Abstract
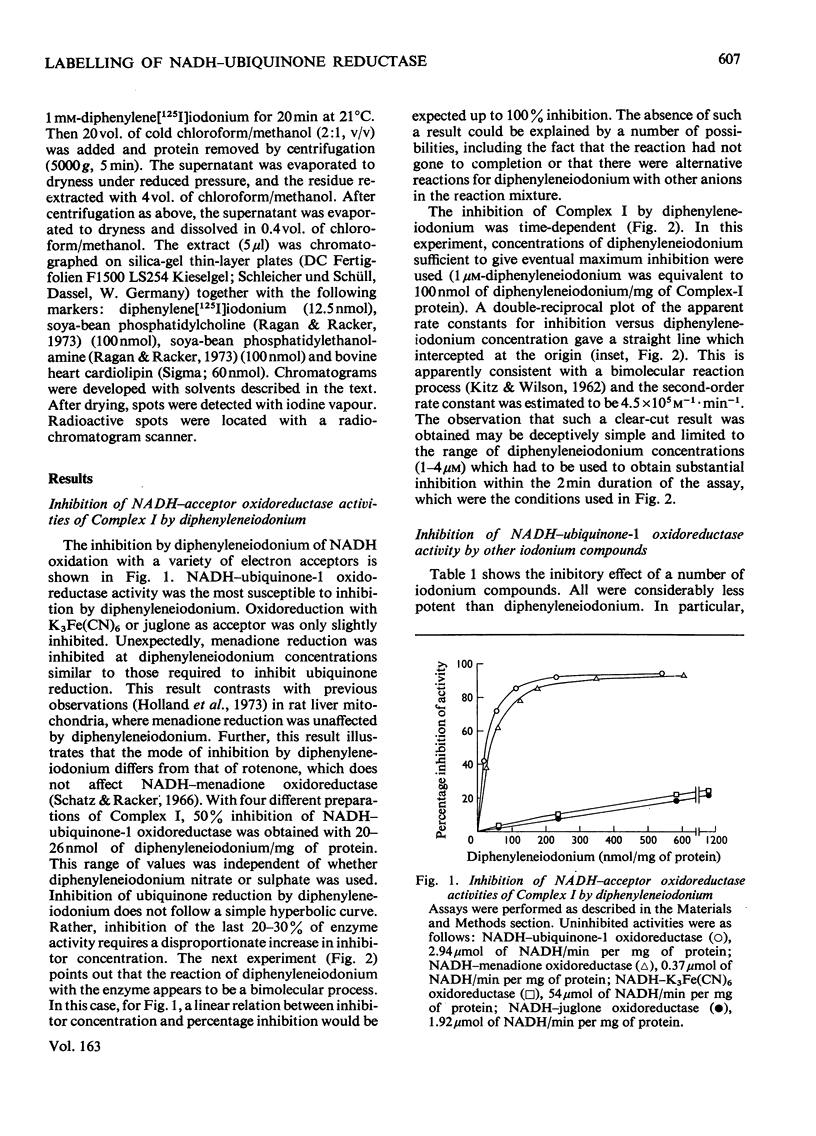
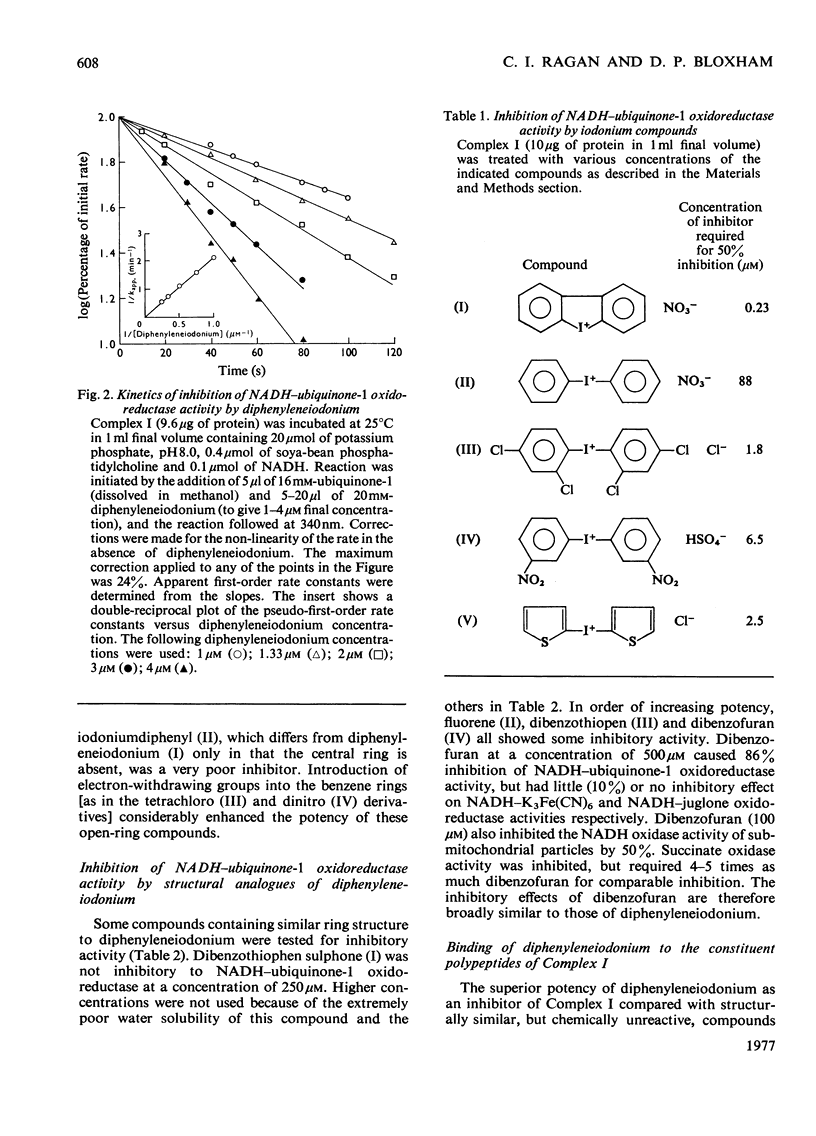
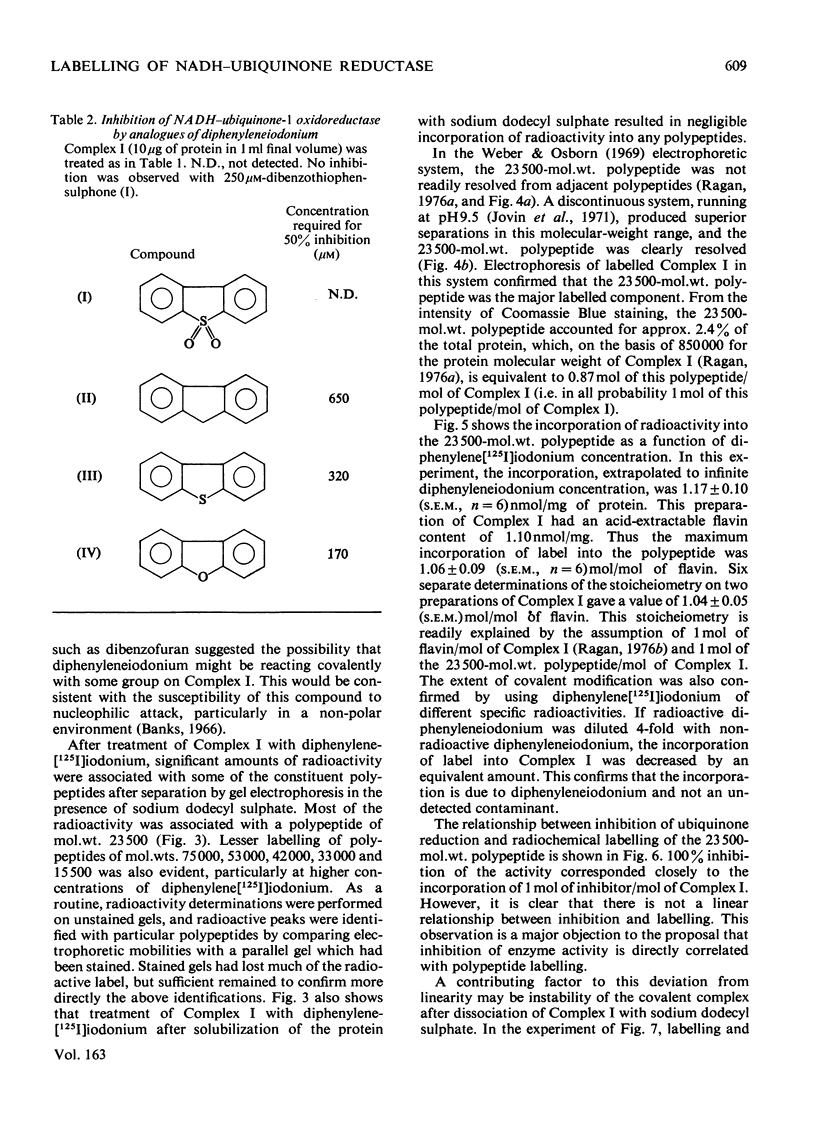
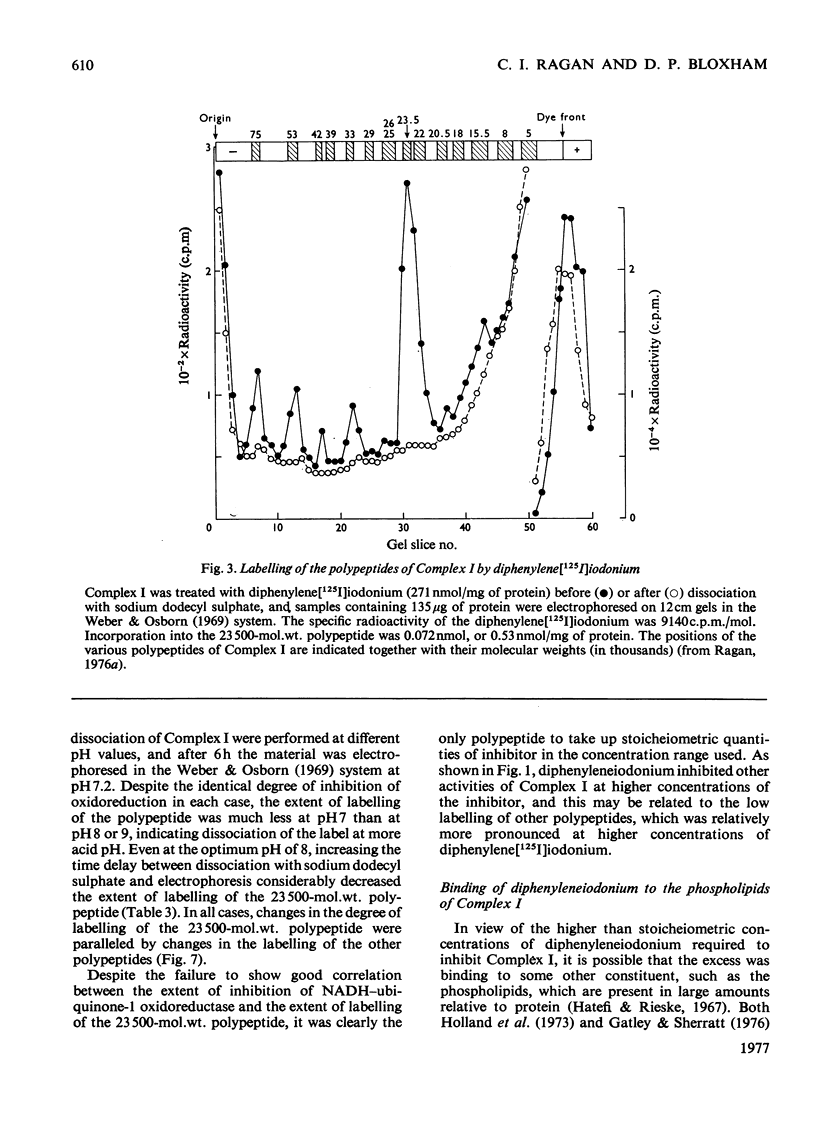
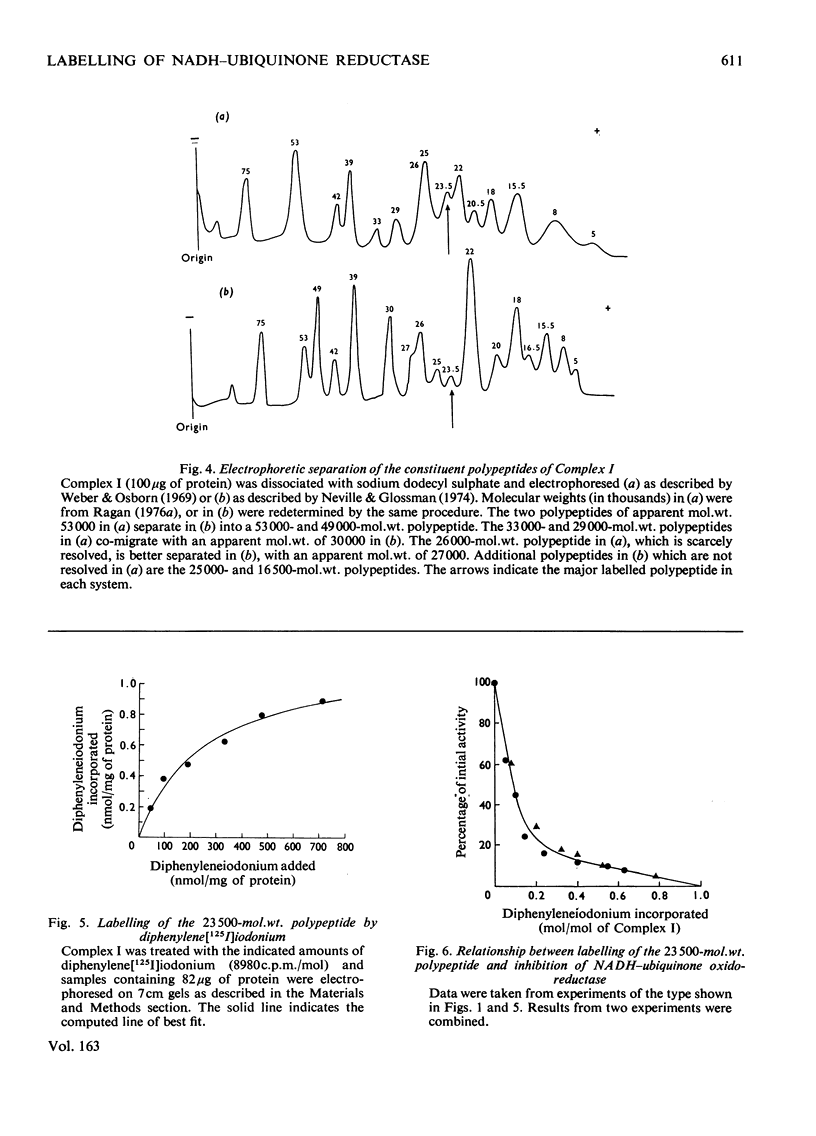
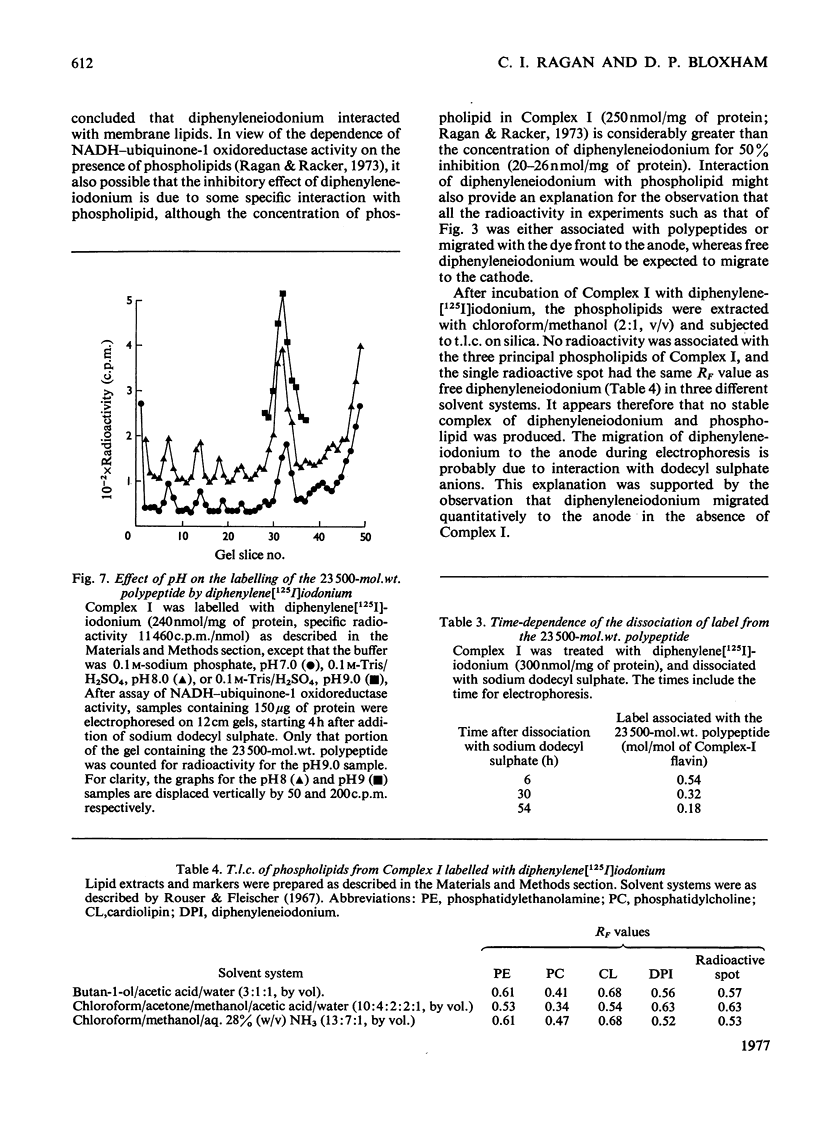
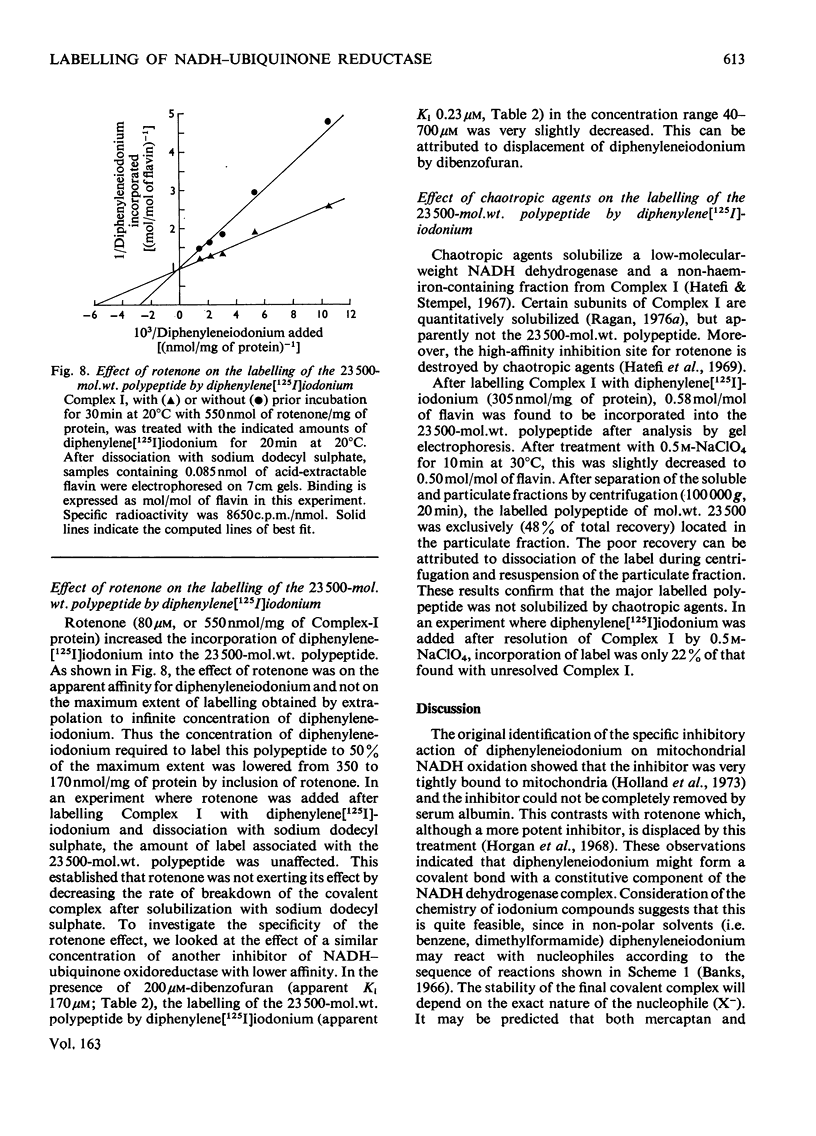
1. NADH-ubiquinone-1 and NADH-menadione reductase activities of Complex I were inhibited by diphenyleneiodonium (apparent Ki 23 and 30 nmol/mg of protein respectively). Reduction of K3Fe(CN)6 and juglone was relatively unaffected. 2. Iodoniumdiphenyl and derivatives were much less effective inhibitors. Compounds with similar ring structures to diphenyleneiodonium, in particular dibenzofuran, were inhibitors of NADH-ubiquinone-1 oxidoreductase. 3. Diphenylene[125I]iodonium specifically labelled a polypeptide of mol.wt. 23500. Maximum incorporation was 1 mol/mol of Complex-I flavin or 1 mol/mol of the 23500-mol.wt. polypeptide. 