Abstract
Human plasma alpha1 proteinase inhibitor is the body's principal modulator of serine proteinases (such as those released from phagocytic cells). Cysteine-active-site proteinases, which are not inhibited, have now been found to inactivate this important inhibitor by proteolytic cleavage of a scissile peptide bond. Papain carries out this inactivation catalytically, whereas cathepsin B1 acts stoicheiometrically. Thus thiol proteinases could easily disrupt the delicately regulated balance between serine proteinases and alpha1 proteinase inhibitor.
Full text
PDF
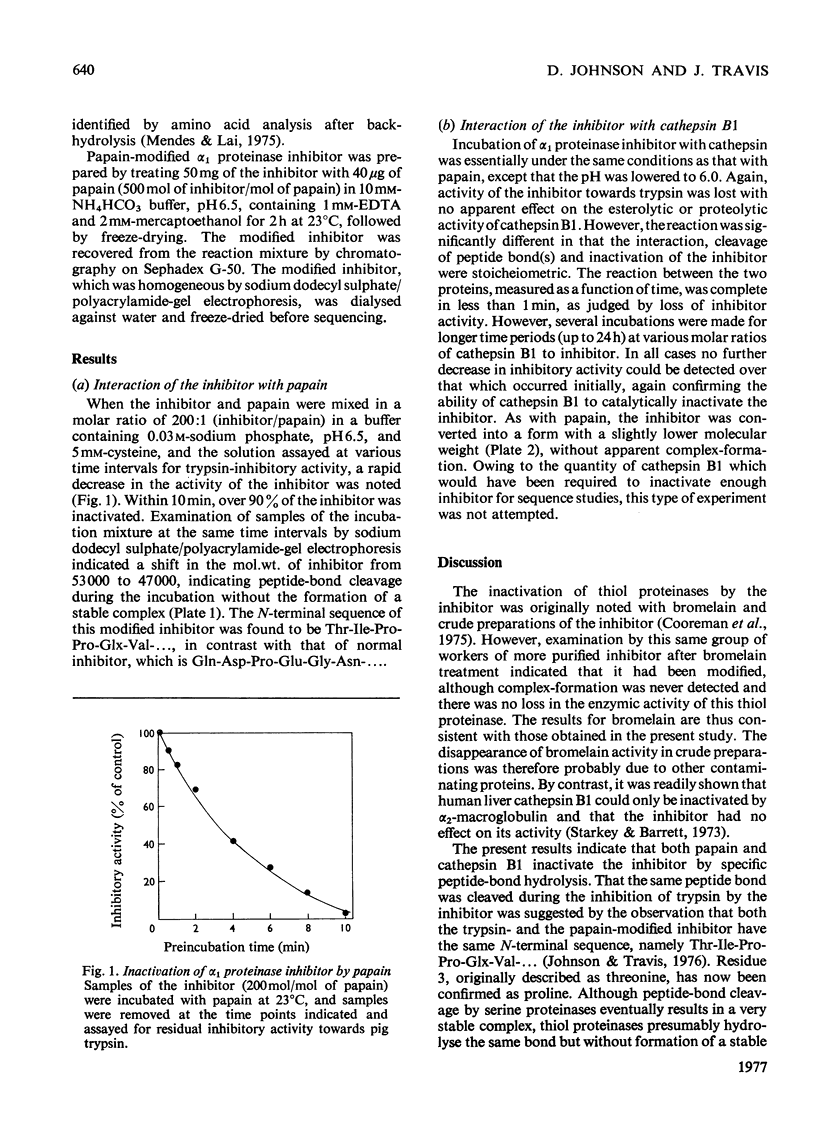
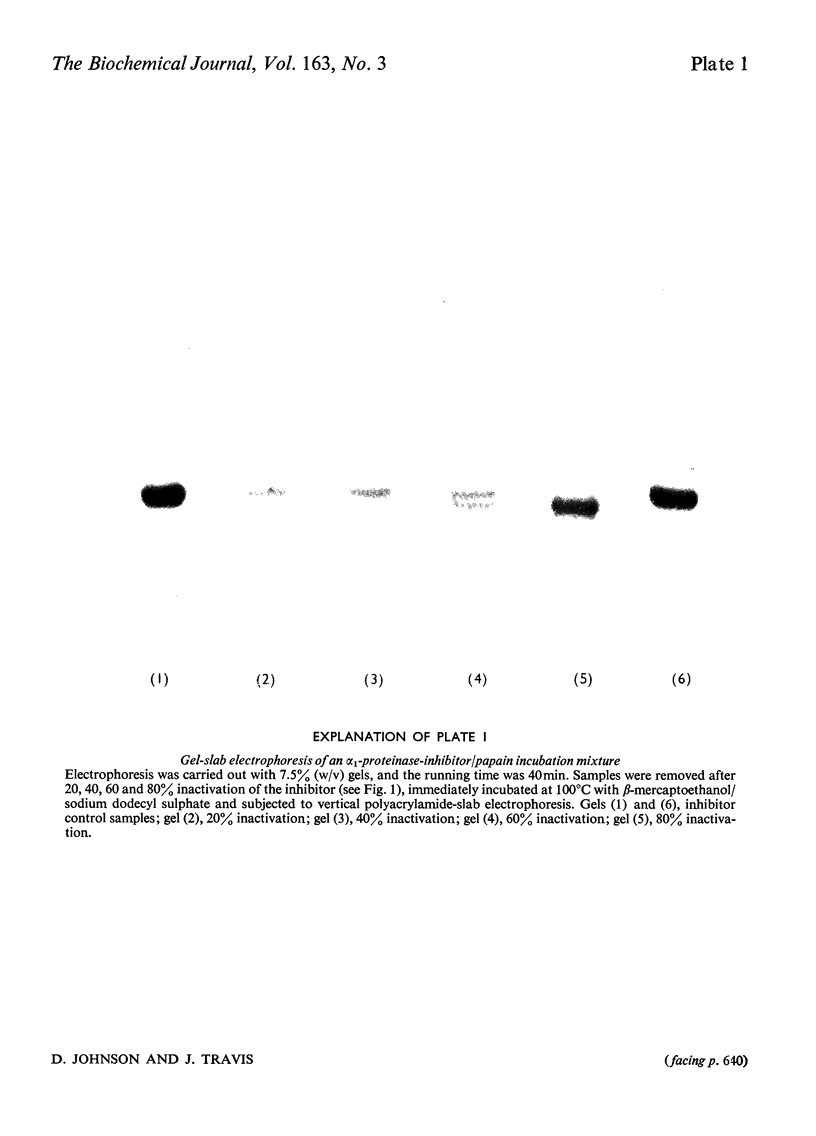
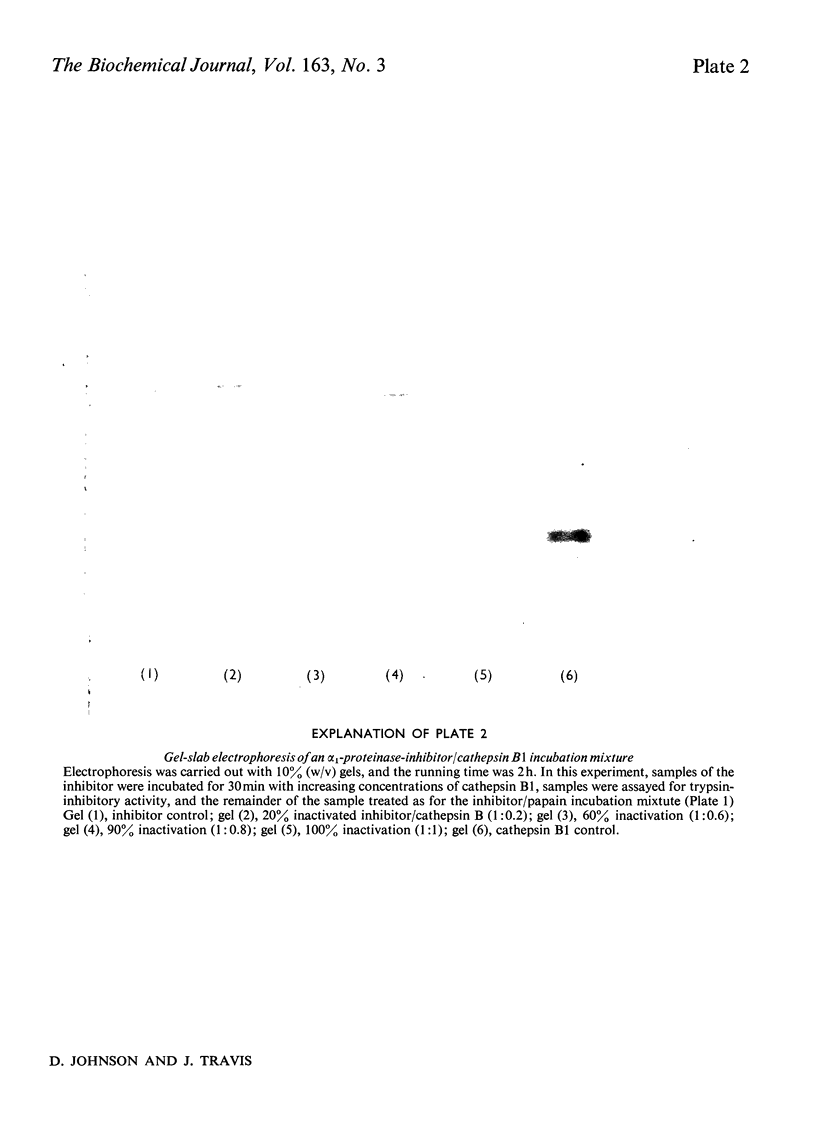

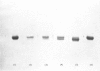
