Abstract
Introduction
Tracheomalacia (TM) often occurs in children with oesophageal atresia (OA), leading to recurrent respiratory symptoms and in severe cases to blue spells or ultimately respiratory arrest. In some patients, a secondary posterior tracheopexy may then be indicated. This secondary surgery, as well as respiratory morbidity, may be prevented by performing a primary posterior tracheopexy (PPT) concurrent with primary OA correction. The aim of this trial is to determine if a PPT can decrease—or prevent—tracheal collapse in newborns with OA and TM. Additionally, the trial aims to determine whether the potential observed effect of PPT on tracheal stability is sustained over time.
Methods and analysis
This is an international multicentre double-blind randomised controlled trial. Seventy-eight children with OA type C will be randomised 1:1 into the no-PPT group or PPT group. Randomisation will be stratified by centre. The degree and location of TM are assessed during preoperative, intraoperative and two postoperative tracheobronchoscopies. The occurrence of TM will be evaluated during three routine follow-up consultations until the age of 6 months. The primary outcome is the degree of collapse of the tracheal wall during the intraoperative tracheobronchoscopy (after performing the PPT/no-PPT), measured in percentages. The difference in the mean degree of collapse will be compared between the no-PPT and the PPT groups using linear regression, adjusting for centre and the preoperative degree of tracheal collapse at baseline. The adjusted mean difference will be reported as effect size together with its 95% CI.
Ethics and dissemination
Patients will be included after written parental informed consent. The risks and burden associated with the trial are minimal. The institutional review board of the University Medical Center Utrecht has approved this protocol (METC-number 23-256/A). Results will be shared in a peer-reviewed scientific journal and presented at international conferences.
Trial registration number
Keywords: Paediatric thoracic surgery, Paediatric otolaryngology, Randomized Controlled Trial
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is the first randomised controlled trial investigating the efficacy of primary posterior tracheopexy (PPT), with the assessment of the degree of tracheomalacia (TM) at multiple time points, providing new insights into the development of TM over time and the possible influence of the primary oesophageal atresia (OA) correction on TM.
A strength of this trial is the double-blind study design, aiming to obtain unbiased results.
This trial is an international collaboration between European expert centres, thereby creating a strong network focused on optimisation of care for OA patients.
Despite the fact that the European multicentre approach is a strength of this study, this approach simultaneously poses challenges with regard to study coordination, patient recruitment and local legislation.
Although evaluating the difference in clinical symptoms between the no-PPT group and PPT group is essential, this was not chosen as a primary outcome due to the difficulty of objectively documenting these symptoms in newborns.
Introduction
Oesophageal atresia (OA) is a rare congenital anomaly involving interrupted oesophageal development, resulting in a blind-ended oesophagus, often accompanied by a tracheo-oesophageal fistula (TOF). Newborns with this congenital anomaly are unable to swallow feedings and require immediate hospitalisation for parenteral feeding and surgical correction within the first week of life.1 2
Tracheomalacia (TM), characterised by a collapse of the trachea, is seen in 31%–87% of the patients with OA during tracheobronchoscopy (TBS).3,7 TM can cause respiratory problems, ranging from chronic cough, wheezing, recurrent respiratory tract infections (RTIs) to brief resolved unexplained events (BRUEs) and, ultimately, respiratory arrest.8,10 Severe symptoms can occur in 9%–32% of OA patients.6 11 12
The cause of TM in OA patients is most likely multifactorial. Due to the presence of TOF, patients frequently have a wider posterior membrane leading to instability and collapse. Additionally, the tracheal rings in these patients are often U-shaped instead of the regular C-shape, resulting in a flatter trachea and increased collapsibility.5 Furthermore, following the surgical correction of OA and closure of the TOF, TM may be exacerbated. This is believed to occur due to the dissection of surrounding tissues during the surgical procedure. It is hypothesised that these tissues, which enclose the oesophagus and trachea, acted as a natural stent prior to surgical correction, helping maintain airway patency before surgical OA correction.313,15
In some patients, surgical treatment of TM may be necessary.1,416 Surgical treatment options include anterior or posterolateral aortopexy and/or anterior or posterior tracheopexy.17 18 If the primary cause of the malacia is thought to be a flaccid posterior wall of the trachea, then a (secondary) posterior tracheopexy (PT) may be most indicated.19,22 If the primary cause is anterior TM, an aortopexy may be the preferred treatment approach.22 However, most patients suffer from a combined anterior (ie, U-shaped tracheal rings, anterior compression) and posterior (ie, flaccid posterior membrane) TM. Neither aortopexy nor tracheopexy has been proven superior in the treatment of these combined TM patients.18 22 A posterior tracheopexy involves suturing the posterior membrane to the anterior spinal ligament, thereby preventing intrusion of the posterior membrane into the airway and thus keeping the airway open.14 19 20 In a previous study on secondary PT, tracheal collapse decreased from 40% before tracheopexy to 15%–20% after tracheopexy.23 24 A study by Shieh et al showed a tracheal collapse that decreased from 80% to 20% after secondary PT.21 However, performing this secondary PT after OA correction entails a second major operation in the newborn, associated with increased morbidity, as the procedure requires operating in the same surgical field as the original OA repair with extensive adhesions.
