Abstract
This study investigated the effects of addition of Italian ryegrass with multi-enzyme on growth performance, fecal odor, and microbiome. The experiment had a two-factor factorial design, using three levels of Italian ryegrass (0%, 2.5%, and 5%) and two levels of multi-enzymes (no enzyme and commercially recommended level) to formulate experimental diets. In total, 60 crossbred Landrace × Yorkshire × Duroc (LYD) pigs (88.35 ± 2.57 kg) were allocated into six dietary treatments with five replicates. After four weeks, fecal samples were collected via rectal massage for microbiome and odorous compound analysis. Results showed no significant difference (p > 0.05) in growth performance, except for feed intake (p < 0.05), which was higher in enzyme-added diets. Fecal microbiome exhibited no differences (p > 0.05) between treatments, with Firmicutes and Bacteroidetes being the major phyla, similar to the general pig population. Alpha and beta diversity analyses showed no significant differences (p > 0.05). Odorous compounds displayed no significant differences (p > 0.05), except for indoles (p < 0.05) influenced by the enzyme. In conclusion, 5% Italian ryegrass with multi-enzymes can be used as an alternative feed ingredient, having no negative effects on the growth performance, microbiome, and odorous compounds of growing pigs.
Keywords: Growth performance, Italian ryegrass, Manure odor, Microbiome, Non-starch polysaccharide (NSP) enzyme
INTRODUCTION
The demand for alternative feed ingredients is increasing due to unstable costs of universal feed ingredients, such as corn and wheat, caused by unstable international situations such as continuous climate change and wars. In those circumstance, feed formulators should have considered the various alternatives which has low nutrient value or has high price. Thus, we were focused on Italian ryegrass (IRG) as an alternative feed ingredient for pig. Ryegrass has two major species, Lolium perenne, also called English ryegrass and Lolium multiflorum Lam., also called IRG. IRG has good characteristics which high-yielding, rapid seedling establishment, weed suppression, and palatable forage that is tolerant to various environmental conditions [1,2]. Even in 2004, a new cold-tolerant and early-heading variety of IRG called “Kogreen” was developed to be suitable in Korean seasonal conditions [3]. Generally IRG regarded as good ingredient for ruminant without affecting negative effect on the animal. Recent research also suggests that Hanwoo fed IRG improves carcass yield 12.5% higher than control diet [4]. However, there is limited research on IRG diets for non-ruminant animals due to their high fibers. Despite the characteristics of fibers in monogastric animal diet which generally regarded as an anti-nutritional actor because of its lower protein level and energy digestibility, fibers could improve gut health with by-product from bacteria which increasing gut length, mass, and villus height [5]. Non-starch polysaccharide (NSP), some portion of dietary fibers, in diet is also generally considered as anti-nutritional factors for non-ruminant animals because of their characteristics which encapsulate nutrients intracellularly and cannot be degraded by endogenous enzymes of non-ruminant animals [6]. NSPs also have cation exchange capacity, hydration properties, viscosity, and organic compound absorptive properties at animal digestive tract [7]. Because of the characteristics of NSP, Choct, Dersjant-Li [8] suggests that NSP in diet, especially for young monogastric animals, NSP decrease the nutrient digestibility, proliferation of Escherichia coli causing swine dysentery and dehydration of NSP could reduce negative effects of NSP. Therefore, previous dietary NSP studies focused on mitigating the effects of NSPs by adding NSP enzymes, and this addition is suggested to have expected and unexpected health benefits for swine in NSP diets [9]. Following studies, Li et al. [10] suggests weaning pig fed high barley contained diet with NSP enzymes could improves growth performance and increase gamma GT activity in jejunal mucosa and Ao et al. [11] suggests growing pig fed corn-soy based diet with multi NSP enzymes improve average daily gain (ADG), G:F, and improve digestibility of dry matter, nitrogen and amino acids. Besides, the microbiome is also crucial for its host animals, as it can provide various benefits that the host lacks due to its genetic diversity [12]. Animals are born without microbial contamination; however, they are continually exposed to microbes due to their gastrointestinal structure, which resembles an open tube [13]. Therefore, feed ingredients consumed by animals are important for shaping the gut microbial community. However, research of IRG in the monogastric animal diet is very limited. Research by Recharla et al. [14] suggests that IRG, with exogenous enzyme addition, has no impact on growth performance and nutrient digestibility; however, it affects the microbial composition in the hindgut. Park et al. [15] propose that adding a maximum of 1.5% IRG to swine diets can reduce odorous compounds in manure by altering the microbial community, while maintaining consistent growth performance.
Therefore, this study was conducted to estimate the availability, tolerance level, and effects of IRG with NSP enzyme supplementation on growth performance, gut microbiome, blood parameter, and manure odor emission in finisher pigs.