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- GROSS P., BABYAK M. A., TOLKER E., KASCHAK M. ENZYMATICALLY PRODUCED PULMONARY EMPHYSEMA; A PRELIMINARY REPORT. J Occup Med. 1964 Dec;6:481–484. [PubMed] [Google Scholar]
- Goldring I. P., Greenburg L., Ratner I. M. On the production of emphysema in Syrian hamsters by aerosol inhalation of papain. Arch Environ Health. 1968 Jan;16(1):59–60. doi: 10.1080/00039896.1968.10665015. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Travis J. Human alpha-1-proteinase inhibitor mechanism of action: evidence for activation by limited proteolysis. Biochem Biophys Res Commun. 1976 Sep 7;72(1):33–39. doi: 10.1016/0006-291x(76)90956-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martorana P. A., Share N. N. Effect of human alpha-antitrypsin in papain-induced emphysema in the hamster. Am Rev Respir Dis. 1976 May;113(5):607–612. doi: 10.1164/arrd.1976.113.5.607. [DOI] [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Regeneration of amino acids from thiazolinones formed in the Edman degradation. Anal Biochem. 1975 Sep;68(1):47–53. doi: 10.1016/0003-2697(75)90677-6. [DOI] [PubMed] [Google Scholar]
- Pannell R., Johnson D., Travis J. Isolation and properties of human plasma alpha-1-proteinase inhibitor. Biochemistry. 1974 Dec 17;13(26):5439–5445. doi: 10.1021/bi00723a031. [DOI] [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955 Apr;16(4):570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Inhibition by alpha-macroglobulin and other serum proteins. Biochem J. 1973 Apr;131(4):823–831. doi: 10.1042/bj1310823. [DOI] [PMC free article] [PubMed] [Google Scholar]