Several experienced medical centres have incorporated the PT during the initial OA repair to avoid the need for a secondary major surgical procedure.21 24 25 Previous studies have shown that this primary posterior tracheopexy (PPT) is safe and feasible.21 25 A recent prospective study showed a significant decrease in RTIs in a group of OA patients after the introduction of the PPT, compared with a historical OA control group before the introduction of PPT.23 However, these results may be biased since patients were not randomised, were treated in different periods, and treating physicians and parents were not blinded.
We aim to address this knowledge gap by performing a double-blind randomised controlled trial comparing PPT and no-PPT. The routine evaluation of the tracheal diameter after OA correction via TBS has thus far not been used in clinical studies. This method objectively assesses the extent of TM.26 Symptoms of TM are frequently non-specific, posing challenges in quantifying parameters such as decreased pulmonary function in these patients. Hence, an objective evaluation, such as TBS, is warranted. With this trial, we aim to determine whether PPT is superior to the wait-and-see policy currently and historically employed in many centres. Furthermore, we aim to determine whether the effect of the PPT is sustained and whether the TM deteriorates beyond the first 2 months.
Methods and analysis
The PORTRAIT trial is an international, multicentre, double-blind, randomised controlled trial with a 1:1 allocation of patients born with OA and a collapse of the trachea to either (a) no-PPT group or (b) PPT group where tracheopexy is performed concurrently with the standard OA correction. This study will include 78 patients, each undergoing a 6-month follow-up period, irrespective of their randomisation arm. The participating centres are all tertiary medical centres in Europe with expertise in paediatric surgery. The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) reporting guidelines have been used.27
Participants
The patients eligible for participation in this trial are born with OA and a distal TOF. Written parental informed consent will be obtained before surgical OA repair by the local principal investigator (PI) or another member of the local research team. The severity of TM, as well as all other airway anomalies such as laryngeal clefts and vascular rings, will be routinely assessed during the preoperative TBS prior to the OA correction. When primary TM is seen during this TBS, the patient will be included and randomised in either the no-PPT group or PPT group. When no primary TM is seen during the preoperative TBS, the patient is excluded from the study prior to randomisation, and routine treatment is carried out. Exclusion criteria are patients without a distal TOF, premature neonates born <34 weeks, the use of an endotracheal tube size <3.0, patients with Cormack-Lehane score 3 or 4,28 patients with cyanotic congenital heart disease, and a lack of parental consent.
Sample size calculation
In a previous pilot study, the average collapse of the trachea was approximately 32% before tracheopexy,23 with an SD of 14%. Postoperative evaluation of tracheal patency using TBS was not performed; only symptoms were assessed. In a retrospective study by Shieh et al,21 postoperative tracheal evaluation using TBS showed a decreased tracheal collapse of approximately 22% after PPT. However, this study involved a selected cohort of patients, predominantly featuring a high prevalence of long-gap OA, who underwent surgical OA correction between 1 and 4 months of age.21
Based on these studies, the SD of the outcome measure was assumed to be 14% and it was decided that an absolute difference of 10% in mean intraoperative degree of tracheal collapse could be considered a clinically relevant difference. To obtain an 80% power with a two-sided significance level of 5%, 31 patients are needed in each study group.
To account for 20% dropouts, the total number of patients planned for inclusion is set at 78 patients (39 per group). Dropouts can occur due to the inability to obtain the desired data during the intraoperative TBS (primary outcome measure), due to clinical deterioration, or if parents/caretakers withdraw consent for participation in the trial.
