Summary
Background
Glucocorticoids (GC) are potent entrainers of the circadian clock. However, their effects on biological rhythms in chronic human exposure have yet to be studied. Endogenous hypercortisolism (Cushing's Syndrome, CS) is a rare condition in which circadian disruption is sustained by a tumorous source of GC excess, offering the unique opportunity to investigate GC's chronic effects in vivo.
Methods
In a 12-month prospective case–control multicentre trial, the daily fluctuations in the number of circulating peripheral blood mononuclear cells (PBMCs) and the time-specific expression of clock-related genes were analysed in a cohort of 68 subjects, 34 affected by CS and 34 matched controls. Cosinor mixed effects model, rhythmicity algorithms and machine learning techniques were applied to the multi-level dataset.
Findings
Multiple, 5-point daily sampling revealed profound changes in the levels, amplitude, and rhythmicity of several PBMC populations during active CS, only partially restored after remission. Clock gene analyses in isolated PBMCs showed a significant flattening of circadian oscillation of CLOCK, PER1, PER2, PER3, and TIMELESS expression. In active CS, all methods confirmed a loss of rhythmicity of those genes which were circadian in the PBMCs of controls. Most, but not all, genes regained physiological oscillation after remission. Machine learning revealed that while combined time-course sets of clock genes were highly effective in separating patients from controls, immune profiling was efficient even as single time points.
Interpretation
In conclusion, the oscillation of circulating immune cells is profoundly altered in patients with CS, representing a convergence point of circadian rhythm disruption and metabolic and steroid hormone imbalances. Machine learning techniques proved the superiority of immune profiling over parameters such as cortisol, anthropometric and metabolic variables, and circadian gene expression analysis to identify CS activity.
Funding
The research leading to these results has received funding from the European Union in the context of the National Recovery and Resilience Plan, Investment PE8 – Project Age-It: “Ageing Well in an Ageing Society”. This resource was co-financed by the Next Generation EU [DM 1557 11.10.2022], the PRecisiOn Medicine to Target Frailty of Endocrine-metabolic Origin (PROMETEO) project (NET-2018-12365454) by the Italian Ministry of Health, and through internal funding to Sapienza University of Rome.
Keywords: Cushing's syndrome, Hypercortisolism, Circadian rhythm, Glucocorticoids, Immune system
Research in context.
Evidence before this study
Excessive glucocorticoid (GC) exposure is associated with poor health outcomes, and the number of patients developing signs and symptoms due to hypercortisolism is increasing. Endogenous hypercortisolism (Cushing's Syndrome, CS) is a rare disease (2–3/million/year) characterised by a loss of circadian cortisol rhythmicity, which triggers multiple complications and ultimately increases mortality. Despite CS being a unique human model of circadian disruption from an endogenous cause, few studies have characterised the associated alterations in circadian clock genes and dynamics of circulating immune cells.
Added value of this study
In this multicentre clinical trial, we provide an extensive and thorough analysis of time-course clock genes and immune and hormonal parameters in a large cohort of patients with CS before and after remission. We also described circadian clock genes and immune parameters in an inclusive control cohort of mixed ages and sexes. First, we showed that patients with hypercortisolism have a unique signature alteration of the immune system and impaired rhythmicity of immune cells. The analyses of circadian genes revealed a complete loss of rhythmicity during the active phase that almost completely normalised during remission. Conversely, not all immune parameters reached levels comparable to controls. The restoration of clock gene expression in immune cells, following the normalisation of cortisol rhythmicity, confirms a causality link between the two. In contrast, the persistence of some alterations in immune cell profiles suggests a more complex interaction with a carry-over effect explaining the symptoms complained by cured patients. We included bioinformatic techniques to provide a multi-level analysis of active and cured CS, identifying the immune system and circadian genes as powerful tools for monitoring hypercortisolism.
Implications of all the available evidence
Finding a reliable peripheral biomarker of GC action has been a conundrum for physicians for over a century. Given the availability of novel, effective medical therapies able to normalise the 24-h cortisol levels in CS, the importance of restoring a healthy circadian rhythm is gaining momentum. The results of this trial demonstrate that circadian immune profiling is a sensitive biomarker of CS activity and endogenous rhythm disruption. Gene expression in peripheral blood mononuclear cells offers a rapid and readily available display for chronobiological interventions in managing conditions associated with circadian disruption, such as exogenous GC therapies, metabolic diseases, and cancer. However, restoring clock gene rhythmicity does not necessarily clear the immune phenotype, revealing different temporal and hierarchical levels. All these findings directly impact the clinical management of patients chronically exposed to GC and, more generally, for chrono-pharmacological interventions.
Introduction
The relevance of night and day rhythmicity, known since ancient times, has only recently acquired a clinical meaning. Such delay is probably the result of the disruptive influence of modern lifestyle, commodities, artificial lights, constant food availability, and drugs, which overcome the resilience of the inner biological clocks, providing the basis for circadian disruption and its damaging implications.1,2
Working categories such as night shift workers and frequent fliers prone to altered circadian rhythm have shown an increased risk for metabolic,3 neoplastic diseases,4,5 or both.6 However, earlier than behavioural factors, some rare clinical conditions had provided a model for the disruption of endogenous rhythm. Cortisol is one of the most circadian hormones, peaking just before awakening and falling to undetectable levels at night. Cortisol is also a potent entrainer for most of our body's tissues by synchronising peripheral clocks with the central circadian pacemaker.7,8 Cushing's Syndrome (CS) is a rare condition with an incidence ranging between 2 and 3/million9 to 8/million10 annually, offering a unique model of circadian rhythm disruption.
In CS, cortisol rhythm is precociously lost because of an abnormal hormonal secretion from the pituitary or ACTH-secreting ectopic tumours (ACTH-dependent CS) or the adrenal gland (ACTH-independent CS). Compared to other conditions affecting daily rhythms, in CS, the cause of disruption is endogenous and prolonged. The abnormal cortisol secretion, lasting several months or years before the diagnosis is established,11 turns the potent hormonal entrainer into a factor chronically desynchronising day–night activities from hormonal profiles and clock-related gene expression. For ethical reasons, such a model cannot be otherwise reproduced in humans and offers the advantage of overcoming the “buffer” mechanisms that arise in human night-shift, sleep disturbance (spontaneous or experimentally induced) or jet-lag studies.
Patients with CS often develop multi-organ complications and comorbidities, including cardiovascular diseases, hypercoagulability, hypertension, osteoporosis, infertility, central obesity, myopathy and infectious diseases.12, 13, 14 Most complications share immune15,16 and metabolic features and mechanisms,12 since, for example, visceral adipose tissue deposition leading to central obesity recruits inflammatory monocytes from peripheral blood, or neutrophil alterations can lead to hypercoagulability and atherosclerosis.13,15 Even after remission, patients can experience long-term effects of chronic exposure to increased GC.17 However, the role of circadian rhythm in the development of these complications and their reversal after treatment has not yet been elucidated because of the disease's rarity and the complexity of human models. Specifically, an in-depth analysis of daily variations of immune cells in CS is still lacking.