MATERIALS AND METHODS
Italian ryegrass evaluation
Following AOAC methods [16], samples were initially pre-dried at 60°C in a drying oven and ground. The feed ingredient was assessed for gross energy using a bomb calorimeter (Model C2000, IKA, Staufen, Germany), ether extract, crude fiber (CF), ash, neutral detergent fiber (NDF), and acid detergent fiber (ADF). Calcium was determined using an atomic absorption spectrophotometer (Perkin Elmer 3300, Perkin Elmer, Waltham, MA, USA). Phosphorus content in the feed ingredients, experimental diets, and feces was measured using a spectrophotometer (Optizen 2120 UV, Mecasys, Daejeon, Korea). The calculated composition of IRG was as follows: 1,447.25 kcal/kg metabolizable energy, 12.1% crude protein, 3.6% ether extract, 22.3% crude fiber, 0.2% phosphate, and 0.4% calcium.
Experimental diet, design, and animal housing
Six experimental diets were formulated and fed to the animals. The diets consisted of two enzyme levels (non and recommended) and three dietary IRG levels (0%, 2.5%, and 5%). All experimental diets were formulated to match equal nutrient levels of metabolizable energy (ME) and crude protein (CP). The formulation and calculated values of experimental diets are presented in Table 1. In total, 60 crossbred Landrace × Yorkshire × Duroc (LYD) pigs were used to assess the effects of dietary IRG and multi-enzyme addition. Multi-enzymes were added to the diet to achieve the commercially recommended level (255 U/kg cellulase; 1,250 U/kg beta-mannanase; 6,000 U/kg xylanase). The pigs were randomly assigned to six dietary treatments (two enzyme levels × three IRG levels), with each treatment replicated five times, and each pen containing two pigs. The average body weight (BW) of the allocated pigs was 88.35 ± 2.57 kg. All pigs are allotted with completely randomized block design. The experiment lasted for four weeks, during which the experimental diet and fresh water were provided ad libitum via metal troughs and nipple drinkers.
Table 1. Feed formulation for the Italian ryegrass experiment for finisher pigs.
| Ingredients | Control | IRG2.5 | IRG5 |
|---|---|---|---|
| Corn | 77.96 | 75.07 | 72.16 |
| SBM | 18.00 | 18.00 | 18.00 |
| IRG | ND | 2.50 | 5.00 |
| Tallow | 1.00 | 1.50 | 2.00 |
| DCP | 1.30 | 1.25 | 1.20 |
| Limestone | 0.80 | 0.75 | 0.70 |
| Lys | 0.27 | 0.26 | 0.26 |
| Met | 0.01 | 0.01 | 0.02 |
| Thr | 0.06 | 0.06 | 0.06 |
| Salt | 0.30 | 0.30 | 0.30 |
| Vit-min premix1) | 0.30 | 0.30 | 0.30 |
| Calculated value | |||
| Gross energy | 3,848 | 3,842 | 3,835 |
| Metabolizable energy | 3,348 | 3,326 | 3,303 |
| Crude fiber | 2.73 | 3.66 | 4.59 |
| Ether extract | 3.91 | 4.32 | 4.73 |
| Ash | 2.16 | 2.37 | 2.57 |
| NDF | 8.87 | 10.36 | 11.84 |
| ADF | 3.44 | 4.48 | 5.51 |
| Crude protein | 14.63 | 14.62 | 14.62 |
| Lys | 0.9 | 0.9 | 0.9 |
| Met | 0.26 | 0.26 | 0.26 |
| Met+Cys | 0.52 | 0.51 | 0.51 |
| Ca/P | 1.23 | 1.21 | 1.22 |
Vitamin-Mineral premix provided the following nutrients per kg: Vitamin A, 15,000 IU; Vitamin D3, 2850 IU; Vitamin E, 75 IU; Vitamin K3, 4.5 mg; Vitamin B1, 3.375 mg; Vitamin B2, 7.5 mg; Vitamin B6, 4.875 mg; Vitamin B12, 56.250 mg; Calcium D-Pantothenate, 25.5; Endox, 18 mg; Niacin, 48 mg; Folic acid, 1.875; Iodine, 0.6 mg; Iron, 150 mg; Magnesium, 54 mg; Zinc, 90 mg; Copper, 39 mg; Biotin, 0.45 mg.
SBM, soybean meal; IRG, Italian ryegrass; ND, not detected; DCP, di-calcium phosphate; Lys, lysine; Thr, threonine; NDF, neutral detergent fiber; ADF, acid detergent fiber; Met, methionine; Cys, cysteine.
Sample collection and analyses
Growth performance data were collected to determine the effects of IRG and multi-enzymes present in the diet on finisher pig. All measurements were taken on a pen basis. Initial and final body weights were recorded on the first and last days of the experiment. Average daily feed intake (ADFI) was determined by measuring the total feed consumption. ADG and feed efficiency (FE) were calculated using the measured body weights and feed intake.
Fecal samples were analyzed to estimate the effects of IRG and multi-enzymes on fecal microbiome and odorous compounds. Fecal samples were collected from the anus after rectal massage and stored in a −80°C deep fridge with 50-mL conical tube.
