Abstract
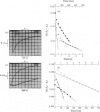
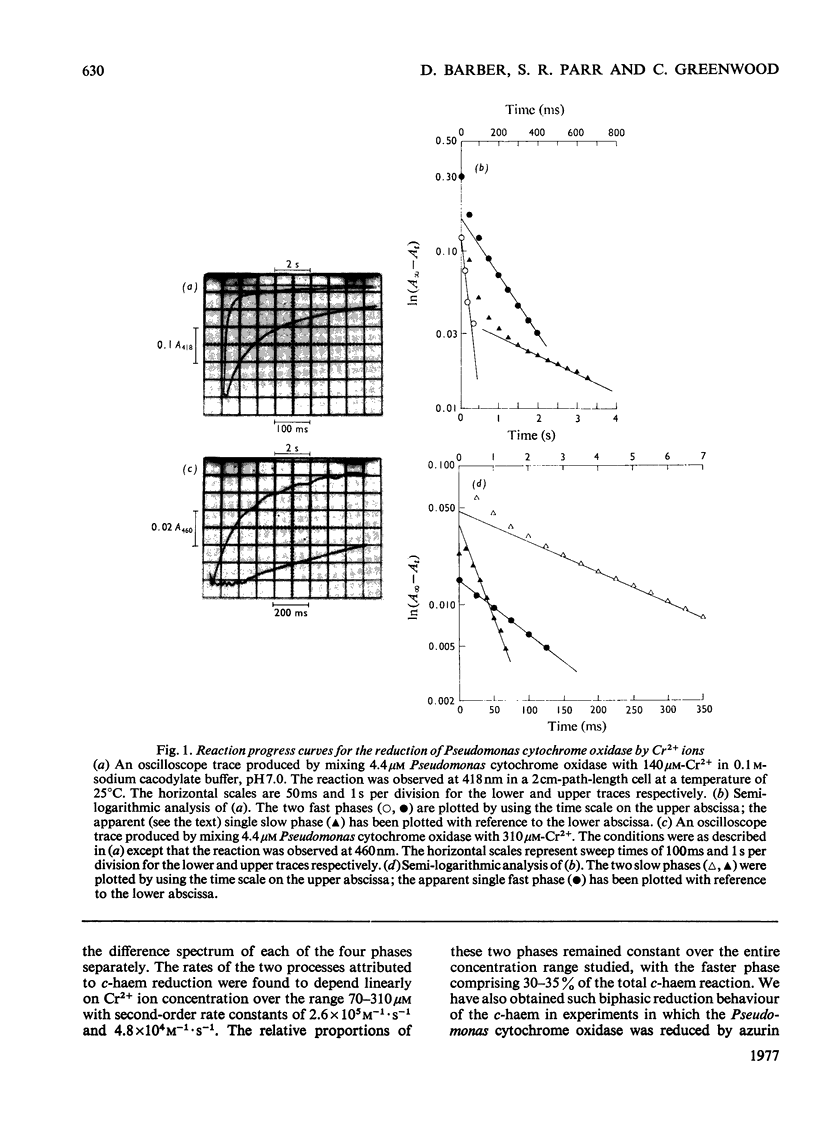
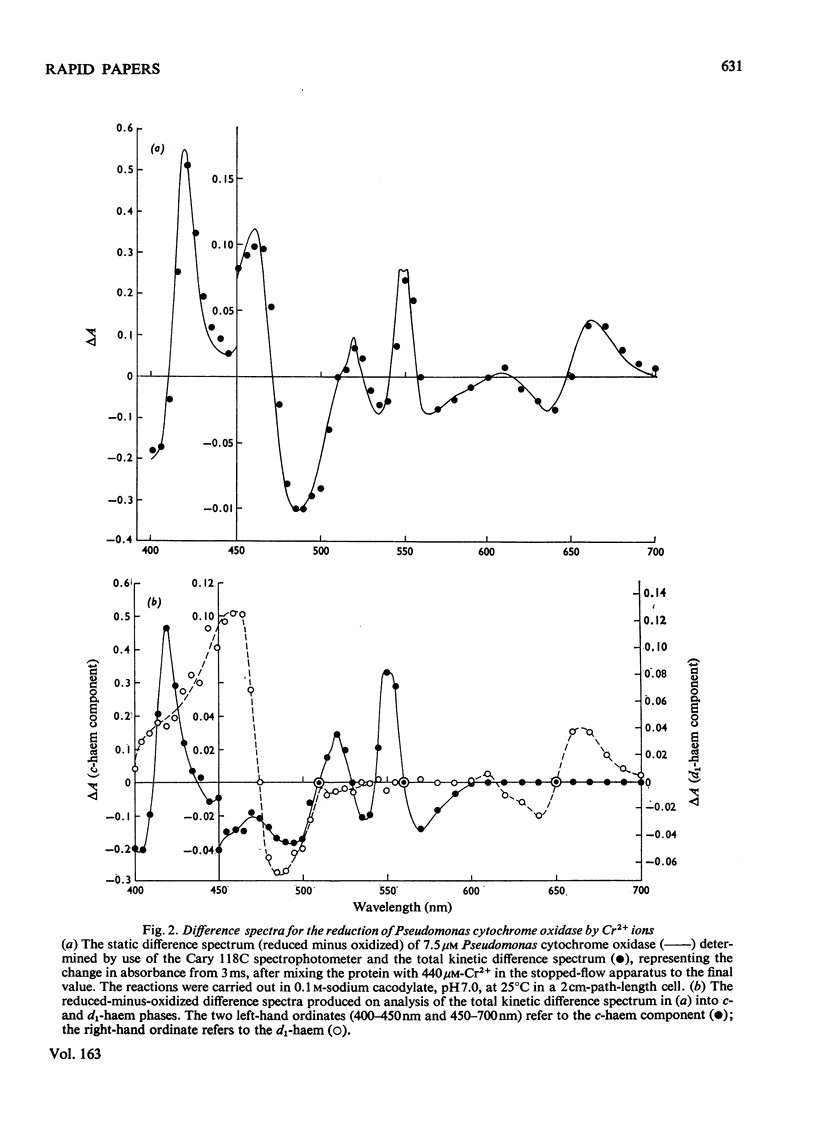
The reduction of cytochrome c551 oxidase from Pseudomonas aeruginosa by Cr2+ ions was followed in the stopped-flow apparatus at a number of wavelengths. The c-haem reduction proceeded in a biphasic fashion with second-order rate constants of 2.6 X 10(5)M-1-S-1 and 4.8 X 10(4)M-1-S-1 at 25 degrees C, whereas the biphasic reduction of the d1-haem appeared to be independent of reductant concentration with rate constants of approx. 1.0S-1 and 0.25S-1 respectively. The kinetically determined difference spectra (reduced minus oxidized) for the c- and d1-haems are presented.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber D., Parr S. R., Greenwood C. Some spectral and steady-state kinetic properties of Pseudomonas cytochrome oxidase. Biochem J. 1976 Aug 1;157(2):431–438. doi: 10.1042/bj1570431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain T., Greenwood C. Kinetic studies on mammalian cytochrome c modified with 2-hydroxy-5-hydroxy-5-nitrobenzyl bromide. Biochem J. 1975 Jul;149(1):179–185. doi: 10.1042/bj1490179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain T., Wilson M. T., Greenwood C. The reduction of