To investigate the endogenous clock in CS as a biomarker of disease activity and severity, its role in the development of complications and the effects of treatment and remission, we designed a multi-level approach to a multicentre clinical trial, enrolling a large cohort of patients with CS during the active phase and after remission, compared with healthy controls. Bioinformatic analyses have proven superior to conventional methods in recognising circadian patterns,18 especially in human studies, thanks to their ability to reduce experimental noises, interindividual differences and confounding factors. Taking advantage of such an approach, we provided a combined analysis of this rare disease's circadian genes, immunity, and cortisol profiling.
Methods
Study design and participants
The longitudinal, prospective, case–control clinical trial was conducted in four Italian centres (Sapienza University of Rome, Federico II University of Naples, “Ospedali Riuniti” University Hospital of Ancona and University Hospital of Padova). The sample size was calculated based on published literature on CLOCK and ARNTL relative gene expression in patients affected by circadian-disrupting conditions, such as alcoholism19 or adrenal insufficiency.20 Based on mean relative gene expression in freshly isolated PBMC, assuming a pooled SD of 0.981 (for CLOCK), a sample size respectively of 21 or 27 in each group would have had an 80% or 90% power to detect an effect size of 0.90 with a 5% two-sided significance level. Assuming a drop-out rate of 15% for potential clinical and technical issues in sample processing and quality analysis based on our previous experience, a sample size of 32 subjects for each group was considered the ideal recruitment. The calculated sample size is consistent with published studies on circadian gene expression in patients with Cushing's Syndrome21 or healthy subjects.22,23 A post-hoc power analysis based on CLOCK expression using CircaPower,24 a useful tool to estimate power analysis in circadian studies, with power set to 90% or 80% estimated a minimum sample requirement of 8 controls and 11 patients with CS or 7 controls and 9 patients with CS, respectively. Eligible patients were men and women aged 18–80 with CS, diagnosed according to guidelines.9,25 Age- and sex-matched healthy controls were enrolled with a 1:1 ratio at the coordinating centre. Controls were invited to enter the current study by random selection from a clinical trial26 that collected a large number of subjects referring to the coordinating center for screening procedures (thyroid nodule screening, andrological screening).
Exclusion criteria at baseline for patients were significant renal, hepatic and respiratory diseases, not-compensated diabetes mellitus (HbA1c ≥ 7.5%), neoplasms (except for pituitary/adrenal adenomas and ACTH-secreting neoplasms), surgery, severe infective or rheumatologic diseases in the 3 months before study evaluation, pregnancy, concomitant medications interfering with protocol analyses (anti-inflammatory, melatonin and other sleep modulating drugs, glucocorticoids other than replacement for adrenal insufficiency after remission, and medical treatment of hypercortisolism). Exclusion criteria for controls were significant renal, hepatic and respiratory diseases, not-compensated diabetes mellitus (HbA1c ≥ 7.5%), neoplasms, surgery, severe infective or rheumatologic diseases in the 3 months before study evaluation, pregnancy, concomitant medications interfering with protocol analyses (anti-inflammatory, melatonin and other sleep modulating drugs, glucocorticoids), sleep disturbances and night-shift working.
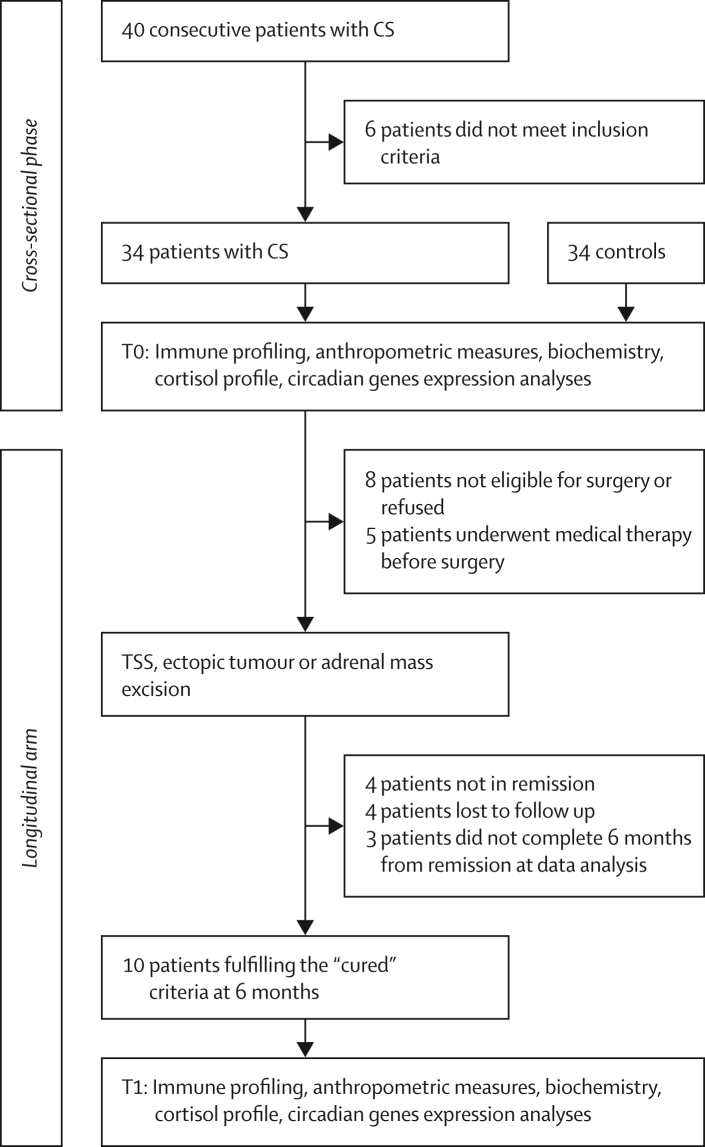
Patients with CS were evaluated at baseline and six months (± one month) after remission. Remission criteria for patients with CS was postoperative (24–48-h) serum cortisol ≤50 nmol/L. Before the 6 months procedures, patients underwent routine clinical and biochemical re-evaluation to exclude recurrence of disease and baseline hormone analyses or dexamethasone suppression test were performed if needed. No patient presented recurrence of disease within the study timeframe. Patients with disease recurrence, medical treatment before surgery, or those who did not achieve remission were excluded from the longitudinal evaluation. The study flowchart is reported in Fig. 1.
Fig. 1.
Study flow chart: study flow chart for the clinical trial.
Ethics
The trial was approved by the ethical review board of the Policlinico Umberto I Hospital (4945/18), published on public registries (NCT04374721), and conducted under the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all patients. The trial was first approved in 2018, enrolment started in 2020 and ended in 2021.