A G-spinTM genomic DNA extraction kit was used to extract genomic DNA from fecal samples. The processed DNA was analyzed using 16S rRNA [17]. All processed sequences were clustered at a 97% similarity threshold using QIIME (v.1.8.0). The alpha diversity of the microbiome at the IRG level was assessed by calculating amplicon sequence variants (ASV), Chao1, Shannon, and Gini-Simpson diversity indices, whereas beta diversity was evaluated using the Bray–Curtis distance, and principal coordinate analysis (PCoA) was employed to visualize it.
Fecal volatile fatty acids, phenols, and indoles were analyzed following the procedure described by Flickinger et al. [18]. Volatile fatty acids were determined using a gas chromatograph (GC, 6890N, Agilent, Santa Clara, CA, USA). Phenols and indoles were extracted by mixing 2 g of feces with 5 mL of methanol containing 2,000 ppm of 5-chloroindole (internal standard). The feces–methanol mixture was sealed with parafilm, thoroughly mixed, and incubated for 1 h at 4°C with frequent mixing. Thereafter, the tubes were centrifuged at 29,000×g for 20 min at 4°C, and the supernatant was collected. The remaining pellet was mixed again with 5 mL of methanol and extracted as described above. The two supernatant fractions were combined for gas-liquid chromatography analysis. The individual concentrations of indole, phenol, p-cresol, and 4-ethylphenol were determined using a gas chromatograph (GC, 6890N, Agilent).
Statistical analysis
All collected and calculated data were analyzed using two-way analysis of variance (ANOVA) in SPSS 26.0 (SPSS, Chicago, IL, USA). The pen and individual pig were considered the statistical unit for growth performance analysis and manure analysis, respectively. A p < 0.05 was considered statistically significant. In cases where significant differences were found, Tukey’s post hoc test was employed to examine the differences between the groups.
RESULTS AND DISCUSSION
Table 2 shows the impact of dietary IRG and multi-enzymes on growth performance. Initial BW, final BW, ADG, and FE showed no significant differences in terms of p-value, IRG, enzyme, or interaction effects. However, ADFI had a significant difference (p < 0.05), with enzymes influencing it. Exogenous carbohydrase enzymes are considered enhancers, improving energy availability and nutrient digestibility by breaking down indigestible bonds in swine diets. They may also enhance intestinal microbiomes, potentially improving host health [19–21]. Previous research suggests that adding NSP-degrading enzymes to swine diets can boost feed intake [22,23]. Combining these enzymes with others can enhance swine diet performance more effectively than using only one type of enzyme [24]. Our results align with previous studies indicating that exogenous NSP-degrading enzymes increase feed intake. However, no significant differences in any growth performance parameters were observed until IRG was added at a 5% level. In general, ingredients with higher fiber content are considered to have lower nutritional value for non-ruminants because fibers cannot be broken down by the host animal’s endogenous enzymes [25]. Previous studies also agreed that a high fiber diet can negatively affect the growth performance of pigs at various growth stages [26–29]. However, the effect of dietary fiber on growth performance remains controversial. Some research suggests that high fiber diets decreases growth performance in growing pigs but have a limited effect on finisher pigs [30,31], whereas other studies propose that high fiber diets have no impact on growing pigs [32,33]. There are very limited results of IRG in swine diet; however, these suggest that a small amount of IRG does not affect the growth performance [14,15]. Agyekum et al. [29] also suggests that the conflicting results of high fiber effects on swine does not depend only on the fiber amount, such as CF, NDF, ADF, and NSP, but on the ratio between the fiber components. The absence of interaction effects between IRG and enzymes may be due to the limited role of 5% IRG as an anti-nutritional factor in the diet.
Table 2. Effects of Italian ryegrass and multi-enzymes in finisher pig diet on the growth performance during the experimental period.
| Treatment | C−1) | C+ | IRG2.5− | IRG2.5+ | IRG5− | IRG5+ | SEM | p-value | IRG | Enzyme | Interaction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial BW | 88.49 | 88.29 | 88.38 | 88.43 | 88.44 | 88.53 | 2.463 | 1.000 | 0.999 | 0.992 | 0.998 |
| Final BW | 109.90 | 112.82 | 113.62 | 112.54 | 111.06 | 110.84 | 2.517 | 0.899 | 0.673 | 0.795 | 0.708 |
| ADG | 1.020 | 1.168 | 1.202 | 1.148 | 1.077 | 1.062 | 0.062 | 0.305 | 0.229 | 0.604 | 0.246 |
| ADFI | 2.988 | 3.438 | 3.259 | 3.397 | 3.091 | 3.307 | 0.094 | 0.016 | 0.338 | 0.002 | 0.245 |
| FE | 0.342 | 0.341 | 0.368 | 0.337 | 0.348 | 0.320 | 0.016 | 0.457 | 0.505 | 0.137 | 0.592 |
C, control; IRG2.5, diet contains 2.5% IRG; IRG5, diet contains 5% IRG; −, without enzyme; +,with enzyme.
IRG, Italian ryegrass; BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; FE, feed efficiency.
