The PALOH experience
The Pharmacogenetics to Avoid Loss of Hearing (PALOH) Study evaluated the first point-of-care (POC) genetic test (GeneDrive) in the neonatal acute setting.1 The GeneDrive POC test can detect the m.1555A>G genetic variant which is present in 1 in 500 individuals. This gene predisposes the individual to profound hearing loss following the administration of aminoglycoside antibiotics, such as gentamicin which is commonly given as first-line antibiotic for early onset neonatal sepsis.2 Approximately, 7%–13% of all newborn infants receive a septic screen.3 Once the decision has been made to screen the infant, antibiotics should be administered promptly.2 Therefore, previously it has not been possible to perform genetic testing for the m.1555A>G genetic variant within the 60 min target for antibiotic administration as traditional genetic tests take weeks, or even months, to provide a result.4 The GeneDrive test is innovative in that it is a POC genetic test providing a result in less than 30 min.4 If the m.1555A>G genetic variant is present, the clinical team can individualise the care provided by selecting alternative antibiotics, thus avoiding aminoglycosides and preventing iatrogenic hearing loss.
In 2020, the PALOH Study was launched across Manchester University National Health Service (NHS) Foundation Trust (MFT) and Liverpool Women’s Hospitals NHS Trust. The PALOH Study followed an opt-out model of consent. MFT was successful in recruiting 751 infants. An additional three sets of parents opted out of the PALOH Study. The study lasted 11 months (January to November 2020) and showed that POC genetic testing is possible and is a desirable development for clinicians and patients.
Why was an opt-out consenting model approved?
The opt-out consent model was selected due to the practicalities and time constraints of providing urgent care to the infant. Many infants admitted to the neonatal intensive care unit (NICU) will require screening for infection in accordance with National Institute of Clinical Excellence risk criteria (NG 195).2 Due to competing priorities (provision of clinical care, obtaining intravenous access, prescribing and administering vital fluids and medications), it would not have been possible to also ensure that every parent was formally consented to the PALOH Study by a Good Clinical Practice trained member of staff within 1 hour of the decision to treat for suspected sepsis. The benefit of the GeneDrive test was felt to be clearly advantageous to the infant and an opt-out model was granted by the ethics review panel.
The GeneDrive test was performed for every infant on admission to NICU during the study period. Parents were subsequently informed of the study and had the option to opt out. While the infant’s data could be removed from the study, the GeneDrive test would have already been performed and the m.1555A>G genetic variant status of the infant would have been determined. In the case of GeneDrive the implications of the presence or absence of the m.1555A>G genetic variant is restricted to susceptibility to aminoglycoside-related hearing loss only. Currently, there are no other known implications of this genetic variant. Once the presence of the m.1555A>G genetic variant is known, medical staff cannot ethically administer aminoglycoside antibiotics to that patient where there is an available alternative, and this information would be entered into the patient medical record to inform future treating healthcare professionals over the patient’s lifetime.
Wider context of opt-out consent in genetic testing
The PALOH Study has set the precedent that to use an opt-out model of consent for genetic research the test must yield clinically vital information that can inform immediate management choices. The genetic variant detected by the GeneDrive test is not currently linked to any other conditions and therefore, has no other implications for the individual other than avoidance of aminoglycosides. However, one would predict that as POC genetic testing expands, detection of collateral information of significance could become problematic. For example, it becomes harder to justify opt-out consenting if the detected genetic variant has associations beyond the immediate clinical question. If the GeneDrive test had detected a genetic variant associated with aminoglycoside-related hearing loss and this variant was also associated with an additional condition, such as early onset dementia, then there is direct conflict between immediate and longer-term benefits and harms of the genetic information to that individual. For some individuals, living with sensorineural hearing loss since infancy would not present any significant issue, but living with the knowledge that their adult life would be affected by early onset dementia would be extremely traumatic. The relative value of information and the trade-offs this information may involve are deeply personal and will differ between individuals.5 Genetic testing with potential lifelong or life-altering implications would be ideally reserved until the infant reaches adulthood and can make their own decision about the relative value of the information. This has been seen in the case of genetic testing for Huntington’s disease.6 However, as bedside genetic testing expands and may provide information which is clinically significant in the neonatal period—as in the case with GeneDrive—there may not be time to wait until the infant reaches adulthood and can determine their own individual risk preferences. In the pursuit of harm avoidance through genetic testing we may well encounter harm through undesired information exposure in cases where the genetic variants have collateral implications.
