Abstract
ABSTRACT
Background
The knowledge about the prevalence of schizophrenia among people with intellectual disabilities (ID) is sparse, particularly concerning the distribution in different age groups.
Aims
To investigate the prevalence of diagnoses in the schizophrenia spectrum among people with ID compared with the general population (gPop).
Methods
This was an 8-year longitudinal register study. The participants were all residents of Skåne on 1 January 2014. People with a diagnosis of ID (F7 in International Statistical Classification of Diseases and Related Health Problems 10th Revision) or Down syndrome (DS; Q90), or service and support for people with ID/autism spectrum disorder (ASD) comprised the ID cohort (n=14 716). After excluding family members of people in the ID cohort, the remaining population of Skåne comprised the gPop cohort (n=1 226 955).
The primary outcome measure was having at least one diagnosis in the schizophrenia spectrum (F20-F29). Secondary outcomes were single diagnoses within the schizophrenia spectrum.
Results
The prevalence of schizophrenia spectrum diagnoses was 7.2% in the ID cohort. This was more than an eightfold increase compared with the gPop (relative risk (RR) 8.45; 95% CI 7.94 to 9.00). The risk was also high among children (aged 0–18 years at the start of the study period; RR 9.42; 95% CI 7.36 to 12.05). In the subcohort comprising those with a diagnosis of DS, the risk of schizophrenia diagnosis was more than twice as high as in gPop. Concomitant ASD or genetic syndrome did not carry an excess risk among people with ID when compared with the gPop.
Conclusions
The findings of the present study support earlier assumptions that people with vulnerable brains develop psychotic disorders more frequently and that the onset age is lower than among people in the gPop. Habilitation services for children and adolescents, as well as general mental health services, should keep in mind that schizophrenia may be present when children and adolescents show severely decreased functioning, anxiety or aggressive behaviour.
Keywords: schizophrenia, autism spectrum disorder, prevalence, cohort studies, intellectual disability
WHAT IS ALREADY KNOWN ON THIS TOPIC
Knowledge about the prevalence of severe mental illness, particularly schizophrenia, in persons with intellectual disability has been scarce.
To our knowledge, only two population studies have been published in 2007 and 2008, both of which found a prevalence of about 3%-4%.
Data in both studies were collected about 20 years ago when knowledge about the assessment of severe mental illness in people with intellectual disabilities was sparse.
WHAT THIS STUDY ADDS
Diagnoses in the schizophrenia spectrum among people with intellectual disabilities are more prevalent than previously believed.
This is particularly true for children, whose risk is about ten times higher than among children in the general population.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Awareness among clinicians of the high prevalence of schizophrenia among people with intellectual disability may result in earlier detection, which in turn may prevent individual suffering and reduce costs.
Introduction
Knowledge of schizophrenia in people with intellectual disabilities (ID), including phenomenology (appearances and characteristics of symptoms) and diagnostic assessment, has increased in recent years.1 Although there is considerable variation in the methodologies, settings and sample sizes in previous studies of schizophrenia in ID, the prevalence is found to be at least threefold compared with the general population (gPop).2 3 Thus, schizophrenia occurs more frequently in people with ID compared with the general population, where the lifetime prevalence of schizophrenia has been estimated to be about 0.5%.4
Advances in diagnoses in the schizophrenia spectrum (F20-F29) from the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) to ICD-11th Revision include removal of the emphasis on first-rank symptoms, as well as the schizophrenia subtypes—paranoid, disorganised and catatonic—besides the categories for unspecified schizophrenia.5 Schizophrenia is currently defined as a serious mental illness that affects cognition, emotions and behaviour. It carries prominent disturbances in multiple mental modalities, including thinking, perception, self-experience, cognition, volition and affect.5 The core symptoms include delusions, hallucinations, disorganisation, negative symptoms and cognitive impairments, which must have been present for at least 1 month.6 Schizophrenia may be difficult to diagnose in people with ID due to diagnostic overshadowing: the phenomenon that core symptoms of schizophrenia may be interpreted as part of the ID condition rather than representing an additional disorder.7 Moreover, people with more severe ID may not be able to report delusions or hallucinations.8 Hence, knowledge about the phenomenology of this mental disorder in ID8 and a possible over-representation among people with ID may encourage clinicians to consider schizophrenia in people with ID who experience a severe fall in practical and cognitive abilities and appear to be confused and alienated.1 Recent research indicates that it may also be feasible to use the ICD-10 criteria for schizophrenia when diagnosing people with ID, even when they experience severe deterioration regarding practical skills, language and regulation of emotions.1 However, as patients with ID may have problems with expressing symptoms, which are aggravated by severe mental illness,1 observations of changes in behaviour and the use of behavioural equivalents to conventional symptoms may be necessary.8
People with schizophrenia are at increased risk of co-occurring physical disorders, early death9 and poor physical health.10 As ID itself carries an increased risk of physical disorders and early death,11 people with both ID and schizophrenia may have an exceptionally high risk of this. Hence, it is important to detect schizophrenia in people with ID to be able to provide the correct treatment and reduce these risks.
Several genetic syndromes are common among people with ID or may even be the cause of the ID. Some of these syndromes have been associated with schizophrenia in the gPop. The most closely associated is 22 q 11 Deletion Syndrome, also called DiGeorge syndrome.12 Among other sex chromosome abnormalities, both Klinefelter syndrome and Turner syndrome have been suggested to increase the risk of schizophrenia.13 14 Although no association has been found between Fragile X syndrome and schizophrenia,15 the use of antipsychotic medication was found to be more than four times higher in those with Fragile X syndrome than among people in the gPop,15 suggesting that schizophrenia may indeed be more common in this group.