Recruitment
Patients will be recruited from four expert paediatric surgery hospitals in Europe, all tertiary medical centres (Great Ormond Street Hospital (GOSH), London, UK; Karolinska University Hospital (KUH), Stockholm, Sweden; Erasmus University Medical Center Sophia Children’s Hospital (EMC), Rotterdam, the Netherlands; University Medical Center Utrecht Wilhelmina Children’s Hospital (UMCU), Utrecht, the Netherlands). The collaboration between these hospitals should make patient recruitment feasible despite the rarity of the disease. Moreover, including several centres throughout Europe will decrease the bias of including patients in a single centre or country.
Randomisation, blinding and treatment allocation
Randomisation will be performed using a permuted blocked randomisation list stratified by participating centre. The allocation ratio will be 1:1 for the no-PPT and PPT group. The randomisation sequence will be generated using the Castor EDC database system (www.castoredc.com), automatically randomising study participants after inclusion and ensuring concealed allocation for future randomisation blocks. Randomisation is stratified by participating centre to reduce the impact of differences between centre-specific protocols. Randomisation will be carried out by the paediatric surgeon performing the OA correction.
All participants, parents/caregivers, investigators and treating healthcare personnel, except for the surgical team performing the OA correction, will be blinded to which study arm the participant is assigned. None of the data gathered are affected by the unblinded surgical team since the primary and key secondary outcome measures are evaluated based on pseudonymised video footage. This video footage is assessed by otolaryngologists who are blinded to the patient and study arm. Moreover, follow-up and documentation of the secondary endpoints are typically performed by clinicians who are not involved in the surgical OA repair and/or TBSs of the child.
Whether or not a PPT has been performed will not be documented in the surgery report to prevent accidental unblinding. The surgery report will describe that the patient participates in the PORTRAIT trial and that randomisation for no-PPT or PPT has occurred.
This blinding process ensures that knowledge of the intervention does not influence the parents’/caregivers’ and treating physician’s behaviour and helps minimise potential biases during the trial.
Unblinding will take place after all patients have been enrolled, their follow-up is completed, and the trial has concluded. The only exception occurs when it is clinically necessary for the patient’s treatment to know whether PPT has taken place or not. In that case, the treating healthcare personnel and the parents/caretakers will be informed of the study arm allocation. In case of unblinding, the patient will not be excluded from the trial, as the primary outcome, the degree of TM during the intraoperative TBS, is recorded before any unblinding may occur.
Study intervention
For the patients randomised to the PPT group, the treatment is as follows: after ligation of the TOF, one to three non-absorbable sutures are placed through the posterior tracheal membrane and the anterior longitudinal spinal ligament. The sutures fixate the posterior membrane to this spinal ligament, thereby pulling the trachea open.25 The sutures are placed through the posterior tracheal membrane but do not penetrate the lumen of the trachea. Subsequently, an intraoperative TBS is performed through the tube to assess tracheal collapse. Patients are mechanically ventilated during this TBS due to the administration of muscle relaxants. Performing an intraoperative TBS is routine patient care in all PPT patients and no-PPT patients with ventilatory problems. In the other no-PPT patients, this TBS is a study procedure. Subsequently, the anastomosis of the oesophagus is performed as in routine patient care.25
A flexible TBS is performed on the paediatric or neonatal intensive care unit (ICU) through the ventilation tube during extubation to assess the direct postoperative effect of the PPT and TM. This is a study procedure for all participating patients. To assess if the effect of the PPT is sustained after several months, a second postoperative TBS under general anaesthesia and with spontaneous breathing is performed at 2–6 months of age by the paediatric otolaryngologist in the presence of the anaesthesiology team. In two participating centres, GOSH and KUH, this TBS is routinely performed in all patients with TM. In the UMCU and EMC, TBS is conducted as routine patient care in approximately half of patients based on clinical indications, such as RTIs or respiratory events.