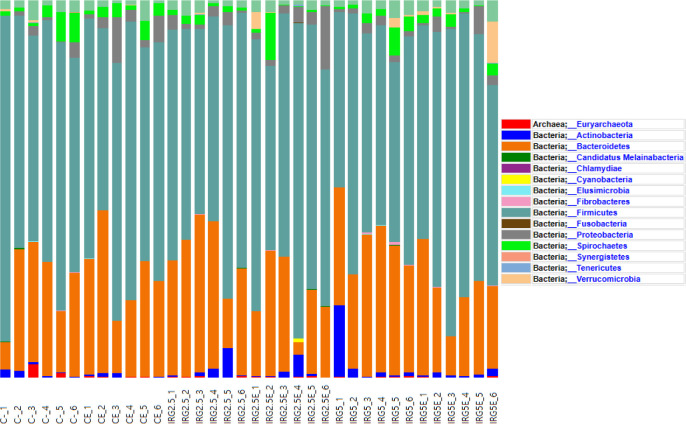
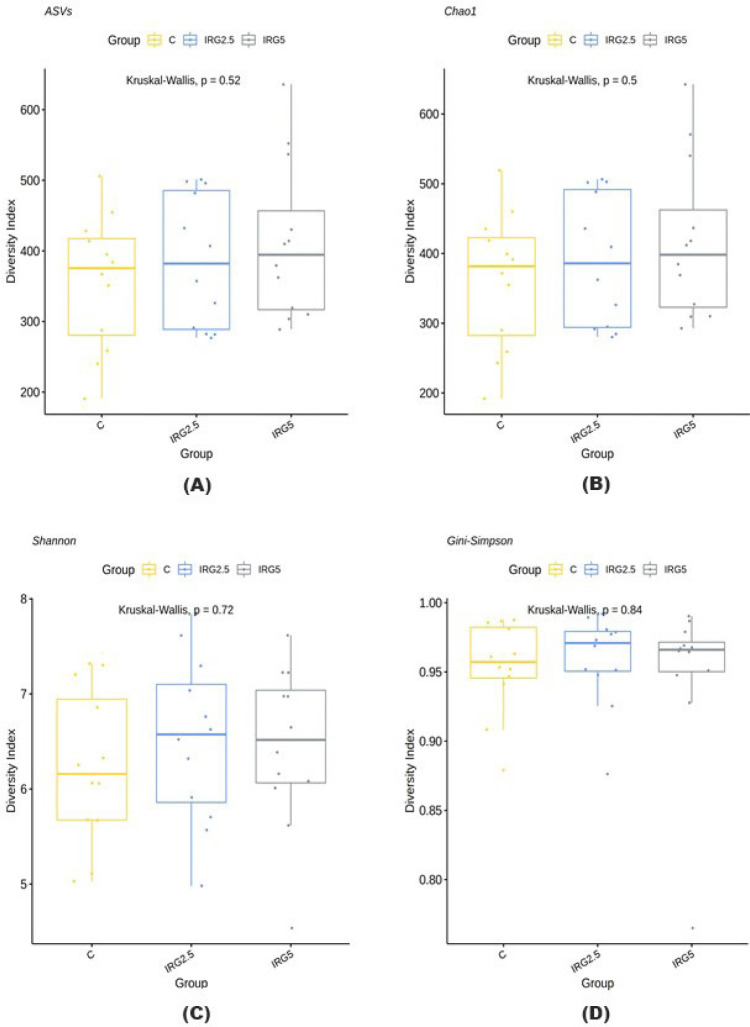
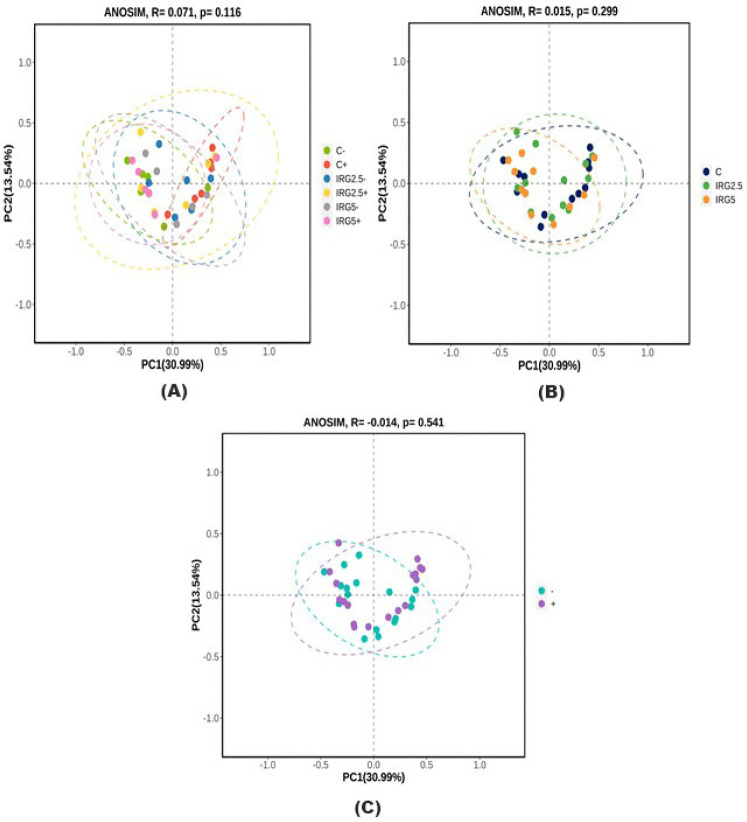
Taxonomic assignment at the phylum level, alpha diversity, and beta diversity are analyzed and illustrated in Figs. 1, 2, and 3 to check the effect of dietary fibers on the gut microbiome. According to Kim et al. [34], the majority of bacteria can be classified into five phyla: Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Spirochaetes, with Firmicutes and Bacteroidetes making up 90% of the bacterial population. These phyla are known to interact with resistant starch [35], and Bacteroidetes possess high carbohydrate enzyme gene activity for breaking down plant cell wall components like glucuronoxylans, xyloglucans, and pectin [36]. As reported by Patil et al. [37], the addition of dietary NSP stimulates the commensal microbiome to produce short-chain organic acids, lowering the gut pH. Consequently, we aimed to investigate potential changes in microbiome diversity with the addition of dietary IRG. Alpha diversity analysis employed ASVs, Chao1, Shannon, and Gini-Simpson methods. However, our results did not indicate any significant differences in alpha diversity. Similarly, beta diversity showed no significant distinctions between the groups. Agyekum and Nyachoti [5] suggested about the ambivalence of the dietary fiber in swine gut which even in the same soluble fiber research which some are suggests that soluble fiber in swine diet contribute the risk of colibacillosis and swine dysentery by colonized pathogenic bacteria caused by the low viscosity but the others research are not. Zhang et al. [38] also suggested that NSP enzyme addition did not impact alpha diversity in growing pigs. Consequently, the results of this study suggested that 5% IRG in the diet with NSP enzymes can be managed without negatively altering microbial composition. The results of the analysis of fecal odorous compounds are shown in Table 3. Fecal odorous compounds analysis was decided because of believes that the dietary NSP could affect the gut microbiome. The major odorous compounds in swine manure are volatile organic compounds, indoles, and phenols [39–41]. Furthermore, whether microbiomes affected by substrates in diet, the major end-products in manure are acetate, propionate, and butyrate [42]. Based on a previous research, volatile fatty acids produced by Eubacterium and Clostridium are the major contributor to odorous compounds. However, various studies presented that the gut microbiome is affected by dietary ingredients, especially fibers [43–45]. Additionally, the gut microbial metabolism of tryptophan results in the production of indole compounds [46]. Therefore, IRG with additional multi-enzymes could possibly change the odorous compounds by reducing the precursor of volatile organic compounds, but no significant difference was observed, except for the indole level influenced by the enzyme. We speculate that this result arises from the degradation of the fiber structure, leading to the release and utilization of entrapped nutrients. From those results, we suggest that adding 5% IRG in finisher pig diets can help achieve the desired goals without negatively impacting the growth performance. Also adding enzymes in high fiber diet can also enhance feed intake and reduce indole compounds by degrading the fiber source.
Fig. 1. Fecal microbiome classification at the phylum level.
Fig. 2. Alpha diversity analysis with ASVs, Chao1, Shannon, and Gini-Simpson method.
(A) AVSs, (B) Chao1, (C) Shannon, (D) Gini-Simpson. ASVs, amplicon sequence variants; C, control diet; IRG2.5, 2.5% IRG added diet; IRG5, 5% IRG added diet.
Fig. 3. Beta diversity analysis using Bray–Curtis distance method.
(A) between all treatment, (B) IRG effect, (C) enzyme effect. C, control diet; IRG2.5, 2.5% IRG added diet; IRG5, 5% IRG added diet; +, commercially recommended level of enzyme added in diet; −, no enzyme added in diet.
Table 3. Effects of Italian ryegrass and multi-enzymes in finisher pig diet on the chemical composition of manure.
| Treatment | C−1) | C+ | IRG2.5− | IRG2.5+ | IRG5− | IRG5+ | SEM | p-value | IRG | Enzyme | Interaction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetate | 3,090.13 | 2,746.45 | 3,029.37 | 2,987.57 | 2,890.46 | 3,002.88 | 178.465 | 0.797 | 0.875 | 0.535 | 0.435 |
| Propionate | 3,085.49 | 2,638.21 | 2,969.10 | 2,627.27 | 2,672.42 | 3,120.61 | 223.005 | 0.381 | 0.905 | 0.535 | 0.100 |
| Isobuty | 175.97 | 172.61 | 165.03 | 161.11 | 132.96 | 150.86 | 16.635 | 0.484 | 0.152 | 0.795 | 0.757 |
| Norbutyrate | 1,860.73 | 1,836.86 | 1,975.28 | 1,881.73 | 1,619.05 | 2,346.27 | 200.761 | 0.230 | 0.799 | 0.220 | 0.086 |
| Isovaleic | 339.95 | 323.18 | 306.50 | 296.02 | 233.05 | 268.67 | 39.139 | 0.441 | 0.124 | 0.931 | 0.767 |
| Norvaleic | 682.64 | 935.21 | 740.34 | 647.21 | 469.45 | 755.29 | 164.573 | 0.516 | 0.491 | 0.274 | 0.449 |
| Phenol | 0.03 | 0.04 | 0.04 | 0.04 | 0.04 | 0.03 | 0.003 | 0.693 | 0.685 | 0.789 | 0.338 |
| pCrezol | 35.68 | 37.31 | 34.82 | 25.95 | 24.17 | 30.68 | 5.452 | 0.432 | 0.246 | 0.956 | 0.361 |
| Indole | 1.26 | 0.15 | 0.37 | 0.34 | 0.93 | 0.32 | 0.328 | 0.143 | 0.528 | 0.035 | 0.270 |
| Skatole | 3.84 | 4.36 | 3.18 | 2.54 | 2.45 | 6.63 | 2.046 | 0.716 | 0.698 | 0.420 | 0.475 |
C, control; IRG2.5, diet contains 2.5% IRG; IRG5, diet contains 5% IRG; −, without enzyme; +, with enzyme.