Procedures
Patients and controls were instructed to conduct stable sleep-night schedules (according to their own preferences) for one week before study procedures. For menstruating participants, blood was sampled during the early follicular phase.27 On the day of admission, patients and controls underwent blood withdrawal by intravenous cannulation to reduce the stress from repeated punctures at 8:00 Ante Meridiem (AM) (before breakfast), 12:00 AM (noon, before lunch), 4:00 Post Meridiem (PM) (after lunch), 8:00 PM (before dinner), and 12:00 PM (midnight). Standard meals with specific timing were administered to avoid interference with circadian entrainment. Bedtime and wake-up time was up to the study participants. Biochemical analyses were performed using standard methods at the central laboratories of each study centre. Hormone assays for cortisol levels were conducted at “Sapienza” University with High Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS/MS). Detailed methods of steroidomic analysis have been described elsewhere.28
Outcomes
The study's primary outcome was comparing patients' and controls' relative expression of CLOCK and ARNTL genes in freshly isolated PBMCs. Secondary outcomes included comparing the relative expression of circadian genes and circadian immune and cortisol profiling between patients and controls at baseline and after remission from CS.
Cytometry and gating strategy
All primary and secondary outcomes analyses were centralised (Sapienza University). PBMCs were isolated from fresh whole blood and gated. Briefly, the monocyte and lymphocyte gate were analysed for CD14 and CD16 expression to identify CD14++CD16-classical monocytes, CD14+CD16+ intermediate monocytes, and CD14+CD16++ non-classical monocytes. Absolute cell counts were derived from the total cell counts provided by the haematological analyser (SYSMEX Roche, Indianapolis, IN, USA). The lymphocyte gate was also analysed for CD3 and CD56 expression to identify CD56+CD3- natural killer cells and CD56−CD3+ T lymphocytes. Natural killer (NK) cells were defined as CD14−CD19−CD3−CD56+ and re-analysed for CD16 expression to identify CD16+ NK cells. NK cells were further analysed based on CD56 density to identify CD56bright cells. Flow cytometry gating strategies are available in the Supplementary (Fig. S1) and RRID tags are provided for all commercial antibodies used in Supplementary Table S1 (Table S1).
Circadian genes analysis
For circadian genes mRNA analyses, samples were obtained at 8:00 AM, 12:00 PM, 4:00 PM, 8:00 PM and 12:00 AM. RNA was extracted from freshly isolated PBMCs and reverse transcribed for PCR amplification. RNA was extracted with an Aurum Total RNA Mini Kit (Bio-Rad, Hercules, CA), followed by a DNase digestion step to remove genomic DNA contamination. Total RNA concentration was quantified with a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA), and purity was estimated by 260 nm/280 nm absorption. We reverse-transcribed 2 μg of RNA from each sample using iScript Reverse Transcription Kit (Bio-Rad). The total cDNA pool obtained served as the template for subsequent PCR amplification in a real-time PCR assay 96-well panel modified for use with SYBR Green circadian rhythms (PrimePCR; Bio-Rad). Primers (including housekeeping genes) were lyophilised in each well using SsoAdvanced Universal SYBR® Green Supermix. The quantitative reverse transcription polymerase chain reaction was run on CFX Connect (Bio-Rad). We selected 19 circadian genes based on our previous work on circadian rhythm in glucocorticoid disorders.20 In addition to the 19 genes tested (ARNTL, CLOCK, MAPK1, PER1, PER2, PER3, TIMELESS, WEE1, AANAT, CAMK2D, CREB1, CREB3, MAT2A, PRKAR1A, PRKAR2A, CSNK1A1, CSNK1E, OPN3, PRF1), each plate contained housekeeping genes for quantitative analyses (GAPDH, ACTB) and specific controls for genomic DNA contamination, RNA quality, and efficiency. Only assays that passed internal controls were included in the database. For data analysis, the Cq expression of housekeeping genes was tested by CFX Manager software (Bio-Rad) to identify the most stable reference gene based on the geometric mean of expression. Most of the reference genes passed the test, with GAPDH and ACTB being the most stably expressed among samples and thus selected for normalisation. No rhythmicity was observed in GAPDH and ACTB expression within our dataset. All gene expression results are expressed as relative expression levels normalised against housekeeping genes.
Statistics
Continuous variables are reported as mean (95% confidence interval) or median (interquartile range) or (25th—75th percentile) when appropriate. Normally distributed variables were assessed using the Shapiro–Wilk test and non-normally distributed variables were corrected by square root or logarithm conversion if possible. Differences between patients with CS and age- and sex-matched controls were evaluated using the Student's t-test for normally distributed variables and the non-parametric Mann–Whitney test for non-normally distributed variables. Differences between the binomial proportions of the two independent groups of a dichotomous-dependent variable were assessed for homogeneity using the chi-square test. A p-value of <0⋅05 was regarded as significant. For circadian analyses, cosinor-based rhythmometry was applied to time-course variables.29 We employed a mixed effects cosinor model to analyse longitudinal periodic data using the R package “cosinoRmixedeffects”, where estimation and hypothesis testing for the non-linear circadian parameters are conducted through bootstrapping. When interpolation of additional time points was needed for improved curve fitting, imputation was also considered. Time series were analysed both as pooled data and individual data sets. The results are reported for individual patients' data sets compared between study groups. The zero-amplitude test was performed to assess rhythmicity. Sex and age interactions in group differences were also explored. Circadian genes were also analysed by a version of the Jonckheere-Terpstra-Kendall (JTK) algorithm designed for the detection of circadian rhythmicity, the JTK_CYCLE algorithm.30 The JTK algorithm was developed by combining the Jonckheere-Terpstra (JT) test and Kendall's tau for optimal detection of monotonic orderings of data across ordered independent groups. The JTK_CYCLE algorithm applies the JTK algorithm to alternative hypothesised group orderings corresponding to a range of user-defined period lengths and phases. The JTK_CYCLE algorithm finds the optimal combination of period and phase that minimises the exact p-value of Kendall's tau correlation between an experimental time series and each tested cyclical ordering. Each minimal p-value is Bonferroni-adjusted for multiple testing. Benjamini-Hochberg correction for multiple comparisons was applied to all tests. Statistical analyses were performed using SPSS, version 27.0 (IBM Corp., Armonk, NY, USA).