Patient timeline
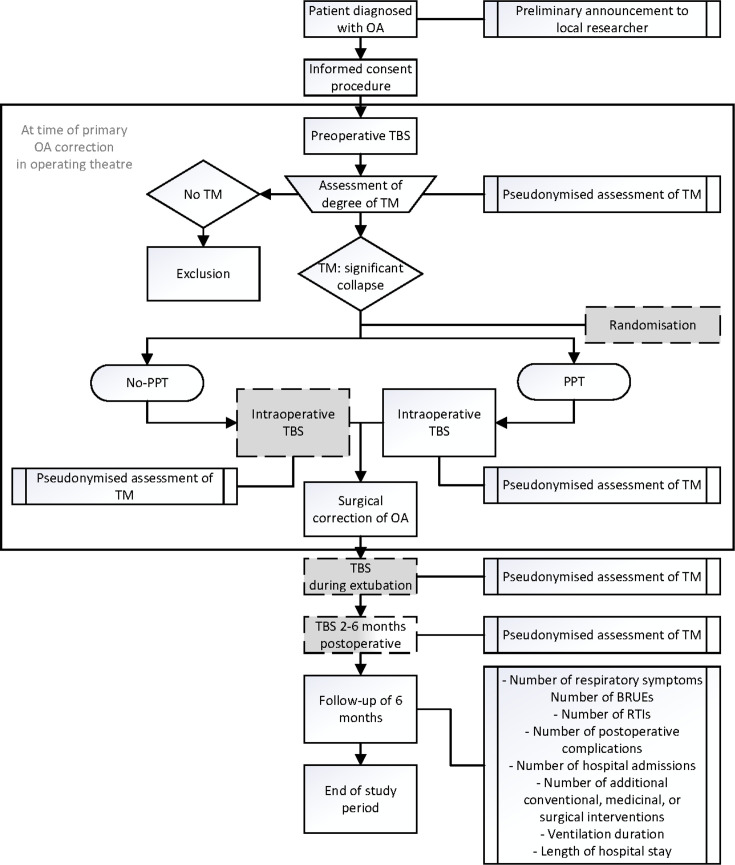
The study timeline is shown in figure 1. All parents of OA patients with a distal TOF will be asked for informed consent to the study during the preoperative consultation with the paediatric surgeon. A routine preoperative flexible and rigid TBS are performed during the induction of the OA correction under general anaesthesia, with spontaneous breathing and without muscle relaxants to assess the location of the TOF and degree of TM in all patients. The patient’s eligibility for inclusion will be determined based on the presence of a significant tracheal collapse (TM), as seen during the preoperative TBS. Subsequently, randomisation will take place for the patients with TM. After the PPT is carried out or not, and the TOF is transected, an intraoperative TBS through the ventilation tube is performed under muscle relaxants and mechanical ventilation. Surgical correction of the OA is performed according to routine patient care and according to the study arm (i.e. with or without the PPT).
Figure 1. Flow chart of study design. White: routine care; grey: study procedures; white/grey: routine or study procedure, depending on the participating centre. BRUEs, brief resolved unexplained events; OA, oesophageal atresia; PPT, primary posterior tracheopexy; RTIs, respiratory tract infections; TBS, tracheobronchoscopy; TM, tracheomalacia.
The participating patients will undergo two postoperative TBSs:
During extubation in the ICU, a flexible TBS is performed through the ventilation tube without muscle relaxants and with a spontaneous breathing trigger.
At 2–6 months of age, a TBS is performed under general anaesthesia, with spontaneous breathing and without muscle relaxants.
Once all patients have been included, all pseudonymised video footage will be blindly reviewed and assessed twice by the same rater and by two different raters (all paediatric otolaryngologists), resulting in four assessments per TBS. The average degree of TM of these assessments will be used for analysis. The collapse site will be assessed during the video assessment, that is, the upper, middle or lower third of the trachea, the posterior or anterior wall, and the collapse degree. The PPT-sutures are placed through the posterior tracheal membrane but do not penetrate the lumen of the trachea. The physicians blindly assessing the footage of the intraoperative and postoperative TBS will, therefore, not be able to see if a PPT was performed.
During routine follow-up consultations at 2–4 weeks, 3 months and 6 months, data will be obtained regarding respiratory symptoms, the number of RTIs and BRUEs, the number of postoperative complications, the number of hospital admissions, and the number of additional conventional, medicinal or surgical interventions, the duration of ventilatory or respiratory support, and the length of ICU and hospital stay. Parameters will also be extracted from the electronic patient records.
Outcome parameters
The primary outcome parameter is the difference in the mean degree of collapse of the tracheal wall between the no-PPT and PPT group measured in percentages during the intraoperative TBS after TOF ligation and dissection (and, in the case of PPT group, after the PPT) but before the surgical correction of OA, through the ventilation tube in the operating theatre.