IRG, Italian ryegrass; BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; FE, feed efficiency.
In conclusion, 5% IRG with multi NSP enzymes could be an option for replacing corn in swine diet and following research which set the maximum IRG level without affecting growth performance and benefits of replacing IRG to corn by economic analyzing are required.
Acknowledgements
This study was supported by 2024 the RDA Fellowship Program of National Institute of Animal Science, Rural Development Administration, Korea.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ014968)” Rural Development Administration, Republic of Korea and this study was supported by 2023 the RDA Fellowship Program of Agriculture Science and Technology Development, Rural Development Administration, Korea.
Availability of data and material
Upon reasonable request, the datasets of this study can be available from the corresponding author.
Authors’ contributions
Conceptualization: Jeong YD, Choi YH.
Data curation: Hong JS, Park HJ.
Formal analysis: Kim C, Back SH.
Methodology: Min YJ.
Software: Kim DW.
Validation: Kim YM, Kim JE.
Investigation: Hong JS, Park HJ.
Writing - original draft: Hong JS, Jeong YD.
Writing - review & editing: Hong JS, Jeong YD, Park HJ, Choi YH, Min YJ, Kim C, Back SH, Kim DW, Kim YM, Kim JE.
Ethics approval and consent to participate
This study was approved by IACUC of Rural Development Administration (No. NIAS-2020-0471).
REFERENCES
- 1.Grogan D, Gilliland T. A review of perennial ryegrass variety evaluation in Ireland. Ir J Agric Food Res. 2011;50:65–81. [Google Scholar]
- 2.Jung GA, Van Wijk AJP, Hunt WF, Watson CE. Ryegrasses. In: Moser LE, Buxton DR, Casler MD, editors. Cool‐season forage grasses. Madison, WI: American Society of Agronomy; 1996. pp. 605–41. p. [DOI] [Google Scholar]
- 3.Choi GJ, Lim YC, Rim YW, Sung BR, Kim MJ, Kim KY, et al. A cold-tolerant and early-heading Italian ryegrass new variety, ‘Kogreen’. J Korean Grassl Sci. 2006;26:9–14. doi: 10.5333/KGFS.2006.26.1.009. [DOI] [Google Scholar]
- 4.Kim WH, Kang SN, Arasu MV, Chu GM, Kim DH, Park JH, et al. Profile of Hanwoo steer carcass characteristics, meat quality and fatty acid composition after feeding Italian ryegrass silage. Korean J Food Sci Anim. 2015;35:299–306. doi: 10.5851/kosfa.2015.35.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agyekum AK, Nyachoti CM. Nutritional and metabolic consequences of feeding high-fiber diets to swine: a review. Engineering. 2017;3:716–25. doi: 10.1016/J.ENG.2017.03.010. [DOI] [Google Scholar]
- 6.Masey O’Neill HV, Smith JA, Bedford MR. Multicarbohydrase enzymes for non-ruminants. Asian-Australas J Anim Sci. 2014;27:290–301. doi: 10.5713/ajas.2013.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach Knudsen KE. The nutritional significance of “dietary fibre” analysis. Anim Feed Sci Technol. 2001;90:3–20. doi: 10.1016/S0377-8401(01)00193-6. [DOI] [Google Scholar]
- 8.Choct M, Dersjant-Li Y, McLeish J, Peisker M. Soy oligosaccharides and soluble non-starch polysaccharides: a review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Asian-Australas J Anim Sci. 2010;23:1386–98. doi: 10.5713/ajas.2010.90222. [DOI] [Google Scholar]
- 9.Petry AL, Patience JF. Xylanase supplementation in corn-based swine diets: a review with emphasis on potential mechanisms of action. J Anim Sci. 2020;98:skaa318. doi: 10.1093/jas/skaa318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li WF, Feng J, Xu ZR, Yang CM. Effects of non-starch polysaccharides enzymes on pancreatic and small intestinal digestive enzyme activities in piglet fed diets containing high amounts of barley. World J Gastroenterol. 2004;10:856–9. doi: 10.3748/wjg.v10.i6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ao X, Meng QW, Yan L, Kim HJ, Hong SM, Cho JH, et al. Effects of non-starch polysaccharide-degrading enzymes on nutrient digestibility, growth performance and blood profiles of growing pigs fed a diet based on corn and soybean meal. Asian-Australas J Anim Sci. 2010;23:1632–8. doi: 10.5713/ajas.2010.10123. [DOI] [Google Scholar]