Machine learning analyses
High-volume data is often scarcely manageable with conventional statistical techniques, requiring alternative approaches for dimensionality reduction. Our investigation started with applying dimensionality reduction techniques, specifically Principal Component Analysis (PCA) and t-distributed Stochastic Neighbour Embedding (t-SNE). These techniques help distil essential features from the extensive dataset, optimising computational efficiency and model interpretability. Then, we used two machine learning algorithms: k-Nearest Neighbours (k-NN) and Support Vector Machines (SVM). These algorithms were selected for their suitability on small datasets and their ability to uncover patterns and make predictions, complementing each other in the analysis. The first task was the recognition of CS among all study participants while blinding the dataset to the diagnoses, achieved by visual and non-visual separation of positive (patients with CS) and negative (controls) subjects. Then, we explored categories of variables that best identified the two groups. Details on pre-processing and method setups are available in the Supplementary Methods section, along with more information on other bioinformatic analyses.
Role of funders
The funders had no role in the study design, data collection, data analyses, interpretation, or writing of the report.
Results
Study population
Overall, 40 patients with CS were screened, and 34 were prospectively enrolled (Fig. 1), along with 34 sex and age-matched controls. All patients and controls confirmed that their perceived gender coincided with their sex. The mean age of patients at enrolment was 47⋅4 ± 10⋅8 years; 23 were females. 52⋅9% of patients had ACTH-dependent pituitary CS, 38⋅2% had ACTH-independent CS, and 8⋅8% had ACTH-dependent CS due to ectopic ACTH secretion from small cell lung carcinoma. After baseline evaluation, 8 patients were not eligible for the follow-up study or refused surgery; the remaining 26 patients underwent trans-sphenoidal surgery, adrenal cortisol-secreting adenoma or ACTH-secreting ectopic tumour excision. Five patients started medical treatment to control cortisol secretion before surgery and, therefore, were excluded from the follow-up analysis. Of the 21 treatment-naïve operated patients, four were not cured by surgery, four were subsequently lost at follow-up, and three did not complete the six-month remission assessment within the study time frame. Ultimately, ten out of the 21 eligible operated patients (48%) fulfilled the “cured” inclusion criteria at the six-month assessment (Fig. 1). Of those, two patients had regained endogenous cortisol secretion at study evaluation, while eight were under glucocorticoid replacement (two under modified-release hydrocortisone, six under cortisone acetate twice daily).
Patients and controls were age- and sex-matched. As expected, they were significantly different regarding BMI, waist circumference, systolic and diastolic arterial pressure, HbA1c, and triglycerides. Patients with CS had hypertension in 67⋅6% of cases, dyslipidaemia in 61⋅8%, diabetes mellitus in 38⋅2%, osteoporosis in 26⋅5% and hypercoagulability in 32⋅4%. The baseline characteristics of the study population are summarised in Table 1. Baseline 8 AM fasted immune profiling and full blood counts are in the Supplementary.
Table 1.
Baseline characteristics of the study cohort.
| Patients with CS (n = 34) | Controls (n = 34) | pa | |
|---|---|---|---|
| Age (years) | 47.4 (43.8–51.2) | 42.4 (37.6–47.9) | 0.111 |
| Sex (F/M) | 23/11 | 20/14 | 0.204 |
| BMI (kg/m2) | 27.97 (26.15–29.96) | 23.64 (22.56–24.69) | 0.004 |
| Waist circumference (cm) | 99.4 (94.8–104.8) | 77.2 (74.0–80.2) | <0.001 |
| Systolic (mmHg) | 133 (125–140) | 117 (113–120) | 0.001 |
| Diastolic (mmHg) | 83 (79–87) | 76 (73–79) | 0.011 |
| Aetiology of CS | |||
| Pituitary CS n (%) | 18 (52.9) | ||
| Adrenal CS n (%) | 13 (38.2) | ||
| Ectopic CS n (%) | 3 (8.8) | ||
| Comorbidities | |||
| Hypertension n (%) | 23 (67.6) | ||
| Diabetes n (%) | 13 (38.2) | ||
| Dyslipidaemia n (%) | 21 (61.8) | ||
| Osteoporosis n (%) | 9 (26.5) | ||
| Hypercoagulability n (%) | 11 (32.4) | ||
| Biochemistry | |||
| Glycaemia (mg/dL) | 85.27 (80.56–90.83) | 85.77 (83.33–88.57) | 0.864 |
| HbA1c (%) | 5.90 (5.62–6.23) | 5.27 (5.17–5.40) | 0.006 |
| Total cholesterol (mg/dL) | 203 (188–217) | 186 (174–200) | 0.101 |
| HDL cholesterol (mg/dL) | 62 (56–67) | 62 (56–68) | 0.988 |
| LDL cholesterol (mg/dL) | 120 (109–132) | 106 (96–116) | 0.086 |
| Triglycerides (mg/dL) | 114 (100–127) | 92 (77–108) | 0.043 |
Values are expressed as mean (lower-upper limit of 95% CI), median (25th–75th percentile), or percentages (%) as appropriate. n = 34 for active CS and n = 34 for controls for each variable except for systolic and diastolic blood pressure (n = 32 for CS) and HbA1c% (n = 30 for CS and n = 28 for controls).
p value for Mann–Whitney test.
Circadian genes analysis
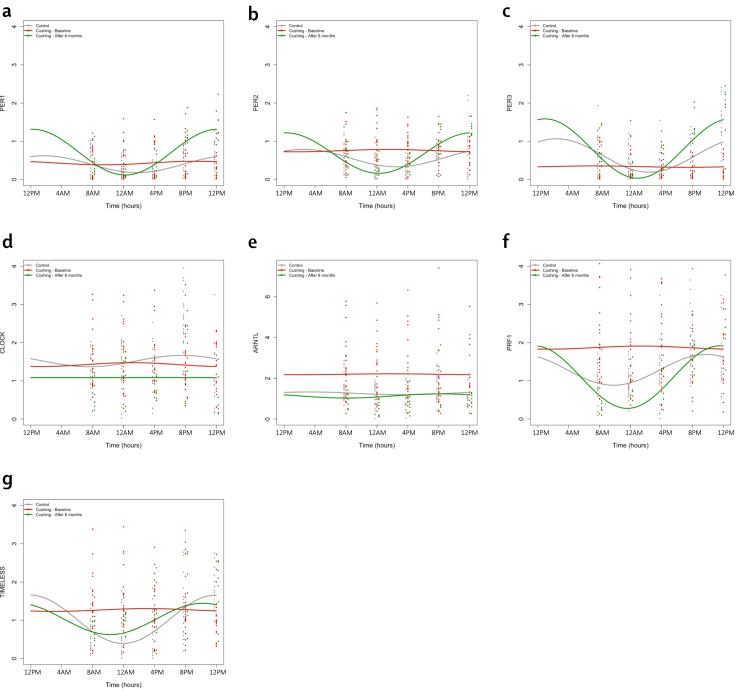
To investigate the effects of CS on peripheral clocks, we applied the mixed effects cosinor model to a set of pre-specified clock-related genes expressed by PBMCs. For each patient, three values were extracted: acrophase (time of peak, when rhythmic), MESOR (Midline Estimating Statistic of Rhythm the mid-value throughout the curve), and amplitude (the distance between the mesor and the highest/lowest value) of the interpolated curve. All results are summarised in Table 2. Collective data are presented in Fig. 2, while standardised coefficients are summarised in Supplementary Table S3 (Table S3). Patients with active CS showed increased mean levels of expression of ARNTL and PRF1, while PER3 was markedly downregulated. Mesor expression of ARNTL and PRF1 normalised after remission, while levels of PER3 increased significantly over control levels. Interestingly, patients with CS in remission had an increased mesor of expression of PER1 and CLOCK compared to controls. The amplitude of several circadian genes was also affected in CS. Specifically, a reduction was observed in PER1, PER2, PER3, and TIMELESS. All the clock genes showed a significant increase in amplitude after remission, reaching levels similar to controls. No differences were observed in acrophase between patients in remission and controls, while acrophase in active CS was not extracted due to loss of rhythmicity in most patients. Age and sex did not affect results except for an age-related effect on CLOCK expression levels (p = 0.030 [interaction analysis]) and amplitude (p = 0.011 [interaction analysis]) in the overall study population. To further investigate the circadian nature of the variables of our data set, we decided to perform additional tests.