carboxymethyl-cytochrome c by chromous ions. Biochem J. 1974 Aug;141(2):455–461. doi: 10.1042/bj1410455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes C. J., Piszkiewicz D., Fleischer E. B. Electron transfer reactions in biological systems: the reduction of ferricytochrome c by chromous ions. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1408–1412. doi: 10.1073/pnas.71.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., YAMANAKA T., MATSUBARA H., OKUNUKI K. Purification and properties of cytochrome oxidase from Pseudomonas aeruginosa. J Biol Chem. 1961 Mar;236:944–951. [PubMed] [Google Scholar]
- Kowalsky A. A study of the mechanism of electron transfer in cytochrome c. Chromium as a probe. J Biol Chem. 1969 Dec 25;244(24):6619–6625. [PubMed] [Google Scholar]
- Kuronen T., Ellfolk N. A new purification procedure and molecular properties of Pseudomonas cytochrome oxidase. Biochim Biophys Acta. 1972 Sep 20;275(3):308–318. doi: 10.1016/0005-2728(72)90212-5. [DOI] [PubMed] [Google Scholar]
- Parr S. R., Barber D., Greenwood C. A purification procedure for the soluble cytochrome oxidase and some other respiratory proteins from Pseudomonas aeruginosa. Biochem J. 1976 Aug 1;157(2):423–430. doi: 10.1042/bj1570423. [DOI] [PMC free article] [PubMed] [Google Scholar]