Key secondary outcome parameters are the difference in the degree of collapse of the tracheal wall between the no-PPT and the PPT group measured in percentages during:
The postoperative flexible TBS through the ventilation tube during routine extubation in the paediatric or neonatal ICU.
The (routine) second postoperative flexible and rigid TBS conducted under general anaesthesia in the operating theatre after approximately 2–6 months. This TBS aims to determine whether the effect of the PPT is sustained and/or whether the TM deteriorates beyond the first 2 months.
The no-PPT group and PPT group will further be compared in terms of the following exploratory secondary endpoints: the number of respiratory symptoms, the number of RTIs and BRUEs, the number of postoperative complications, the number of hospital admissions and the number of additional conventional, medicinal or surgical interventions, the ventilation duration and the length of hospital stay in the first 6 months of life. In addition, the preoperative degree of TM will be compared with the outcomes of the primary and key secondary endpoints (the intraoperative and postoperative degree of TM).
Statistical analysis
The statistical analyses, including the definitions of the analysis population sets, will be described in detail in the statistical analysis plan, which will be finalised before the database lock and unblinding of the trial. Primary and key secondary endpoints will be analysed in the intention-to-treat population consisting of all randomised patients. Primary and key secondary endpoints will be compared between the treatment groups (as-randomised) using linear regression with the treatment group as the dependent variable of interest, adjusting for centre as a stratification factor and for the preoperative degree of the tracheal collapse at baseline. Other secondary outcomes will be analysed with models for count data (Poisson or negative binomial models). Baseline data will be described and summarised by means, SD, medians and IQRs as appropriate for continuous data or by numbers and percentages for categorical data. We expect missing data will be limited for most outcomes so multiple imputations will not be needed. Sensitivity analyses to investigate the impact of missing outcomes will be described in the statistical analysis plan. All statistical analyses will be performed by using IBM SPSS Statistics 29.0.1 software or R statistical computing. A two-sided significance level of 5% will be used for all statistical tests.
Patient and public involvement
A representative of the Dutch patients association, de Vereniging voor Ouderen en Kinderen met een Slokdarmafsluiting (VOKS), was involved in developing this research protocol. The VOKS closely cooperates with the Esophageal Atresia Global Support Groups. Also, patients and the public will be informed of study results through patient societies and social media.
Adverse events and auditing
Adverse events will be handled according to the guidelines of the institutional review board (IRB) of the University Medical Center Utrecht. All adverse events will be registered during the study. Serious adverse events will be reported to the sponsor immediately and registered appropriately within 24 hours. All participating sites will be audited once a year by an independent monitor, and a written monitor report will be submitted to the sponsor afterwards.
Benefits and risk assessment
The risk associated with participation in this study is considered to be low. Severe symptoms of TM develop in approximately 11%–33% of OA patients. In these patients, surgical treatment of TM may be necessary, such as a secondary PT.1,416 This secondary PT entails a second major surgical procedure and can be accompanied by a risk of damaging the anastomosis of the OA and requiring hours of surgery because of extensive adhesions.
It is hypothesised that the PPT can prevent or reduce these symptoms and complications and decrease postoperative reinterventions. Therefore, the PPT has been implemented routinely in some centres, reducing respiratory morbidity.21 23 24 A drawback of the PPT lies in the fact that all OA patients with TM undergo the PPT, while some may not have developed respiratory morbidity.
It remains unclear if newborns benefit more from the wait-and-see policy (no-PPT), with the possible risk of having to undergo a more extensive and invasive surgery later on (eg, secondary PT or aortopexy), or if they benefit more from the PPT, possibly preventing respiratory morbidity. Both the PPT and the wait-and-see policy (possibly including secondary PT or aortopexy) have been proven effective and safe and are currently used. Therefore, all patients participating in our trial will receive adequate treatment.23 24
Performing intraoperative and postoperative TBSs for this trial may pose a potential risk (ie, transient desaturations or bradycardia). However, a diagnostic TBS is a routine procedure commonly used to safely assess the condition of the trachea, even in newborns. Complications during a TBS, such as cough, brief tachycardia or bradycardia, and laryngospasms or bronchospasms, are rare.4
After the OA correction, newborns are extubated in the neonatal or paediatric ICU within a few days. During this extubation process, the first postoperative TBS is conducted through the ventilation tube by a paediatric otorhinolaryngologist, with the presence of either the ICU specialist or ICU nurse. Therefore, the burden and risk associated with the TBS procedure are considered negligible, given the controlled and supervised environment in which it is performed.