- 12.Isaacson R, Kim HB. The intestinal microbiome of the pig. Anim Health Res Rev. 2012;13:100–9. doi: 10.1017/S1466252312000084. [DOI] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Gordon JI. An invitation to the marriage of metagenomics and metabolomics. Cell. 2008;134:708–13. doi: 10.1016/j.cell.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Recharla N, Kim D, Ramani S, Song M, Park J, Balasubramanian B, et al. Dietary multi-enzyme complex improves in vitro nutrient digestibility and hind gut microbial fermentation of pigs. PLOS ONE. 2019;14:e0217459. doi: 10.1371/journal.pone.0217459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Cho S, Hwang O. Effects of Italian ryegrass (IRG) supplementation on animal performance, gut microbial compositions and odor emission from manure in growing pigs. Agronomy. 2020;10:647. doi: 10.3390/agronomy10050647. [DOI] [Google Scholar]
- 16.AOAC [Association of Official Agricultural Chemists] International . Official methods of analysis of AOAC International. 20th ed. Rockville, MD: AOAC International; 2016. Method 930.29 948.15, 952.08, 985.29, 992.23. [Google Scholar]
- 17.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flickinger EA, Schreijen EMWC, Patil AR, Hussein HS, Grieshop CM, Merchen NR, et al. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J Anim Sci. 2003;81:2008–18. doi: 10.2527/2003.8182008x. [DOI] [PubMed] [Google Scholar]
- 19.Kiarie E, Nyachoti CM, Slominski BA, Blank G. Growth performance, gastrointestinal microbial activity, and nutrient digestibility in early-weaned pigs fed diets containing flaxseed and carbohydrase enzyme. J Anim Sci. 2007;85:2982–93. doi: 10.2527/jas.2006-481. [DOI] [PubMed] [Google Scholar]
- 20.Emiola IA, Opapeju FO, Slominski BA, Nyachoti CM. Growth performance and nutrient digestibility in pigs fed wheat distillers dried grains with solubles-based diets supplemented with a multicarbohydrase enzyme. J Anim Sci. 2009;87:2315–22. doi: 10.2527/jas.2008-1195. [DOI] [PubMed] [Google Scholar]
- 21.de Vries S, Pustjens AM, Schols HA, Hendriks WH, Gerrits WJJ. Improving digestive utilization of fiber-rich feedstuffs in pigs and poultry by processing and enzyme technologies: a review. Anim Feed Sci Technol. 2012;178:123–38. doi: 10.1016/j.anifeedsci.2012.10.004. [DOI] [Google Scholar]
- 22.Walsh MC, Geraert PA, Maillard R, Kluess J, Lawlor PG. The effect of a non-starch polysaccharide-hydrolysing enzyme (Rovabio® Excel) on feed intake and body condition of sows during lactation and on progeny growth performance. Animal. 2012;6:1627–33. doi: 10.1017/S1751731112000237. [DOI] [PubMed] [Google Scholar]
- 23.Choct M, Selby EAD, Cadogan DJ, Campbell RG. Effect of liquid to feed ratio, steeping time, and enzyme supplementation on the performance of weaner pigs. Aust J Agric Res. 2004;55:247–52. doi: 10.1071/AR03106. [DOI] [Google Scholar]
- 24.O’Shea CJ, Mc Alpine PO, Solan P, Curran T, Varley PF, Walsh AM, et al. The effect of protease and xylanase enzymes on growth performance, nutrient digestibility, and manure odour in grower–finisher pigs. Anim Feed Sci Technol. 2014;189:88–97. doi: 10.1016/j.anifeedsci.2013.11.012. [DOI] [Google Scholar]
- 25.Bedford MR, Schulze H. Exogenous enzymes for pigs and poultry. Nutr Res Rev. 1998;11:91–114. doi: 10.1079/NRR19980007. [DOI] [PubMed] [Google Scholar]
- 26.Varel VH, Pond WG, Yen JT. Influence of dietary fiber on the performance and cellulase activity of growing-finishing swine. J Anim Sci. 1984;59:388–93. doi: 10.2527/jas1984.592388x. [DOI] [PubMed] [Google Scholar]
- 27.Wenk C. The role of dietary fibre in the digestive physiology of the pig. Anim Feed Sci Technol. 2001;90:21–33. doi: 10.1016/S0377-8401(01)00194-8. [DOI] [Google Scholar]
- 28.Owusu-Asiedu A, Patience JF, Laarveld B, Van Kessel AG, Simmins PH, Zijlstra RT. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. J Anim Sci. 2006;84:843–52. doi: 10.2527/2006.844843x. [DOI] [PubMed] [Google Scholar]
- 29.Agyekum AK, Sands JS, Regassa A, Kiarie E, Weihrauch D, Kim WK, et al. Effect of supplementing a fibrous diet with a xylanase and β-glucanase blend on growth performance, intestinal glucose uptake, and transport-associated gene expression in growing pigs. J Anim Sci. 2015;93:3483–93. doi: 10.2527/jas.2015-9027. [DOI] [PubMed] [Google Scholar]