Where possible, bedside genetic tests should be defined and discrete to test for the minimum amount of information required for the immediate clinical question. This is particularly relevant to the neonatal and paediatric population, where decisions made on their behalf could otherwise have life-long implications due to collateral risk information being uncovered. This poses potential harm through psychological trauma that the unwanted information may hold, and potential financial and career effects if the collaterally detected genetic variants have additional implications, for example, impacts on health insurance or consequent prevention of application to certain career paths.7 8
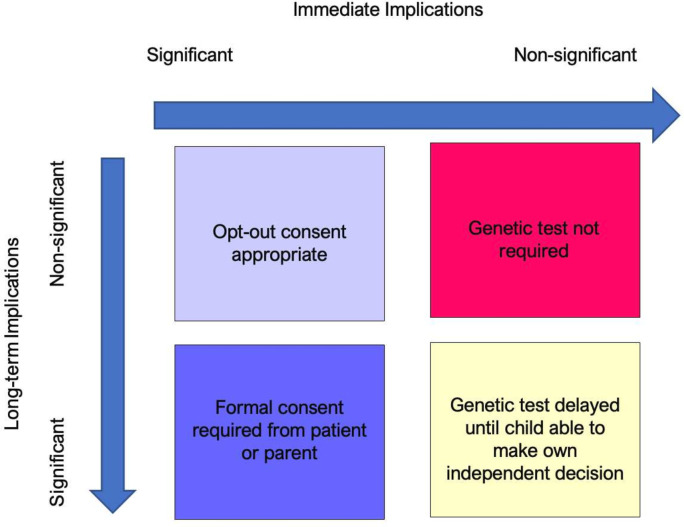
In cases where the genetic information can change immediate clinical management to prevent lifelong harm with no other implications of that genetic information, opt-out consent seems entirely appropriate. However, if the immediate implications of a genetic test are less significant, or will not change immediate clinical management, and/or there may be significant collateral implications of that gene, then formal consent must be sought (figure 1).
Figure 1. Proposed consenting models for genetic testing depending on implications of the genetic information.
Implications for the future
Opt-out consenting ensures streamlined recruitment and equity of access to study interventions that need to be deployed in rapid, time-pressured circumstances. This model does have the potential to erode or prevent trust being established between parent and clinician. However, as the PALOH Study demonstrates, an opt-out model can be effective in specific circumstances and can be acceptable to parents. As the field of bedside genetic testing develops and the complexity of information that genetic testing can yield progresses, it must be ensured that a balancing act is traversed between the clinical need for the genetic information in the immediate period to optimise the individual’s clinical management, with the significance and certainty of any collateral genetic information implications in the longer term. It will be interesting to see where we, as an ethical community of professionals and parents, set our limits for opt-in and opt-out consenting models for future research.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Provenance and peer review: Not commissioned; internally peer reviewed.
Contributor Information
Jennifer LH Peterson, Email: jennifer.peterson@hotmail.co.uk.
Nicola Booth, Email: nicola.booth@mft.nhs.uk.
Ajit Mahaveer, Email: Ajit.mahaveer@mft.nhs.uk.
References
- 1.McDermott JH, Mahaveer A, James RA, et al. Rapid Point-of-Care Genotyping to Avoid Aminoglycoside-Induced Ototoxicity in Neonatal Intensive Care. JAMA Pediatr. 2022;176:486–92. doi: 10.1001/jamapediatrics.2022.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence (NICE) Neonatal infection: antibiotics for prevention and treatment. nice guideline [ng195] 2021. [8-Feb-2024]. https://www.nice.org.uk/guidance/ng195 Available. Accessed. [PubMed]
- 3.Simonsen KA, Anderson-Berry AL, Delair SF, et al. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27:21–47. doi: 10.1128/CMR.00031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott JH. Genetic testing in the acute setting: a round table discussion. J Med Ethics. 2020;46:531–2. doi: 10.1136/medethics-2019-106043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson KE, Eberly S, Marder KS, et al. The choice not to undergo genetic testing for Huntington disease: Results from the PHAROS study. Clin Genet. 2019;96:28–34. doi: 10.1111/cge.13529. [DOI] [PubMed] [Google Scholar]
- 6.Huntingtons’s Disease Association Genetic testing. [13-Mar-2022]. https://www.hda.org.uk/getting-help/if-youre-at-risk/genetic-testing#:~:text=Deciding%20to%20have%20the%20test,the%20test%20will%20be%20performed Available. Accessed.
- 7.Royal Air Force Medical conditions that preclude entry. [15-Mar-2022]. https://www.raf.mod.uk/recruitment/media/1652/medical-conditions-that-preclude-entry.pdf Available. Accessed.
- 8.Godard B, Raeburn S, Pembrey M, et al. Genetic information and testing in insurance and employment: technical, social and ethical issues. Eur J Hum Genet. 2003;11:S123–42. doi: 10.1038/sj.ejhg.5201117. [DOI] [PubMed] [Google Scholar]