The second postoperative TBS may present a burden due to the need for readmission and administration of general anaesthesia in the operating theatre. However, a routine TBS in the operating theatre is routinely performed in all (GOSH and KUH) or half (UMCU and EMC) of the OA patients. Thus, this study procedure entails a TBS performed as a trial procedure only in a small percentage of patients. Moreover, since most of these latter patients undergo a therapeutic oesophagogastroscopy under general anaesthesia for clinical reasons (eg, dilatation/eosinophilic oesophagitis), these patients will undergo the second postoperative TBS combined with this oesophagogastroscopy. Conducting a second postoperative TBS enables the identification of patients with deteriorated TM. The ability to identify such patients may outweigh the associated risks or added burden of the procedure. The findings of the TBS, in combination with symptoms, can assist the treating physician in finding the optimal treatment. This TBS under general anaesthesia is an objective measure to evaluate the degree of collapse in these patients.26 29 30
Data management
Data will be handled confidentially and anonymously using the Castor EDC database system for data collection (Castor EDC, USA), thus complying with ICH E6 Good Clinical Practice. All patient data will be coded using a subject identification code list. The local PI safeguards the code’s key; the sponsor will have access to these codes. Other than that, access to personal patient data is only possible for monitoring purposes, audits or evaluation by the IRB and the Healthcare Inspectorate. The local PI will only have access to patients’ data from their own centre; the sponsor will have access to the final trial dataset. All of this has been stated in a clinical trial site agreement signed by all participating sites. The handling of personal data will comply with the General Data Protection Regulation (GDPR, https://gdpr.eu/). All data will be kept for 15 years.
Ethics and dissemination
The IRB of the UMCU approved this study protocol for the implementation of this trial in both the UMCU and EMC (protocol version 2 d.d. 12-01-2024; METC number 23-256/A; NL84862.041.23). The protocol will shortly be submitted for ethical approval at GOSH and KUH. In addition, the trial protocol is registered with ClinicalTrials.gov.
In case of any protocol modifications, an official amendment will be submitted to the IRB. Approved changes will be communicated to all relevant parties according to the rules of the IRB. This trial’s informed consent and assent process aligns with the Good Clinical Practice guideline.31
No interim analyses for efficacy or futility are planned. However, external experts have been assembled in the form of a data safety monitoring board (DSMB). This committee consists of an otolaryngologist, a paediatric surgeon and a statistician. All experts are independent of the sponsor and participating centres so competing interests will be avoided. The DSMB will meet once at the start of the trial and once a year, or more often if deemed necessary. The DSMB will monitor the safety of the study subjects during the trial.
The trial results will be published in an international peer-reviewed scientific journal as soon as possible after the end of the follow-up period of the last included patient. Furthermore, we aim to present the results at several major international conferences.
Acknowledgements
We want to thank the following study clinicians for their contribution to the development of the trial and conducting trial procedures: Saskia Coenraad, Kim van Loon, Ellen Reuling, Robert Stokroos, and Johannes Verweij (University Medical Center Utrecht); Andreas Andersson and Manja Nilsson (Karolinska University Hospital Stockholm).
Footnotes
Funding: This work is supported by the For Wis(h)dom Foundation, grant number WF/2022/1310.
Prepublication history for this paper is available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-087272).
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Marit J B van Stigt, Email: m.j.b.vanstigt-3@umcutrecht.nl.
Anne-Fleur R L van Hal, Email: a.r.l.vanhal@erasmusmc.nl.
Arnold J N Bittermann, Email: a.j.n.bittermann@umcutrecht.nl.
Colin R Butler, Email: colin.butler@gosh.nhs.uk.
Ilse Ceelie, Email: i.ceelie-2@umcutrecht.nl.
Daniela Cianci, Email: D.Cianci@umcutrecht.nl.
Paolo de Coppi, Email: paolo.decoppi@gosh.nhs.uk.
Caroline Gahm, Email: caroline.gahm@regionstockholm.se.
Julia E Hut, Email: j.e.hut@umcutrecht.nl.
Koen F M Joosten, Email: k.joosten@erasmusmc.nl.