Table 2.
Cosinor-based rhythmometry analysis of circadian genes.
| Mesor (relative expression) |
Amplitude (relative expression) |
Acrophase (hours) |
Percentage of rhythmic subjects |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Active CS | CS in remission | Controls | Active CS | CS in remission | Controls | CS in remission | Controls | Active CS | CS in remission | ||
| ARNTL | 1.35 (0.81–1.50) | 1.71 (1.30–3.04)∗∗ | 0.99 (0.60–0.99)°° | 0.55 (0.28–0.75) | 0.33 (0.22–0.63) | 0.34 (0.21–0.49) | 01:03 (22:27–04:47) | 00:22 (20:54–01:03) | 36% | 41% | 38% | |
| CLOCK | 1.46 (1.19–1.69) | 1.37 (0.89–1.68) | 0.95 (0.63–1.12)## | 0.54 (0.36–0.65) | 0.21 (0.15–0.30) | 0.45 (0.32–0.55) | 01:00 (20:49–02:46) | 00:55 (19:40–01:54) | 46% | 33% | 12% | |
| PER1 | 0.34 (0.24–0.49) | 0.16 (0.11–0.70) | 0.66 (0.51–0.77)## | 0.22 (0.18–0.29) | 0.11 (0.05–0.20)∗ | 0.34 (0.22–0.50)° | 03:18 (21:35–05:01) | 19:55 (19:06–21:50) | 73% | 30% | 88% | |
| PER2 | 0.50 (0.32–0.66) | 0.69 (0.47–0.99) | 0.67 (0.57–0.77) | 0.32 (0.19–0.46) | 0.15 (0.09–0.21)∗∗ | 0.30 (0.25–0.34)°° | 02:39 (20:51–04:10) | 22:21 (21:08–00:17) | 55% | 30% | 75% | |
| PER3 | 0.49 (0.28–0.70) | 0.21 (0.12–0.47)∗ | 0.87 (0.69–0.16)°°;# | 0.28 (0.21–0.42) | 0.17 (0.06–0.27)∗ | 0.38 (0.35–0.47)°° | 22:05 (19:49–00:20) | 21:27 (20:15–23:22) | 82% | 41% | 75% | |
| PRF1 | 1.15 (0.91–1.46) | 1.58 (1.08–1.58)∗ | 1.29 (1.07–1.58) | 0.42 (0.25–2.45) | 0.29 (0.13–0.42) | 0.44 (0.27–0.58) | 21:47 (19:40–00:02) | 23:06 (21:24–01:33) | 73% | 26% | 88% | |
| TIMELESS | 0.89 (0.75–1.23) | 1.13 (0.85–1.55) | 1.25 (0.93–1.45) | 0.42 (0.2–0.67) | 0.18 (0.10–0.32)∗∗ | 0.44 (0.40–0.63)°° | 22:57 (20:59–01:21) | 22:05 (19:25–01:10) | 77% | 30% | 63% | |
Results of cosinor-based rhythmometry analysis on the time-course relative expression of circadian genes and percentage of subjects with circadian rhythmicity. Results are expressed as median (25th—75th percentiles). ∗: active CS vs. Controls; °: CS in remission vs. active CS; #: CS in remission vs. controls. ∗,°,#p < 0.05; ∗∗,°°,##p < 0.010; ∗∗∗,°°°,###p < 0.001 (Mann–Whitney test).
Fig. 2.
Cosinor-based rhythmometry analysis of circadian genes. Results are presented as relative expression compared to housekeeping genes. Figures include patients with active CS (red, n = 34) in remission (green, n = 10) against controls (grey, n = 34). Dots are single measurements for each time point. Curves are obtained from the pooled data. Genes included in the figure are: PER family (a, b, c), CLOCK (d), ARNTL (e), PRF1 (f) and TIMELESS (g). p values for comparisons of cosinor-based rhythmometry parameters are available in Table 2.
Rhythmicity analysis
To study the derangement and possible resumption of circadian rhythmicity in CS, we ran the JTK_CYCLE algorithm that investigates the presence of 24-h patterns in gene expression time course. We applied the algorithm to controls and patients with CS during the active phase and after remission. Of the 19 analysed clock-related genes, 11 showed a significant circadian rhythm in controls. During the active phase, all genes lost their rhythmicity. The genes that regained rhythmicity in cured CS were AANAT, CAMK2D, CLOCK, CREB1, PER1, PER2, PER3, PRF1 and TIMELESS. On the other hand, CREB3 and MAT2A did not regain a robust rhythm in remission. All results are summarised in Table 3. We compared these findings with those obtained by the zero-amplitude test from the cosinor mixed effects analysis. Results largely overlapped, showing 11 genes with a consistent circadian rhythm (AANAT, CSNK1A1, CREB1, CREB3, MAT2A, PER1, PER2, PER3, PRKAR2A, TIMELESS and WEE1) and two that approached significance (CLOCK, p = 0.062 [cosinor analysis]; CAMK2D p = 0.061 [cosinor analysis]). In patients with active CS, all genes had lost their rhythmicity. After remission, a robust rhythm was restored in most genes except CREB3, MAT2A, and WEE1. Surprisingly, a tendency toward oscillation was observed in a few genes which were not circadian in controls, that however, gained rhythmicity after CS remission (MAPK1 p = 0⋅001 [cosinor analysis]; PRF1 p = 0⋅001 [cosinor analysis]; PRKAR2A p = 0⋅068 [cosinor analysis]). The latter suggests that when the clock is reset by effectively removing the cause of disruption, it frees a robust synchronous input to the oscillator that also reflects on non-canonical circadian genes.
Table 3.
