumin were produced by peptic digestion of the native protein [Geisow & Beaven (1977) Biochem. J. 161, 619-625]. Fragment P44 represents residues 1-386 and fragments P29 and P31 represent residues 49-307 and residues 308-584 respectively of the albumin molecule. The large N-terminal fragment P44 has a similar percentage of alpha-helix to stored defatted albumin, although the alpha-helix content of all the fragments is significantly less than that of freshly prepared albumin. The fragment P44 appears to account for all the binding of the hydrophobic probe 8-anilinonaphthalene-1-sulphonate to albumin. N-Acetyl-L-tryptophan binds to this fragment and displaces one of the bound molecules of 8-anilinonaphthalene-1-sulphonate. Bilirubin binds to fragments P44 and P29, and the complexes show similar circular-dichroism spectra to that of the complex between bilirubin and whole albumin. These results are in agreement with affinity-labeling work on albumin with reactive ligands where substitution occurs in the N-terminal region of the molecule. The sharp conformational transitional transition in albumin which is observed between pH4 and 3.5 was absent from the fragments. This isomerization, usually called the N-F transition, probably occurs in intact albumin as a result of the unfolding or separation of the C-terminal third of the protein from the remainder of the molecule.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. O., Rehnström A., Eaker D. L. Studies on "nonspecific" binding. Eur J Biochem. 1971 Jun 11;20(3):371–380. doi: 10.1111/j.1432-1033.1971.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Blauer G., Wagnière G. Conformation of bilirubin and biliverdin in their complexes with serum albumin. J Am Chem Soc. 1975 Apr 2;97(7):1949–1954. doi: 10.1021/ja00840a057. [DOI] [PubMed] [Google Scholar]
- Breaven G. H., D'Albis A., Gratzer W. B. The interaction of bilirubin with human serum albumin. Eur J Biochem. 1973 Mar 15;33(3):500–509. doi: 10.1111/j.1432-1033.1973.tb02709.x. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- Gambhir K. K., McMenamy R. H., Watson F. Positions in human serum albumin which involve the indole binding site. Sequence of 107-residue fragment. J Biol Chem. 1975 Sep 10;250(17):6711–6719. [PubMed] [Google Scholar]
- Geisow M. J., Beaven G. H. Large fragments of human serum albumin. Biochem J. 1977 Mar 1;161(3):619–625. doi: 10.1042/bj1610619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmatz D., Blauer G. Optical properties of bilirubin-serum albumin complexes in aqueous solution. A comparison among albumins from different species. Arch Biochem Biophys. 1975 Oct;170(2):375–383. doi: 10.1016/0003-9861(75)90132-0. [DOI] [PubMed] [Google Scholar]
- King T. P. Limited pepsin digestion of bovine plasma albumin. Arch Biochem Biophys. 1973 Jun;156(2):509–520. doi: 10.1016/0003-9861(73)90300-7. [DOI] [PubMed] [Google Scholar]
- Ma J. K., Jun H. W., Luzzi L. A. Determination of equilibrium constants and binding capacities using a modified Scatchard method in drug-protein binding studies. J Pharm Sci. 1973 Dec;62(12):2038–2040. doi: 10.1002/jps.2600621233. [DOI] [PubMed] [Google Scholar]
- McMENAMY R. H., ONCLEY J. L. The specific binding of L-tryptophan to serum albumin. J Biol Chem. 1958 Dec;233(6):1436–1447. [PubMed] [Google Scholar]
- Meloun B., Morávek L., Kostka V. Complete amino acid sequence of human serum albumin. FEBS Lett. 1975 Oct 15;58(1):134–137. doi: 10.1016/0014-5793(75)80242-0. [DOI] [PubMed] [Google Scholar]
- Reed R. G., Feldhoff R. C., Clute O. L., Peters T., Jr Fragments of bovine serum albumin produced by limited proteolysis. Conformation and ligand binding. Biochemistry. 1975 Oct 21;14(21):4578–4583. doi: 10.1021/bi00692a004. [DOI] [PubMed] [Google Scholar]
- WEBER G., YOUNG L. B. FRAGMENTATION OF BOVINE SERUM ALBUMIN BY PEPSIN. I. THE ORIGIN OF THE ACID EXPANSION OF THE ALBUMIN MOLECULE. J Biol Chem. 1964 May;239:1415–1423. [PubMed] [Google Scholar]
- Walker J. E. Lysine residue 199 of human serum albumin is modified by acetylsalicyclic acid. FEBS Lett. 1976 Jul 15;66(2):173–175. doi: 10.1016/0014-5793(76)80496-6. [DOI] [PubMed] [Google Scholar]