Petra M A Lemmers, Email: p.lemmers@umcutrecht.nl.
Dhanya Mullassery, Email: Dhanya.Mullassery@gosh.nhs.uk.
Reema Nandi, Email: reema.nandi@gosh.nhs.uk.
Bas Pullens, Email: b.pullens@erasmusmc.nl.
Lonneke M Staals, Email: l.staals@erasmusmc.nl.
Jan F Svensson, Email: jan.f.svensson@regionstockholm.se.
Stefaan H A J Tytgat, Email: s.tytgat@umcutrecht.nl.
Peter M van de Ven, Email: p.m.vandeven-3@umcutrecht.nl.
René M H Wijnen, Email: r.wijnen@erasmusmc.nl.
John Vlot, Email: john.vlot@erasmusmc.nl.
Maud Y A Lindeboom, Email: m.y.a.lindeboom@umcutrecht.nl.
References
- 1.Gross RE. Atresia of the esophagus. Am J Dis Child (1911) 1947;74:369. [PubMed] [Google Scholar]
- 2.Pedersen RN, Calzolari E, Husby S, et al. Oesophageal atresia: prevalence, prenatal diagnosis and associated anomalies in 23 European regions. Arch Dis Child. 2012;97:227–32. doi: 10.1136/archdischild-2011-300597. [DOI] [PubMed] [Google Scholar]
- 3.Dodge-Khatami A, Deanovic D, Sacher P, et al. Clinically relevant tracheomalacia after repair of esophageal atresia: the role of minimal intra-operative dissection and timing for aortopexy. Thorac Cardiovasc Surg. 2006;54:178–81. doi: 10.1055/s-2005-872954. [DOI] [PubMed] [Google Scholar]
- 4.Thakkar H, Upadhyaya M, Yardley IE. Bronchoscopy as a screening tool for symptomatic tracheomalacia in oesophageal atresia. J Pediatr Surg. 2018;53:227–9. doi: 10.1016/j.jpedsurg.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Usui N, Kamata S, Ishikawa S, et al. Anomalies of the tracheobronchial tree in patients with esophageal atresia. J Pediatr Surg. 1996;31:258–62. doi: 10.1016/s0022-3468(96)90010-x. [DOI] [PubMed] [Google Scholar]
- 6.Slany E, Holzki J, Holschneider AM, et al. [Tracheal instability in tracheo-esophageal abnormalities] Trachealinstabilitat bei tracheo-osophagealen Fehlbildungen. Z Kinderchir. 1990;45:78–85. doi: 10.1055/s-2008-1042555. [DOI] [PubMed] [Google Scholar]
- 7.Cartabuke RH, Lopez R, Thota PN. Long-term esophageal and respiratory outcomes in children with esophageal atresia and tracheoesophageal fistula. Gastroenterol Rep (Oxf) 2016;4:310–4. doi: 10.1093/gastro/gov055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sistonen SJ, Pakarinen MP, Rintala RJ. Long-term results of esophageal atresia: Helsinki experience and review of literature. Pediatr Surg Int. 2011;27:1141–9. doi: 10.1007/s00383-011-2980-7. [DOI] [PubMed] [Google Scholar]
- 9.Hysinger EB, Panitch HB. Paediatric Tracheomalacia. Paediatr Respir Rev. 2016;17:9–15. doi: 10.1016/j.prrv.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Chetcuti P, Phelan PD. Respiratory morbidity after repair of oesophageal atresia and tracheo-oesophageal fistula. Arch Dis Child. 1993;68:167–70. doi: 10.1136/adc.68.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spitz L, Kiely E, Brereton RJ. Esophageal atresia: five year experience with 148 cases. J Pediatr Surg. 1987;22:103–8. doi: 10.1016/s0022-3468(87)80420-7. [DOI] [PubMed] [Google Scholar]
- 12.Lacher M, Froehlich S, von Schweinitz D, et al. Early and long term outcome in children with esophageal atresia treated over the last 22 years. Klin Padiatr. 2010;222:296–301. doi: 10.1055/s-0030-1249610. [DOI] [PubMed] [Google Scholar]
- 13.Kovesi T, Rubin S. Long-term complications of congenital esophageal atresia and/or tracheoesophageal fistula. Chest. 2004;126:915–25. doi: 10.1378/chest.126.3.915. [DOI] [PubMed] [Google Scholar]
- 14.Fraga JC, Jennings RW, Kim PCW. Pediatric tracheomalacia. Semin Pediatr Surg. 2016;25:156–64. doi: 10.1053/j.sempedsurg.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 15.van der Zee DC, Straver M. Thoracoscopic aortopexy for tracheomalacia. World J Surg. 2015;39:158–64. doi: 10.1007/s00268-014-2798-2. [DOI] [PubMed] [Google Scholar]