Unlike earlier assumptions of the low risk of developing schizophrenia or psychosis in people with Down syndrome (DS), Dykens et al16 found an increased risk of psychosis in DS compared with those with other types of ID. However, the study by Dykens et al used a clinical sample from a psychiatric centre. Hence, there is still a need for larger-scale studies to assess a possible association between DS and schizophrenia.
Autism spectrum disorder (ASD) is a neurodevelopmental condition with onset in early childhood6 and a prevalence of about 1% in the gPop.17 ASD is considered as highly hereditary as well as highly prevalent in people with ID.18 There is reason to believe that people with ASD more often develop severe mental disorders, especially disorders on the schizophrenia spectrum. However, there is a lack of research on the potentially increased risk of developing schizophrenia in individuals with ID and co-occurring ASD.
ID is diagnosed when the individual’s intellectual and adaptive development differs significantly from typical development.6 The level of ID is usually diagnosed in four levels: mild (intelligence quotient (IQ) 50–69), moderate (IQ 35–49), severe (IQ 20–34) and profound (IQ <20). There are also diagnoses in the ICD-10 for unknown and other ID available for those without a specified severity. People with ID constitute a largely heterogeneous group regarding cognitive abilities, personalities, interests and the co-occurrence of challenges such as mental health and physical disorders.
Previous research indicates that schizophrenia is caused by complex and heterogeneous interactions between different biopsychosocial factors, including genetic, perinatal, neuroanatomic, neurochemical and other biological factors.19 There is a need for more knowledge regarding schizophrenia in the ID population to increase the detection of schizophrenia among people with ID, who then may be provided adequate treatment and care. Prevalence studies and research based on representative populations have been sparse, particularly those that also compare findings with the gPop. Most reports on prevalence are either review studies or studies based on data from 20 years ago or more.3 4 Although psychotic symptoms may be hard to detect in people with ID, research after 2000 shows that using observations of symptoms (behavioural equivalents to conventional symptoms) will facilitate the diagnostic assessment of schizophrenia in people with ID.8 Representative studies of the prevalence of schizophrenia in people with ID may contribute to more updated knowledge on the co-occurrence of ID and schizophrenia.
The overall aim of the present study was to investigate the prevalence of diagnoses in the schizophrenia spectrum among people with ID compared with the gPop. Consequently, these research questions were formulated: What is the prevalence of schizophrenia diagnoses among people with ID compared with the gPop? Moreover, does the prevalence vary with the severity of ID, DS and the co-occurrence of ASD and genetic syndromes?
Methods
This is a prospective, longitudinal register study20 covering the entire population of Skåne, the southernmost region of Sweden, from 2014 to 2021.
Data sources
The data in this study are collected from one national and one regional register.
The national LSS (The Swedish Act Concerning Support and Service for Persons with Certain Functional Impairments) register is maintained by the Swedish National Board and Welfare. It comprises data on all support provided according to the Act Concerning Support and Service for Persons with Certain Functional Impairments (LSS Act21). The LSS Act states that service and support should be provided to promote equality in living conditions and full participation in social life. People with the right to such support are those with (1) developmental disability, autism or autism-like condition, (2) significant and permanent ID after brain damage in adulthood caused by external violence or physical illness or (3) other permanent physical or mental disabilities that are not due to normal ageing, if they are large and cause significant difficulties in daily life and thus an extensive need for support or services. Although no diagnoses are recorded in the LSS register, it is possible to identify which of the three groups a person belongs.
The regional Skåne Healthcare Register22 collects data from all healthcare contacts with public healthcare providers and private healthcare providers under contract with the county council. In all public care, one primary diagnosis and up to seven secondary diagnoses are recorded using the ICD-10. The primary diagnosis is intended to reflect the reason for the healthcare contact; the secondary diagnoses list disorders and conditions deemed relevant for the treatment of the primary diagnosis. Medical record notes are not available in the register.
Study cohorts
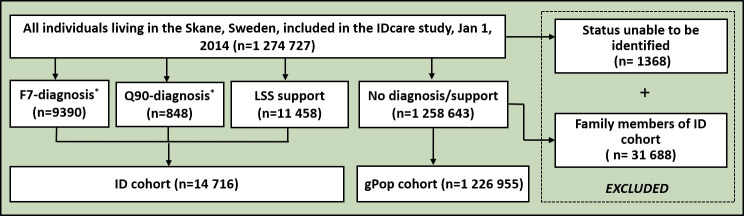
Statistics Sweden identified all people living in Skåne on 1 January 2014. As there is no data source allowing for the identification of people with ID, we used the Skåne Healthcare Register to capture those with a diagnosis of ID (F70-F79 or Q90 in ICD-10) and the LSS register to identify those with administrative ID (ie, those belonging to the first group described above). Thus, having a diagnosis or receiving support was used as a proxy for having ID. Using these two registers, we identified 9390 people with at least one F7 diagnosis, 848 people with at least one Q90 diagnosis and 11 458 people with at least one measure of support. Due to the overlap between these three categories, the ID cohort comprised 14 716 people (figure 1). After excluding people with a family or household member who were included in the ID cohort, the remaining people comprised the gPop cohort (n=1 226 955). All individuals in the two cohorts were categorised based on their age of inclusion (ie, 2014) into children (0–18 years), adults (19–64 years) and older adults (65+ years) (table 1).
Figure 1. Flowchart of IDcare study: inclusion of people in the ID and gPop cohorts. *F7, ID. †Q90, Down syndrome. gPop, general population; ID, intellectual disability; LSS, The Swedish Act Concerning Support and Service for Persons with Certain Functional Impairments.