JTK circadian genes analysis.
| Controls | Active CS | CS in remission | |
|---|---|---|---|
| AANAT | <0.001 | 1.000 | 0.011 |
| ARNTL | 0.360 | 1.000 | 0.400 |
| CAMK2D | 0.013 | 0.217 | 0.022 |
| CLOCK | 0.002 | 1.000 | 0.047 |
| CREB1 | 0.042 | 1.000 | 0.018 |
| CREB3 | 0.032 | 1.000 | 1.000 |
| CSNK1A1 | 0.054 | 1.000 | 0.056 |
| CSNK1E | 0.106 | 1.000 | 0.032 |
| MAPK1 | 0.471 | 1.000 | <0.001 |
| MAT2A | 0.049 | 1.000 | 0.075 |
| OPN3 | 0.538 | 1.000 | 0.144 |
| PER1 | <0.001 | 0.553 | <0.001 |
| PER2 | <0.001 | 1.000 | <0.001 |
| PER3 | <0.001 | 1.000 | <0.001 |
| PRF1 | <0.001 | 1.000 | <0.001 |
| PRKAR1A | 0.378 | 1.000 | 0.137 |
| PRKAR2A | 0.161 | 1.000 | 0.063 |
| TIMELESS | <0.001 | 1.000 | 0.002 |
| WEE1 | 0.242 | 0.906 | 1.000 |
Results from circadian genes time-course analysis with JTK package.
Values < 0.05 indicate circadian rhythmicity (zero amplitude test).
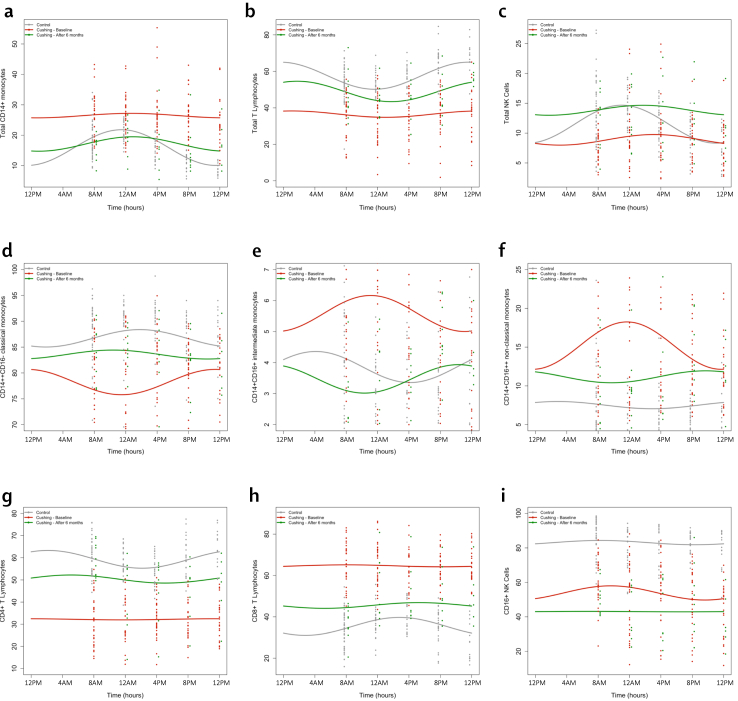
Circadian immune profiling
Circadian fluctuations in the immune profile were input into a mixed effects cosinor model. The results are summarised in Table 4 and Fig. 3. Subgroup analysis for the aetiology of CS did not show major differences in the immune profile between ACTH-dependent and ACTH-independent CS.
Table 4.
Cosinor-based rhythmometry analysis of peripheral blood mononuclear cells.
| Mesor (%) |
Amplitude (%) |
Acrophase (hours) |
||||||
|---|---|---|---|---|---|---|---|---|
| Controls | Active CS | CS in remission | Controls | Active CS | CS in remission | Controls | CS in remission | |
| Total Monocytes | 15.7 (13.8–17.2) | 26.3 (22.4–29)∗∗∗ | 16.8 (14.7–18.4)°° | 3.4 (2.5–1.4) | 2.2 (1.4–3.8) | 4.2 (3.3–7.2)° | 23:53 (22:04–02:07) | 23:01 (21:37–01:23) |
| Classical Monocytes | 89.2 (87.2–90.8) | 81.1 (76.7–83.5)∗∗∗ | 84.2 (81.2–86.6)## | 2.8 (2.1–3.7) | 4.4 (2.3–6.9) | 3.1 (1.8–4.8) | 23:30 (21:11–02:14) | 20:37 (19:11–23:20) |
| Intermediate Monocytes | 3.8 (3.1–4.9) | 5.1 (4.1–7.0)∗∗ | 3.8 (3.3–4.2)° | 1.0 (0.7–1.4) | 1.5 (0.9–2.4) | 0.9 (0.8–2.4) | 23:07 (20:20–01:43) | 02:43 (19:31–04:07) |
| Non-classical Monocytes | 5.0 (3.1–6.2) | 12.6 (9.1–18.2)∗∗∗ | 9,2 (7.3–11.9)## | 1.4 (0.9–2.4) | 3.5 (1.6–5.9)∗∗ | 2.9 (2.3–4.1) | 00:06 (22:34–02:25) | 21:51 (20:08–00:26) |
| Total T Lymphocytes | 57.8 (53.7–61.1) | 35.4 (31.9–44.4)∗∗∗ | 49.6 (42.8–50.7)°;## | 4.8 (2.8–6.5) | 4.4 (2.4–7.9) | 8.1 (6.4–11.1) | 23:48 (20:27–02:36) | 03:29 (01:07–05:01) |
| CD4+ T Lymphocytes | 58.8 (56.0–64.3) | 29.6 (27.9–27.9)∗∗∗ | 53.4 (44–56.8)°°;# | 5.8 (4.4–10.5) | 4.1 (2.6–7.4) | 6.1 (3.7–7.7) | 00:22 (22:43–02:01) | 01:31 (22:38–03:58) |
| CD8+ T Lymphocytes | 36.2 (32.4–40.0) | 67.4 (60.2–69.2)∗∗∗ | 42.2 (39.3–53.2)°°;# | 5.8 (4.6–9.0) | 4.4 (3.0–6.3) | 3.1 (2.8–8.3) | 22:29 (20:02–02:36) | 00:54 (21:40–02:28) |
| Total CD56+ NK cells | 13.0 (11.2–15.4) | 9.3 (6.6–10.7)∗∗∗ | 11.1 (8.7–16.3) | 2.0 (1.3–3.4) | 2.0 (1.3–4.2) | 3.3 (1.5–3.8) | 21:59 (20:24–23:59) | 02:32 (22:37–02:57) |
| CD56bright NK cells | 8.3 (6.6–11.1) | 3.8 (2.6–5.5)∗∗∗ | 2.3 (1.5–3.1)### | 2.4 (1.3–4.1) | 1.7 (1.0–2.4) | 0.7 (0.6–1.1) | 23:08 (20:44–00:46) | 23:04 (23:02–01:12) |
| CD56dim CD16+ NK cells | 87.9 (85.8–91.1) | 54.4 (52.2–62.0)∗∗∗ | 71.3 (56.5–84.6)°°;### | 5.5 (3.1–8.8) | 9.5 (6.1–14.9)∗ | 11.0 (8.1–18.8) | 23:48 (20:43–01:03) | 21:57 (21:02–00:00) |
Results of cosinor-based rhythmometry analysis on time-course percentages of immune cells. Results are expressed as median (25th—75th percentiles). ∗: active CS vs. Controls; °: CS in remission vs. active CS; #: CS in remission vs. controls.