- 16.Macchini F, Parente G, Morandi A, et al. Classification of Esophageal Strictures following Esophageal Atresia Repair. Eur J Pediatr Surg. 2018;28:243–9. doi: 10.1055/s-0037-1598656. [DOI] [PubMed] [Google Scholar]
- 17.Svetanoff WJ, Zendejas B, Frain L, et al. When to consider a posterolateral descending aortopexy in addition to a posterior tracheopexy for the surgical treatment of symptomatic tracheobronchomalacia. J Pediatr Surg. 2020;55:2682–9. doi: 10.1016/j.jpedsurg.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Kamran A, Jennings RW. Tracheomalacia and Tracheobronchomalacia in Pediatrics: An Overview of Evaluation, Medical Management, and Surgical Treatment. Front Pediatr. 2019;7:512. doi: 10.3389/fped.2019.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumida W, Yasui A, Shirota C, et al. Update on aortopexy and posterior tracheopexy for tracheomalacia in patients with esophageal atresia. Surg Today. 2024;54:211–9. doi: 10.1007/s00595-023-02652-6. [DOI] [PubMed] [Google Scholar]
- 20.Shieh HF, Smithers CJ, Hamilton TE, et al. Posterior tracheopexy for severe tracheomalacia. J Pediatr Surg. 2017;52:951–5. doi: 10.1016/j.jpedsurg.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Shieh HF, Smithers CJ, Hamilton TE, et al. Posterior Tracheopexy for Severe Tracheomalacia Associated with Esophageal Atresia (EA): Primary Treatment at the Time of Initial EA Repair versus Secondary Treatment. Front Surg. 2017;4:80. doi: 10.3389/fsurg.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shieh HF, Smithers CJ, Hamilton TE, et al. Descending Aortopexy and Posterior Tracheopexy for Severe Tracheomalacia and Left Mainstem Bronchomalacia. Semin Thorac Cardiovasc Surg. 2019;31:479–85. doi: 10.1053/j.semtcvs.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 23.van Tuyll van Serooskerken ES, Tytgat SHAJ, Verweij JW, et al. Primary Posterior Tracheopexy in Esophageal Atresia Decreases Respiratory Tract Infections. Front Pediatr. 2021;9:720618. doi: 10.3389/fped.2021.720618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasui A, Hinoki A, Amano H, et al. Thoracoscopic posterior tracheopexy during primary esophageal atresia repair ameliorate tracheomalacia in neonates: a single-center retrospective comparative cohort study. BMC Surg. 2022;22:285. doi: 10.1186/s12893-022-01738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tytgat S, van Herwaarden-Lindeboom MYA, van Tuyll van Serooskerken ES, et al. Thoracoscopic posterior tracheopexy during primary esophageal atresia repair: a new approach to prevent tracheomalacia complications. J Pediatr Surg. 2018;53:1420–3. doi: 10.1016/j.jpedsurg.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Koumbourlis AC, Belessis Y, Cataletto M, et al. Care recommendations for the respiratory complications of esophageal atresia-tracheoesophageal fistula. Pediatr Pulmonol. 2020;55:2713–29. doi: 10.1002/ppul.24982. [DOI] [PubMed] [Google Scholar]
- 27.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105–11. [PubMed] [Google Scholar]
- 29.Choi J, Dharmarajan H, Yu J, et al. Diagnostic flexible versus rigid bronchoscopy for the assessment of tracheomalacia in children. J Laryngol Otol. 2018;132:1083–7. doi: 10.1017/S0022215118002050. [DOI] [PubMed] [Google Scholar]
- 30.Hysinger EB, Hart CK, Burg G, et al. Differences in Flexible and Rigid Bronchoscopy for Assessment of Tracheomalacia. Laryngoscope. 2021;131:201–4. doi: 10.1002/lary.28656. [DOI] [PubMed] [Google Scholar]
- 31.ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice. J Postgrad Med. 2001;47:199–203. [PubMed] [Google Scholar]