Table 1. People in the gPop and ID cohorts in 2014 in the IDcare study.
| Total | Children | Adults | Older people | |
| gPop | ||||
| Total | 1 226 955 | 244 769 | 725 334 | 256 852 |
| Male | 606 923 | 125 080 | 364 868 | 116 975 |
| Female | 620 032 | 119 689 | 360 466 | 139 877 |
| ID | ||||
| Total | 14 716 | 5637 | 8313 | 766 |
| Male | 8777 | 3623 | 4735 | 419 |
| Female | 5939 | 2014 | 3578 | 347 |
gPopgeneral populationIDintellectual disability
Intellectual disabilities
Schizophrenia among people with ID was evaluated in three different ways. The first was by analysing the ID cohort, that is, using a diagnosis of ID and/or receiving support, according to the LSS Act, as a proxy for ID. The second way was by assessing subgroups within the ID cohort as defined by the severity of ID and the diagnosis of DS, respectively. When evaluating the severity of ID, we used the most severe type of ID found in the Skåne Healthcare Register. Thus, if a person was diagnosed with F70 (mild ID) and F71 (moderate ID) on different occasions, they were considered as having moderate ID. Using this definition, we identified 3952 people with mild ID, 1086 with moderate ID, 613 with severe/profound ID and 3739 with other/unknown ID (table 2). The third way was to identify a subgroup of 319 people with co-occurring ID and genetic syndromes and another with 5010 people with ID and co-occurring ASD (table 2).
Table 2. Overview of included participants.
| ID cohort characteristics and ICD-10 diagnoses | Total | Children | Adults | Older people |
| Reason for inclusion in ID cohort | N=14 716 | N=5637 | N=8313 | N=766 |
| Diagnosis only | 3258 (22%) | 1590 (28%) | 1423 (17%) | 245 (32%) |
| LSS only | 4844 (33%) | 1982 (35%) | 2736 (33%) | 126 (16%) |
| Diagnosis and LSS | 6614 (45%) | 2065 (37%) | 4154 (50%) | 395 (52%) |
| Diagnoses | N=9872 | N=3655 | N=5577 | N=640 |
| F7 only | 9024 (91%) | 3351 (92%) | 5060 (91%) | 613 (96%) |
| Q90 only | 482 (5%) | 77 (2%) | 383 (7%) | 22 (3%) |
| F7 with Q90 | 366 (4%) | 227 (6%) | 134 (2%) | 5 (1%) |
| Severity of ID | N=9390 | N=3578 | N=5194 | N=618 |
| Mild (F70) | 3952 (42%) | 2011 (56%) | 1844 (36%) | 97 (16%) |
| Moderate (F71) | 1086 (12%) | 666 (19%) | 391 (8%) | 29 (5%) |
| Severe/Profound (F72/F73) | 613 (7%) | 336 (9%) | 246 (5%) | 31 (5%) |
| Other/Unknown (F78/F79) | 3739 (40%) | 565 (16%) | 2713 (52%) | 461 (75%) |
| Concomitant ASD | N=14 716 | N=5637 | N=8313 | N=766 |
| Pervasive developmental disorders (F84, not F84.5) | 3910 (27%) | 2758 (49%) | 1125 (14%) | 27 (4%) |
| Asperger syndrome (F84.5) | 1535 (10%) | 429 (8%) | 1098 (13%) | 8 (1%) |
| Any ASD | 5010 (34%) | 2974 (53%) | 2001 (24%) | 35 (5%) |
| Concomitant genetic syndromes* | N=14 716 | N=5637 | N=9079 | |
| DiGeorge syndrome (D82.1) | 27 (0%) | 18 (0%) | 9 (0%) | |
| Edwards syndrome and Patau syndrome (Q91) | 8 (0%) | |||
| Turner syndrome (Q96) | 13 (0%) | |||
| Other sex chromosome abnormalities, female phenotype (Q97)† | 5 (0%) | |||
| Klinefelter syndrome (Q98.0, Q98.1, Q98.2, Q98.4) | 26 (0%) | 9 (0%) | 17 (0%) | |
| Other chromosome abnormalities (Q99) | 262 (2%) | 165 (3%) | 97 (1%) | |
| Any genetic syndrome | 319 (2%) | 195 (3%) | 124 (1%) |
Number of people with (ID) by age group, diagnosis, severity of ID and concomitant genetic syndromes and (ASD). Numbers by age groups are only presented if all groups include at least five people.
Older people are included in the age group ‘adults’.
Percentage based on number of women in the cohort.
Percentage based on the number of men in the cohort
ASDautism spectrum disorderICD-1010th revision of the International Classification of DiseasesIDintellectual disabilityLSSThe Swedish Act Concerning Support and Service for Persons with Certain Functional Impairments
Genetic syndromes and ASD
To assess the prevalence of schizophrenia among people with ID and concomitant genetic syndromes, we identified all people with at least one diagnosis of DiGeorge syndrome (D82.1), Edwards syndrome and Patau syndrome (Q91), Turner syndrome (Q96), Klinefelter syndrome (Q98.0, Q98.1, Q98.2, Q98.4), other sex chromosome abnormalities, female phenotype, not elsewhere classified (Q97) and other chromosome abnormalities, not elsewhere classified (Q99). Moreover, to evaluate schizophrenia among people with ID and concomitant ASD, we identified all people in the ID cohort with at least one diagnosis of pervasive developmental disorders (F84) (table 2).