4. The label associated with this polypeptide was of limited stability, especially at lower pH. 5. Complete inhibition of ubiquinone reduction was achieved when 1 mol of inhibitor was incorporated/mol of Complex-I flavin, but the relationship between inhibition and labelling was not linear. 6. No evidence for covalent interaction between diphenyleneiodonium and the phospholipids of Complex I was obtained. 7. Rotenone increased the apparent affinity of diphenyleneiodonium for the 23500-mol.wt. polypeptide without affecting the maximum incorporation. 8. The 23500-mol.wt. polypeptide was not solubilized by chaotropic agents. Prior treatment of Complex I with chaotropic agents or sodium dodecyl sulphate prevented incorporation of diphenyleneiodonium into this polypeptide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gatley S. J., Sherratt S. A. The effects of diphenyleneiodonium on mitochondrial reactions. Relation of binding of diphenylene[125I]iodonium to mitochondria to the extent of inhibition of oxygen uptake. Biochem J. 1976 Aug 15;158(2):307–315. doi: 10.1042/bj1580307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman M., Singer T. P., Casida J. E. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. XVII. Reaction sites of piericidin A and rotenone. J Biol Chem. 1970 Apr 25;245(8):1992–1997. [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., GRIFFITHS D. E. Studies on the electron transfer system. XL. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. J Biol Chem. 1962 May;237:1676–1680. [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E., Hanstein W. G. Inhibitors and