∗,°,#p < 0.05; ∗∗,°°,##p < 0.010; ∗∗∗,°°°,###p < 0.001 (Mann–Whitney test).
Fig. 3.
Cosinor-based rhythmometry analysis of peripheral blood mononuclear cells on the study population (n = 68). Results are presented as percentage of immune cells. The panels include patients with active CS (red, n = 34) in remission (green, n = 10) compared to controls (grey, n = 34). Dots are single measurements for each time point. Curves are obtained from the pooled data. Results are presented as percentages of immune subsets over whole PBMCs (a, b), lymphocytes (c, g, h), monocytes (d, e, f) and NK cells (i). p values for comparisons of cosinor-based rhythmometry parameters are available in Table 3.
Monocytes
The circadian profile of monocytes was significantly altered in CS compared to controls. An increase in mesor of total CD14+, intermediate CD14+CD16+ and non-classical CD14+CD16++ monocytes during active CS was mirrored by a significant decrease in classical CD14++CD16- monocytes, confirming that the abnormalities found in the morning were sustained throughout the day and night. After remission, percentages of total CD14+ and intermediate CD14+CD16+ monocytes subsets were restored to levels not different from controls. In contrast, non-classical CD14+CD16++ and classical CD14++CD16- monocytes didn't reach normal levels despite a significant difference compared to the active phase (Fig.3 a, d–f). The cosinor model for non-classical CD14+ CD16++ monocytes showed a substantial flattening of the curve in active CS compared to controls, which improved but failed to normalise after remission. No differences in amplitude were demonstrated in other monocyte subsets, except for a tendency towards flattening in the Total CD14+ population (p = 0.071 [cosinor analysis]). During active CS, all monocyte subsets lost their rhythmicity and didn't regain it entirely after remission. No gender-related differences were observed.
Lymphocytes
As expected, patients with CS had markedly suppressed percentages of T lymphocytes throughout the day. However, despite some improvement from baseline, the low mesors of total CD3+ and CD4+ T lymphocytes persisted to be reduced for up to six months since remission. Conversely, the percentage of CD8+ T lymphocytes was elevated and remained higher than controls after remission (Fig. 3 b, g, h). In terms of amplitude, no significant differences were observed despite a tendency towards flattening the curve of CD8+ T lymphocytes (p = 0.062 [cosinor analysis]). Rhythmicity of total CD3+ and CD4+ T lymphocytes was evident in controls, lost in active CS, and regained after remission. A sex interaction analysis in the mixed effects cosinor model showed that the female sex had a significant impact on total T lymphocytes (estimate 5.82%, SE 2.75%; p = 0.0366 [interaction analysis]) and CD8+ lymphocytes (estimate −6.89%, SE 2.23%; p = 0.003 [interaction analysis]). A sex-by-group interaction analysis confirmed the differences in patients with active CS (p = 0.023 [interaction analysis]).
Natural killer cells
Strengthening the data of morning assessment, the mesor of total CD56+ NK cells was significantly reduced in patients with CS compared to controls, but fully normalised at six months. Other NK cell subsets from patients, including the CD56bright NK cells, and the CD56dimCD16+ NK also exhibited a decreased mesor compared to controls (Fig. 3 c, i). However, while total CD56+ NK cells normalised at six months, both CD56bright NK cells and CD56dimCD16+ NK cells increased but failed to reach normal levels. Interestingly, there was a paradoxical increase in amplitude in the CD56dimCD16+ NK subset. Acrophases were similar between patients in remission and controls, possibly due to the feeble circadian variation of these subset percentages throughout the day. Interestingly, total CD56+ and CD56dimCD16+ NK cells showed circadian rhythmicity in controls that persisted during active CS but were lost after remission. A sex-by-group interaction analysis showed an effect of female sex on CD56dimCD16+ NKs mesor in patients with active CS (estimate −26.74%, SE 11.2%; p = 0.018 [interaction analysis]).
Cortisol profiling
Cortisol levels throughout the day were analysed by mass spectrometry. As expected, results showed profound differences between patients during the active phase and controls at all time points. Complete results of each time-point comparison of cortisol levels are available in Supplementary Table S2 (Table S2). Patients with active CS had increased cortisol levels throughout the day compared to controls. On the other hand, patients in remission showed cortisol levels comparable to controls at 8:00 AM, noon, 4:00 PM and midnight, but higher at 8:00 PM, possibly due to the effect of conventional post-surgical glucocorticoid replacement therapy.
Cosinor-based rhythmometry showed a significantly higher cortisol mesor in active CS than controls and loss of rhythmicity during the active phase (p = 0.079 vs. p = .013 [cosinor analysis]). Interestingly, while a decrease after remission was observed (p = 0.057 [cosinor mixed effects analysis]), the rhythm was apparently not restored.
Machine learning analyses
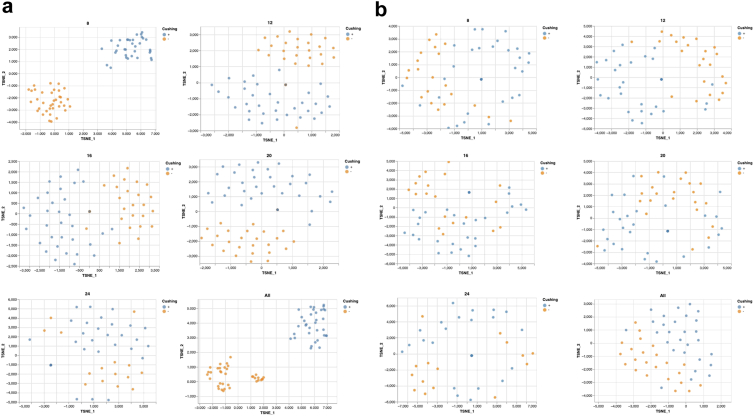
Dimensionality reduction and classification
Results of the two-dimensional visual reduction of the entire dataset by principal component analysis (PCA) and t-Distributed Stochastic Neighbour Embedding (t-SNE) are shown in the Supplementary (Fig. S3). Patients and controls are easily distinguishable. Both linear (PCA) and non-linear (t-SNE) techniques were applied with an exploratory intent to provide a more comprehensive analysis. Having established the potential for automatic detection of positive and negative patients, our focus shifted to the effectiveness of this automated classification.