Schizophrenia, schizotypal and delusional disorders
Through the Skåne Healthcare Register, we identified all diagnoses in the schizophrenia spectrum (schizophrenia, schizotypal and delusional disorders; F20-F29 in the ICD-10), as well as the single diagnoses schizophrenia (F20), schizotypal disorder (F21), persistent delusional disorders (F22), acute and transient psychotic disorders (F23), induced delusional disorder (F24), schizoaffective disorders (F25), other non-organic psychotic disorders (F28) and unspecified non-organic psychosis (F29). This was done for both the ID cohort and the gPop cohort.
Statistical analysis
Using the gPop cohort as a reference, we estimated the risk of the different diagnoses among the three groups of people with ID (ID cohort, ID diagnosis (severity and DS), and ID diagnosis with ASD or genetic syndromes). Analyses were performed for the whole group (ie, all ages) and stratified by age group. In the first case (whole group), all outcomes were assessed, that is, at least one diagnosis in the F20-F29 block and at least one diagnosis in the single diagnoses F20-F29. However, due to small group sizes, only the whole block (F20-F29) was considered in the stratified analyses. Comparisons were made using Poisson regression to estimate relative risks (RRs) with 95% CIs. All analyses were performed in IBM SPSS Statistics V29.0. P values <0.05 were considered statistically significant. To prevent the identification of individuals, results are presented only for groups comprising at least five people.
Results
The overall prevalence of diagnoses in the schizophrenia spectrum (F20-F29) was 7.2% in the ID cohort, which was more than an eightfold increase compared with the gPop (table 3). The prevalence decreased with the severity of ID. However, even among those with severe/profound ID, the risk increase compared with the gPop was more than sixfold. Concomitant ASD did not change the risk increase among people with ID compared with gPop. Although the increased risk, compared with gPop, was lowest among children in all analyses, except for severe/profound ID, it was still high, ranging from RR 6.10 and upwards.
Table 3. Number and percentages of diagnoses in the schizophrenia spectrum.
| ICD-10 diagnoses | gPop | ID cohort | ID versus gPop | |||
| N | % | N | % | RR | 95% CI | |
| ID (n=14 716) | ||||||
| Total | 10 455 | 0.9 | 1060 | 7.2 | 8.45 | 7.94 to9.00 |
| Children | 355 | 0.1 | 77 | 1.4 | 9.42 | 7.36 to 12.05 |
| Adults | 6899 | 1.0 | 880 | 10.6 | 11.13 | 10.38 to 11.94 |
| Older people | 3 201 | 1.2 | 103 | 13.4 | 10.79 | 8.87 to 13.13 |
| Mild ID (n=3952) | ||||||
| Total | 10 455 | 0.9 | 419 | 10.6 | 12.44 | 11.28 to13.72 |
| Children | 355 | 0.1 | 26 | 1.3 | 8.91 | 5.99 to 13.27 |
| Adults | 6899 | 1.0 | 370 | 20.1 | 21.10 | 19.00 to 23.42 |
| Older people | 3201 | 1.2 | 23 | 23.7 | 19.03 | 12.62 to 28.67 |
| Moderate ID (n=1086) | ||||||
| Total | 10 455 | 0.9 | 100 | 9.2 | 10.81 | 8.87 to13.16 |
| Children | 355 | 0.1 | 7 | 1.1 | 7.25 | 3.43 to 15.31 |
| Adults | 6899 | 1.0 | 83 | 21.2 | 22.32 | 17.97 to 27.71 |
| Older people | 3201 | 1.2 | 10 | 34.5 | 27.67 | 14.87 to 51.47 |
| Severe/Profound ID (n=613) | ||||||
| Total | 10 455 | 0.9 | 32 | 5.2 | 6.13 | 4.33 to8.67 |
| Children | 355 | 0.1 | 7 | 2.1 | 14.36 | 6.80 to 30.35 |
| Adults | 6899 | 1.0 | 20 | 8.1 | 8.55 | 5.51 to 13.26 |
| Older people | 3201 | 1.2 | 5 | 16.1 | 12.94 | 5.38 to 31.12 |
| Other/Unknown ID (n=3739) | ||||||
| Total | 10 455 | 0.9 | 233 | 6.2 | 7.31 | 6.42 to8.33 |
| Children | 355 | 0.1 | 5 | 0.9 | 6.10 | 2.52 to 14.75 |
| Adults | 6899 | 1.0 | 177 | 6.5 | 6.86 | 5.91 to 7.96 |
| Older people | 3201 | 1.2 | 51 | 11.1 | 8.88 | 6.73 to 11.71 |
| Down syndrome (n=848) | ||||||
| Total | 10 455 | 0.9 | 18 | 2.1 | 2.49 | 1.57 to3.96 |
| Children | 355 | 0.1 | <5 | – | – | – |
| Adults | 6899 | 1.0 | 16 | 3.1 | 3.25 | 1.99 to 5.31 |
| Older people | 3201 | 1.2 | <5 | – | – | – |
| ID with ASD (n=5010) | ||||||
| Total | 10 455 | 0.9 | 336 | 6.7 | 7.87 | 7.06 to8.77 |
| Children | 355 | 0.1 | 51 | 1.7 | 11.82 | 8.82 to 15.86 |
| Adults | 6899 | 1.0 | 277 | 13.8 | 14.55 | 12.91 to 16.41 |
| Older people | 3201 | 1.2 | 8 | 22.9 | 18.34 | 9.16 to 36.71 |
| ID with genetic syndrome (n=319) | ||||||
| Total | 10 455 | 0.9 | 13 | 4.1 | 4.78 | 2.78 to8.24 |
| Children | 355 | 0.1 | <5 | – | – | – |
| Adults | 6899 | 1.0 | 12 | 9.7 | 10.43 | 5.92 to 18.37 |
| Older people | 3201 | 1.2 | <5 | – | – | – |
Number and percentage with diagnosis of schizophrenia, schizotypal and delusional disorders in a cohort and subcohorts of people with (ID) and a referent cohort from the general population (gPop), with (RRs) and 95% (CIs) for comparisons with the gPop.