activators of the mitochondrial reduced diphosphopyridine nucleotide dehydrogenase. J Biol Chem. 1969 May 10;244(9):2358–2365. [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Resolution of complex I (DPNH-coenzyme Q reductase) of the mitochondrial electron transfer system. Biochem Biophys Res Commun. 1967 Feb 8;26(3):301–308. doi: 10.1016/0006-291x(67)90122-2. [DOI] [PubMed] [Google Scholar]
- Holland P. C., Clark M. G., Bloxham D. P., Lardy H. A. Mechanism of action of the hypoglycemic agent diphenyleneiodonium. J Biol Chem. 1973 Sep 10;248(17):6050–6056. [PubMed] [Google Scholar]
- Horgan D. J., Singer T. P., Casida J. E. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. 13. Binding sites of rotenone, piericidin A, and amytal in the respiratory chain. J Biol Chem. 1968 Feb 25;243(4):834–843. [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- LINDAHL P. E., OBERG K. E. The effect of rotenone on respiration and its point of attack. Exp Cell Res. 1961 Mar;23:228–237. doi: 10.1016/0014-4827(61)90033-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASSEY V., SWOBODA B. E. THE FLAVIN COMPOSITION OF PIG HEART MUSCLE PREPARATIONS. Biochem Z. 1963;338:474–484. [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 1974;32:92–102. doi: 10.1016/0076-6879(74)32012-5. [DOI] [PubMed] [Google Scholar]
- RACKER E. Studies of factors involved in oxidative phosphorylation. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1659–1663. doi: 10.1073/pnas.48.9.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan C. I. NADH-ubiquinone oxidoreductase. Biochim Biophys Acta. 1976 Nov 30;456(3-4):249–290. doi: 10.1016/0304-4173(76)90001-x. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Racker E. Resolution and reconstitution of the mitochondrial electron transport system. IV. The reconstitution of rotenone-sensitive reduced nicotinamide adenine dinucleotide-ubiquinone reductase from reduced nicotinamide adenine dinucleotide dehydrogenase and phospholipids. J Biol Chem. 1973 Oct 10;248(19):6876–6884. [PubMed] [Google Scholar]
- Ragan C. I. The structure and subunit composition of the particulate NADH-ubiquinone reductase of bovine heart mitochondria. Biochem J. 1976 Feb 15;154(2):295–305. doi: 10.1042/bj1540295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool C. G., Nicolaidis S., Akhtar M. The asymmetric distribution of enzymic activity between the six subunits of bovine liver glutamate dehydrogenase. Use of D- and L-glutamyl alpha-chloromethyl ketones (4-amino-6-chloro-5-oxohexanoic acid. Biochem J. 1976 Sep 1;157(3):675–686. doi: 10.1042/bj1570675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. VII. Oxidative phosphorylation in the diphosphopyridine nucleotide-cytochrome b segment of the respiratory chain: assay and properties in submitochondrial particles. J Biol Chem. 1966 Mar 25;241(6):1429–1438. [PubMed] [Google Scholar]
- Singer T. P., Gutman M. The DPNH dehydrogenase of the mitochondrial respiratory chain. Adv Enzymol Relat Areas Mol Biol. 1971;34:79–153. doi: 10.1002/9780470122792.ch3. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yates D. W., Duance V. C. The binding of nucleotides and bivalent cations to the calcium-and-magnesium ion-dependent adenosine triphosphatase from rabbit muscle sarcoplasmic reticulum. Biochem J. 1976 Dec 1;159(3):719–728. doi: 10.1042/bj1590719. [DOI] [PMC free article] [PubMed] [Google Scholar]