- 30.Anguita M, Gasa J, Nofrarias M, Martín-Orúe SM, Pérez JF. Effect of coarse ground corn, sugar beet pulp and wheat bran on the voluntary intake and physicochemical characteristics of digesta of growing pigs. Livest Sci. 2007;107:182–91. doi: 10.1016/j.livsci.2006.09.016. [DOI] [Google Scholar]
- 31.Laitat M, Antoine N, Cabaraux JF, Cassart D, Mainil J, Moula N, et al. Influence of sugar beet pulp on feeding behavior, growth performance, carcass quality and gut health of fattening pigs. Biotechnol Agron Soc Environ. 2015;19:20–31. [Google Scholar]
- 32.Kerr BJ, Gabler NK, Shurson GC. Formulating diets containing corn distillers dried grains with solubles on a net energy basis: effects on pig performance and on energy and nutrient digestibility. Prof Anim Sci. 2015;31:497–503. doi: 10.15232/pas.2015-01445. [DOI] [Google Scholar]
- 33.Millet S, Meyns T, Aluwé M, De Brabander D, Ducatelle R. Effect of grinding intensity and crude fibre content of the feed on growth performance and gastric mucosa integrity of growing–finishing pigs. Livest Sci. 2010;134:152–4. doi: 10.1016/j.livsci.2010.06.123. [DOI] [PubMed] [Google Scholar]
- 34.Kim HB, Borewicz K, White BA, Singer RS, Sreevatsan S, Tu ZJ, et al. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Vet Microbiol. 2011;153:124–33. doi: 10.1016/j.vetmic.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Pandey S, Kim ES, Cho JH, Song M, Doo H, Kim S, et al. Swine gut microbiome associated with non-digestible carbohydrate utilization. Front Vet Sci. 2023;10:1231072. doi: 10.3389/fvets.2023.1231072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu G, Li P, Hou L, Niu Q, Pu G, Wang B, et al. Metagenomic analysis reveals new microbiota related to fiber digestion in pigs. Front Microbiol. 2021;12:746717. doi: 10.3389/fmicb.2021.746717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patil Y, Gooneratne R, Ju XH. Interactions between host and gut microbiota in domestic pigs: a review. Gut Microbes. 2020;11:310–34. doi: 10.1080/19490976.2019.1690363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Tun HM, Li R, Gonzalez BJM, Keenes HC, Nyachoti CM, et al. Impact of xylanases on gut microbiota of growing pigs fed corn- or wheat-based diets. Anim Nutr. 2018;4:339–50. doi: 10.1016/j.aninu.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni JQ, Robarge WP, Xiao C, Heber AJ. Volatile organic compounds at swine facilities: a critical review. Chemosphere. 2012;89:769–88. doi: 10.1016/j.chemosphere.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 40.Zhu J, Jacobson LD. Correlating microbes to major odorous compounds in swine manure. J Environ Qual. 1999;28:737–44. doi: 10.2134/jeq1999.00472425002800030001x. [DOI] [Google Scholar]
- 41.Trabue S, Kerr B, Bearson B, Ziemer C. Swine odor analyzed by odor panels and chemical techniques. J Environ Qual. 2011;40:1510–20. doi: 10.2134/jeq2010.0522. [DOI] [PubMed] [Google Scholar]
- 42.Fan P, Li L, Rezaei A, Eslamfam S, Che D, Ma X. Metabolites of dietary protein and peptides by intestinal microbes and their impacts on gut. Curr Protein Pept Sci. 2015;16:646–54. doi: 10.2174/1389203716666150630133657. [DOI] [PubMed] [Google Scholar]
- 43.Liu P, Zhao J, Guo P, Lu W, Geng Z, Levesque CL, et al. Dietary corn bran fermented by Bacillus subtilis MA139 decreased gut cellulolytic bacteria and microbiota diversity in finishing pigs. Front Cell Infect Microbiol. 2017;7:526. doi: 10.3389/fcimb.2017.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Tsai T, Deng F, Wei X, Chai J, Knapp J, et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome. 2019;7:109. doi: 10.1186/s40168-019-0721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umu ÖCO, Frank JA, Fangel JU, Oostindjer M, Da Silva CS, Bolhuis EJ, et al. Resistant starch diet induces change in the swine microbiome and a predominance of beneficial bacterial populations. Microbiome. 2015;3:16. doi: 10.1186/s40168-015-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taleb S. Tryptophan dietary impacts gut barrier and metabolic diseases. Front Immunol. 2019;10:2113. doi: 10.3389/fimmu.2019.02113. [DOI] [PMC free article] [PubMed] [Google Scholar]