ASDautism spectrum disordergPopgeneral populationIDintellectual disabilityRRsrelative risks
Looking at individual diagnoses within the schizophrenia spectrum, the highest prevalence in the ID cohort was found for unspecified non-organic psychosis (F29; 3.9%) and schizophrenia (F20; 3.1%). The highest increase in risk compared with the gPop was found for unspecified non-organic psychosis (F29; RR 11.31), induced delusional disorder (F24; RR 10.88) and schizotypal disorder (F21; RR 10.58). The prevalence and risk patterns were similar for all severities of ID (table 4).
Table 4. Diagnoses in the schizophrenia spectrum.
| ICD-10 diagnoses | gPop | ID cohort | ID versus gPop | |||
| N | % | N | % | RR | 95% CI | |
| Mild ID (n=3952) | ||||||
| F20 schizophrenia | 3909 | 0.3 | 187 | 4.7 | 14.85 | 12.83 to 17.20 |
| F21 schizotypal disorder | 449 | 0.0 | 23 | 0.6 | 15.90 | 10.46 to 24.18 |
| F22 persistent delusional disorders | 2657 | 0.2 | 74 | 1.9 | 8.65 | 6.86 to 10.89 |
| F23 acute and transient psychotic disorders | 2707 | 0.2 | 85 | 2.2 | 9.75 | 7.86 to 12.10 |
| F24 induced delusional disorder | 46 | 0.0 | <5 | - | - | - |
| F25 schizoaffective disorders | 1159 | 0.1 | 40 | 1.0 | 10.71 | 7.82 to 14.69 |
| F28 other non-organic psychotic disorders | 131 | 0.0 | 6 | 0.2 | 14.22 | 6.27 to 32.23 |
| F29 unspecified non-organic psychosis | 4239 | 0.3 | 228 | 5.8 | 16.70 | 14.62 to 19.08 |
| Moderate ID (n=1086) | ||||||
| F20 schizophrenia | 3909 | 0.3 | 45 | 4.1 | 13.01 | 9.69 to 17.45 |
| F21 schizotypal disorder | 449 | 0.0 | <5 | - | - | - |
| F22 persistent delusional disorders | 2657 | 0.2 | 13 | 1.2 | 5.53 | 3.21 to 9.53 |
| F23 acute and transient psychotic disorders | 2707 | 0.2 | 16 | 1.5 | 6.68 | 4.09 to 10.92 |
| F24 induced delusional disorder | 46 | 0.0 | <5 | - | - | - |
| F25 schizoaffective disorders | 1159 | 0.1 | 9 | 0.8 | 8.77 | 4.55 to 16.90 |
| F28 other non-organic psychotic disorders | 131 | 0.0 | <5 | - | - | - |
| F29 unspecified non-organic psychosis | 4239 | 0.3 | 55 | 5.1 | 14.66 | 11.24 to 19.13 |
| Severe/Profound ID (n=613) | ||||||
| F20 schizophrenia | 3909 | 0.3 | 8 | 1.3 | 4.10 | 2.05 to 8.20 |
| F21 schizotypal disorder | 449 | 0.0 | <5 | - | - | - |
| F22 persistent delusional disorders | 2657 | 0.2 | 6 | 1.0 | 4.52 | 2.03 to 10.07 |
| F23 acute and transient psychotic disorders | 2707 | 0.2 | <5 | - | - | - |
| F24 induced delusional disorder | 46 | 0.0 | <5 | - | - | - |
| F25 schizoaffective disorders | 1159 | 0.1 | <5 | - | - | - |
| F28 other non-organic psychotic disorders | 131 | 0.0 | <5 | - | - | - |
| F29 unspecified non-organic psychosis | 4239 | 0.3 | 19 | 3.1 | 8.97 | 5.72 to 14.08 |
| Other/Unknown ID (n=3739) | ||||||
| F20 schizophrenia | 3909 | 0.3 | 100 | 2.7 | 8.39 | 6.88 to 10.24 |
| F21 schizotypal disorder | 449 | 0.0 | 12 | 0.3 | 8.77 | 4.94 to 15.56 |
| F22 persistent delusional disorders | 2657 | 0.2 | 48 | 1.3 | 5.93 | 4.46 to 7.89 |
| F23 acute and transient psychotic disorders | 2707 | 0.2 | 30 | 0.8 | 3.64 | 2.54 to 5.21 |
| F24 induced delusional disorder | 46 | 0.0 | <5 | - | - | - |
| F25 schizoaffective disorders | 1159 | 0.1 | 19 | 0.5 | 5.38 | 3.42 to 8.46 |
| F28 other non-organic psychotic disorders | 131 | 0.0 | <5 | - | - | - |
| F29 unspecified non-organic psychosis | 4239 | 0.3 | 127 | 3.4 | 9.83 | 8.24 to 11.73 |
| Down syndrome (n=848) | ||||||
| F20 schizophrenia | 3909 | 0.3 | <5 | - | - | - |
| F21 schizotypal disorder | 449 | 0.0 | <5 | - | - | - |
| F22 persistent delusional disorders | 2657 | 0.2 | <5 | - | - | - |
| F23 acute and transient psychotic disorders | 2707 | 0.2 | 6 | 0.7 | 3.21 | 1.44 to 7.14 |
| F24 induced delusional disorder | 46 | 0.0 | <5 | - | - | - |
| F25 schizoaffective disorders | 1159 | 0.1 | <5 | - | - | - |
| F28 other non-organic psychotic disorders | 131 | 0.0 | <5 | - | - | - |
| F29 unspecified non-organic psychosis | 4239 | 0.3 | 11 | 1.3 | 3.75 | 2.08 to 6.78 |
Number and percentage with diagnosis in a cohort of people withIDwith ID) and a referent cohort from the general population (gPop), with (RRs) and 95% (CIs) for comparisons with the gPop.
gPopgeneral populationICD-1010th revision of the International Classification of DiseasesIDintellectual disabilityRRsrelative risks
Discussion
Main findings
The main findings are the high prevalence of diagnoses in the schizophrenia spectrum (ie, F20-F29 diagnoses) among people with ID compared with previous research and the greatly increased risk of diagnoses among people with ID compared with the gPop. In the present study, covering all inhabitants in the south of Sweden, >7% of people in the ID cohort were given such a diagnosis during the 8-year study period. To the best of our knowledge, this is the first population-based study investigating the prevalence of diagnoses in the schizophrenia spectrum in people with ID since the study from the Greater Glasgow Area was published about 15 years ago.3 In the present study, the prevalence was about twice as high, which may be explained by the increased knowledge about the phenomenology of psychotic symptoms in persons with ID.1 8 Another explanation could be the heterogeneity in the cohort characteristics, where, for example, the proportion of people with severe or profound ID and the proportion with DS differed between the two studies. The sample size of the present study, including the entire population in southern Sweden, is a great strength compared with the limited sample size (n=1023) in the study by Cooper et al.3
Traditionally, it has been assumed that schizophrenia cannot occur in people with an IQ lower than approximately 50, as they cannot report on core features of the disorder.23 This is partly supported by the decreasing prevalence of schizophrenia with increasing severity of ID found in the present study. However, even among those with severe/profound ID in the present study, the risk increase relative to the gPop was more than sixfold.
Most studies regarding schizophrenia and other psychotic disorders in intellectually disabled people are restricted to patients with mild ID as opposed to evaluating all levels of ID.1 However, more attention is currently drawn to the phenomenon that people with a vulnerable brain, especially those with more severe ID, as well as people with ASD, dementia and other neurological disorders, may develop psychotic disorders more often than the gPop.24 25 Hence, schizophrenia should be considered when those with more severe ID show a rapid decline in adaptive skills. This means that it is possible to diagnose schizophrenia in people with severe and profound ID and emphasises the need for these people to be included in studies on ID and schizophrenia.
People with mild and moderate ID are able to report core features of psychosis, especially when they are in remission from acute phases.26 As people with more severe ID may not be able to report core symptoms like delusions and hallucinations,1 there may be a need for the use of behavioural equivalents to conventional symptoms for these patients.1 8
Stratifying by age group, the most interesting finding was that among children, the risk of having a diagnosis in the schizophrenia spectrum was about 10 times higher among those with ID than in the gPop. This is very important, as knowledge about the onset of disorders in this spectrum in people with ID is sparse, despite assumptions about early onset or very early onset—often referred to as childhood onset of schizophrenia—in people with ID.27 This finding is supported by a 2020 French study.27
The prevalence of diagnoses in the schizophrenia spectrum among those with concomitant ID and autism was about the same as in the entire ID cohort (about 7%). To the best of our knowledge, no previous population studies have assessed the prevalence of diagnoses in the schizophrenia spectrum in patients with both ID and ASD, which makes the present study unique. Even though we did not find any indication of an increased risk for those with concomitant ID and ASD, it is important to be aware of the risk of misinterpretation of symptoms in this group.8 There is a considerable risk of misinterpreting ASD features as schizophrenia or vice versa, especially as delusions may be taken for autistic idiosyncrasies or the other way around, and negative symptoms may be taken as ASD symptoms.8
Of all 14 716 people in the ID cohort, 320, about 2%, had a genetic syndrome (excluding DS, which was included in the definition of ID). Of these, 13 also had a schizophrenia diagnosis, which corresponds to a prevalence of 4% and an almost fivefold increase compared with the gPop. Since DiGeorge syndrome is particularly known to be associated with a high risk of developing schizophrenia in the gPop,12 this is a somewhat surprising finding. In people with DS, the prevalence of schizophrenia was lower than in the entire ID cohort—approximately 2% of those with ID—and hence twice the proportion as in the gPop. The finding is not in line with the study by Dykens et al,16 who found an increased risk of schizophrenia in DS compared with other ID groups, including other genetic syndromes, birth trauma, neural injuries or unknown causes (accounting for 78% of the sample). A possible explanation for this is the difference in study designs, as Dykens et al used a clinical mental health sample, whereas we analysed population-based data.
The major strength of the present study is the use of register data to establish the study cohorts and collect information on schizophrenia diagnoses. The inclusion of all public healthcare in healthcare registers is regulated by law in Sweden, and individuals do not have the right to decline registration of their data. Thus, the use of register data ensures a large study population with no selection bias.
Limitations
We used two ways to identify people with ID: by diagnosis and service/support for those with ID/ASD. Although it is fair to assume that a person without ID would not get a diagnosis of ID, that is, those identified as having ID based on diagnosis are likely actually to have ID, we cannot rule out that people with ID did not get an ID diagnosis during the 8-year study period. This could, for example, occur among older people, where the original diagnosis was made several years ago, and the person with ID did not have a healthcare contact during the 8-year study period. Thus, by using diagnoses of ID, we may have misclassified some people with ID as not having ID. Therefore, we also used service/support for people with ID/ASD as an inclusion criterion. However, even though this may have captured more people with ID, it also carried the risk of including people with ASD but not ID; it is difficult to estimate the misclassification rate due to this. Still, among the 9390 people with an F7 diagnosis, the prevalence of diagnosis in the schizophrenia spectrum was 8.3%, which is only slightly higher than the 7.2% found in the ID cohort. Thus, the possible inclusion of people with ASD but not ID in the ID cohort should have affected the results only marginally.
Another weakness may be that we used recorded diagnoses during a set time window as a proxy for schizophrenia. Diagnosis does not equal disease. A person may have a disorder without ever getting a diagnosis. This may occur if the person does not contact health services about their symptoms or if the physician fails to recognise the disorder in question. In people with ID, especially those with multiple complex needs, diagnostic assessment is challenging, and setting the wrong diagnosis is assumed to occur often.1 8 Additionally, the diagnostic window in the present study was limited to 2014–2021. Thus, persons with schizophrenia may have been diagnosed outside the time window, but the diagnosis did not appear in the current data. Both these scenarios would result in an underestimation of the prevalence of schizophrenia (disorder, not diagnosis) in the present study. The prevalence of the schizophrenia spectrum in the gPop is similar in the present study (0.9%) compared with lifetime diagnoses estimations in populations in the Western world (0.5%).8 Thus, it seems that the use of diagnosis as a proxy for the prevalence of the disorder does not seem to pose any major threat to internal validity and that the increased risk found for the ID cohort cannot be explained by a low prevalence in the gPop. In the ID cohort, misdiagnoses could also have occurred due to cognitive impairments and communication difficulties. The validity of the diagnostic process may be weaker compared with that of cognitively able people. This may especially be a concern when differentiating the diagnoses in the schizophrenia spectrum from autism, obsessive-compulsive disorder, anxiety or even physical pain.8
Implications
The main clinical implications of the present findings are twofold. First, the significantly increased risk of developing disorders of the schizophrenia spectrum should encourage clinicians in general mental health services, habilitation services and specialised mental health services for people with ID to look for disorders in the schizophrenia spectrum when a decline in global functioning and behavioral problems are observed. Second, the present findings support previous assumptions that early onset, or even very early onset of schizophrenia spectrum disorders occurs more often in individuals with ID compared with the gPop. Hence, schizophrenia should also be considered in adolescents and children with ID when they show severely disturbed behaviour and severe decline in adaptive functioning.
Future research should include investigations of early signs of psychosis in people with ID, which is an understudied topic today. Also, more research is needed to confirm the age of schizophrenia onset in people with ID, as early interventions may positively affect the prognosis. Moreover, as prognosis is generally still an understudied area regarding severe mental illness in people with ID, future research should pay attention to this. A recently published article found that treatment and interventions of schizophrenia in persons with ID and autism found that the patients profited from treatment and interventions used in the gPop when adapted to the level of ID and personal communication style.28
Biography
Trine Lise Bakken is a registered nurse who received her Master's degree of Health Science in 2000 and her PhD in 2010 from the University of Oslo, Faculty of Medicine in Oslo, Norway. She has been working in the Department of Mental Health and Addiction, the Unit for Mental Health in Intellectual Disability (ID) and the National Advisory Unit for Mental Health in ID at Oslo University Hospital since 1995. She is a clinical nurse specialist and the head of research and development for the National Advisory Unit for Mental Health in ID. She is also an associate professor at Oslo Metropolitan University. Her research activities include schizophrenia in ID/autism, and she recently published two papers. She is currently leading two national studies: (1) when young adults with multiple, complex needs, move from their family to group homes and (2) a feasibility study on the new Norwegian SEED-2 version (assessment of emotional development in persons with ID). In addition, she provides counselling to both clinicians and research fellows.

Footnotes
Funding: This study was funded by FORTE (2019-00105 and 2021-01862).
Patient consent for publication: Not applicable.
Ethics approval: Ethical approval was granted by the Swedish Ethical Review Authority (DNR 2021-01444 with amendments 2021-04910 and 2021-06056). The data in this study are collected from one national and one regional register. The national LSS register is maintained by the Swedish National Board and Welfare. It comprises data on all support provided to people with intellectual disabilities and/or autism spectrum disorder, according to the Act Concerning Support and Service for Persons with Certain Functional Impairments.
Provenance and peer review: Not commissioned; internally peer reviewed.
Contributor Information
Trine Lise Bakken, Email: uxtlba@ous-hf.no.
Magnus Sandberg, Email: magnus.sandberg@med.lu.se.
Anna Axmon, Email: anna.axmon@med.lu.se.
Data availability statement
Data are available on reasonable request.
References
- 1.Bakken TL, Kildahl AN, Ludvigsen LB, et al. Schizophrenia in autistic people with intellectual disabilities: symptom manifestations and identification. J Appl Res Intellect Disabil. 2023;36:1076–91. doi: 10.1111/jar.13127. [DOI] [PubMed] [Google Scholar]
- 2.Morgan VA, Leonard H, Bourke J, et al. Intellectual disability co-occurring with schizophrenia and other psychiatric illness: population-based study. Br J Psychiatry. 2008;193:364–72. doi: 10.1192/bjp.bp.107.044461. [DOI] [PubMed] [Google Scholar]
- 3.Cooper S-A, Smiley E, Morrison J, et al. Mental ill-health in adults with intellectual disabilities: prevalence and associated factors. Br J Psychiatry. 2007;190:27–35. doi: 10.1192/bjp.bp.106.022483. [DOI] [PubMed] [Google Scholar]
- 4.Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990─2013: a systematic literature review. BMC Psychiatry. 2015;15:193. doi: 10.1186/s12888-015-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaebel W, Kerst A, Stricker J. Classification and diagnosis of schizophrenia or other primary psychotic disorders: changes from ICD-10 to ICD-11 and implementation in clinical practice. Psychiatr Danub. 2020;32:320–4. doi: 10.24869/psyd.2020.320. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . Geneva: World Health Organization; 2018. The ICD-11 classification of mental and behavioral disorders. Clinical descriptions and diagnostic guidelines. [Google Scholar]
- 7.Jopp DA, Keys CB. Diagnostic overshadowing reviewed and reconsidered. Am J Ment Retard . 2001;106:416–33. doi: 10.1352/0895-8017(2001)106<0416:DORAR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Bakken TL. Behavioural equivalents of schizophrenia in people with intellectual disability and autism spectrum disorder. A selective review. Int J Dev Disabil. 2021;67:310–7. doi: 10.1080/20473869.2021.1925402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019;140:340–8. doi: 10.1111/acps.13076. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Perera G, Shetty H, et al. Body mass index and mortality in patients with schizophrenia spectrum disorders: a cohort study in a South London catchment area. Gen Psychiatr. 2022;35:e100819. doi: 10.1136/gpsych-2022-100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glover G, Williams R, Heslop P, et al. Mortality in people with intellectual disabilities in England. J Intellect Disabil Res. 2017;61:62–74. doi: 10.1111/jir.12314. [DOI] [PubMed] [Google Scholar]
- 12.Patel H, Vadukapuram R, Mansuri Z, et al. Psychiatric comorbidities in adults with DiGeorge syndrome. Clin Psychopharmacol Neurosci. 2022;20:498–503. doi: 10.9758/cpn.2022.20.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cederlöf M, Ohlsson Gotby A, Larsson H. Klinefelter syndrome and risk of psychosis, autism and ADHD. J Psychiatr Res. 2014;48:128–30. doi: 10.1016/j.jpsychires.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Prior TI, Chue PS, Tibbo P. Investigation of Turner syndrome in schizophrenia. Am J Med Genet. 2000;96:373–8. doi: 10.1002/1096-8628(20000612)96:3<373::aid-ajmg26>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Nazareth T, Li N, Marynchenko M, et al. Burden of illness among patients with fragile X syndrome (FXS): a Medicaid perspective. Curr Med Res Opin. 2016;32:405–16. doi: 10.1185/03007995.2015.1119678. [DOI] [PubMed] [Google Scholar]
- 16.Dykens EM, Shah B, Davis B, et al. Psychiatric disorders in adolescents and young adults with Down syndrome and other intellectual disabilities. J Neurodev Disord. 2015;7:1–8. doi: 10.1186/s11689-015-9101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baxter AJ, Brugha TS, Erskine HE, et al. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45:601–13. doi: 10.1017/S003329171400172X. [DOI] [PubMed] [Google Scholar]
- 18.Tonnsen BL, Boan AD, Bradley CC, et al. Prevalence of autism spectrum disorders among children with intellectual disability. Am J Intellect Dev Disabil. 2016;121:487–500. doi: 10.1352/1944-7558-121.6.487. [DOI] [PubMed] [Google Scholar]
- 19.Ayano G. Bipolar disorder: a concise overview of etiology, epidemiology diagnosis and management: review of literatures. SOJP. 2016;3:1–8. doi: 10.15226/2374-6874/3/2/00131. [DOI] [Google Scholar]
- 20.Sandberg M, Kristensson J, Axmon A. IDcare - a longitudinal register study of pre-pandemic and pandemic health care utilization and diagnostic profiles among people with intellectual disabilities in southern Sweden. Eur J Epidemiol. 2024:1063–71. doi: 10.1007/s10654-024-01151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SFS; 1993. Act concerning support and service for persons with certain functional impairments (LSS) [In Swedish: Lag om stöd och service till vissa funktionshindrade (LSS)] p. 387. [Google Scholar]
- 22.Löfvendahl S, Schelin MEC, Jöud A. The value of the Skåne Health-care Register: prospectively collected individual-level data for population-based studies. Scand J Public Health. 2020;48:56–63. doi: 10.1177/1403494819868042. [DOI] [PubMed] [Google Scholar]
- 23.Lund J. The prevalence of psychiatric morbidity in mentally retarded adults. Acta Psychiatr Scand. 1985;72:563–70. doi: 10.1111/j.1600-0447.1985.tb02655.x. [DOI] [PubMed] [Google Scholar]
- 24.Mulugeta A, Suppiah V, Hyppönen E. Schizophrenia and co-morbidity risk: evidence from a data driven phenomewide association study. J Psychiatr Res. 2023;162:1–10. doi: 10.1016/j.jpsychires.2023.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Gordon PC, Kauark RBG, Costa CDM, et al. Clinical implications of the national institute of neurological disorders and stroke criteria for diagnosing psychosis in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2016;28:26–31. doi: 10.1176/appi.neuropsych.15050119. [DOI] [PubMed] [Google Scholar]
- 26.Engebretsen MH, Kildahl AN, Hoy IH, et al. Metyrosine treatment in a woman with chromosome 22q11.2 deletion syndrome and psychosis: a case study. Int J Dev Disabil. 2019;65:116–21. doi: 10.1080/20473869.2017.1401257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coulon N, Godin O, Bulzacka E, et al. Early and very early-onset schizophrenia compared with adult-onset schizophrenia: French FACE-SZ database. Brain Behav. 2020;10:e01495. doi: 10.1002/brb3.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakken TL, Askeland Hellerud JM, Kildahl AN, et al. Schizophrenia in autistic people with intellectual disabilities. Treatment and interventions. J Autism Dev Disord. 2024:1–11. doi: 10.1007/s10803-024-06286-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.