Non-visual classification methods were then applied. Results from K-Nearest Neighbours (k-NN, best configuration 10 components, k = 7) lead to a correct classification in two categories (active CS and controls) with 98⋅6% accuracy and 2⋅9% standard deviation. Results from the Support Vector Machine analysis (the best configuration of 3 components in PCA, sigmoid kernel) lead to similar results: the algorithm successfully distinguished positive and negative subjects with 99⋅3% accuracy and 2⋅1% variance.
Variable analyses
Then, we categorised our variables to evaluate their performance in identifying patients compared to controls. The entire dataset was divided into the following feature groups: anthropometric measurements and vital parameters, biochemical features, complications and comorbidities, concomitant medications, circadian genes, immune profiling, and hormonal levels.
Even though all categories exhibited degrees of variability between patients and controls, the effectiveness was very variable, with performances ranging from 67% (biochemistry) to 97% (immune profiling). Interestingly, groups of features expected to predict CS more reliably, such as hormones (71%), comorbidities (71%) and anthropometric measurements (71%), were less able to identify patients compared to controls compared to other components of our dataset.
We then divided all collected variables into two subsets: those that are known to be associated with CS and through which the clinical diagnosis is made (Group A: anthropometric measurements, vital parameters, glucose metabolism, cortisol levels, complications and comorbidities, concomitant medications) and those less frequently explored in CS (Group B: immune profiling and circadian genes). Spatial distribution with t-SNE of subjects using either group of variables is shown in Supplementary Figure S4 (Fig. S4).
Interestingly, running the k-NN classification algorithm, the latter category of “novel” variables significantly outperformed the same algorithm using “conventional” parameters usually employed in the diagnostic proceeding for CS, with an overall accuracy of 97% and a +20% improvement.
To explore the importance of circadian rhythm in characterising active CS, we narrowed the proposed strategy to time-course variables: immune profiling, hormones (including cortisol profiling), and circadian genes. t-SNE results showed that if we only consider the measurements at a given time point, we can still classify patients as positive or negative. This holds for any given time, but notably, it is valid for the standard 8 AM measurements.
Given that the loss of cortisol circadian rhythm is used to define CS itself, we decided to exclude this specific set and run our analyses. Results on immune profiling and circadian genes still showed a consistent separation between patients and controls. The combination of these two groups turned out to be a powerful detector of CS, especially, again, at 8 AM (98⋅6% accuracy, only a 0⋅7% drop compared to previous model analyses including cortisol) to a level that can be considered a surrogate of cortisol measurement.
Lastly, we decided to analyse immune profiling and circadian genes separately. The immune profiling variables (Fig. 4a) were able to classify affected and non-affected subjects when time points were examined individually and more efficiently when all time points were analysed jointly. The best-performing timepoint was the 8 AM point (99% accuracy), while midnight was the least accurate timepoint, despite retaining a good ability to discern patients from controls (79%).
Fig. 4.
Two-dimensional visualisation of time-course variables. Analyses of controls (orange, n = 34) and patients with active CS (blue, n = 34) for time-course variables immune profiling (a) and circadian genes (b) at each time point and considering all time points (last square for each panel). 2D visualisation is provided by t-SNE.
In contrast, the clock genes variables could best distinguish the two groups when considered jointly within the time series, while individual time points were less reliable (Fig. 4b). So, the predicting value for clock genes is not their level of expression at any time point, but only their variation throughout the day.
We then performed machine learning analyses on CS patients to identify potential variables clustering among different aetiologies. The dataset variables did not show a significant difference between ACTH-dependent and -independent CS, except for hormone values. Therefore, immune profiling and circadian rhythm disruption characterise all patients with CS regardless of the origin of autonomous hormone secretion.
In summary, through a machine learning approach, we successfully reduced the complex matrix of our 24-h multidimensional dataset, including more than 500 variables for each patient, into a single-time-point profiling of circulating immune cells that is capable of predicting circadian rhythm disruption induced by hypercortisolism and can be included in the future diagnostic and management algorithm of CS.
Discussion
Our study demonstrates that chronic hypercortisolism alters the precision of the daily rhythm of innate and adaptive immune cell subpopulations in the absolute number and relative amplitude of their physiological circadian oscillation. The GC dependency has been proven by the reversal of most alterations after the cure of hypercortisolism. The persistence of dysregulated rhythms during remission could contribute to the long-term complications of prolonged exposure to GC and some symptoms complained by cured patients.
Harvey Cushing recognised an increased infectious risk in the first description of the syndrome.31 Our study shows that patients with CS have a unique immune phenotype that combines features of immune depression (lymphopenia, reduced marginalisation of immune cells, decreased inflammatory monocytes) with alterations observed in chronic low-grade inflammation status, such as increased non-classical monocytes and reduced CD16+ NK cells. The immune signature observed in our heterogeneous cohort of patients with CS seems unique to this syndrome, as supported by the fact that a stable, chronic association of NK impoverishment, increased intermediate and non-classical monocytes and low lymphocytes, to our knowledge, is not reported in any other endogenous condition, and by the consequent highly discriminatory efficiency.
GC exposure is associated with increased circulating white blood cell count, chronic lymphopenia,32, 33, 34 and an increased neutrophil-to-lymphocyte ratio.35,36 This results from an anti-inflammatory effect that reduces peripheral dissemination, extravasation and patrolling from monocytes33 and neutrophils37 while interfering with lymphocyte apoptosis.38 These ‘canonical’ alterations have all been confirmed in our cohort of patients. We also showed that, despite the increased number of total circulating monocytes, the percentage of classical (also known as “inflammatory”) monocytes was decreased, and that of non-classical monocytes increased. Classical monocytes are specialised in phagocytosis, innate sensing, immune responses and migration; intermediate monocytes are more involved in antigen presentation, cytokine secretion, apoptosis regulation and differentiation, and non-classical monocytes are specialised in complement and Fc gamma-mediated phagocytosis and adhesion and have been often characterised as “enhancers” of hyperactive immune response in chronic diseases, such as HIV infection.39 Interestingly, metabolic syndrome and obesity also increase non-classical and intermediate monocytes, sustaining the characteristic low-grade inflammation.39 However, our finding of increased non-classical monocytes was accompanied by a decrease in classical monocytes, unlike in obesity.40 The divergence suggests that some monocyte subsets might develop escape mechanisms, sustaining low-grade inflammation and ultimately contributing to the increased cardiovascular risk observed in CS.
Classical monocytes are increased in the visceral fat of patients with CS.41 Therefore, our observation of reduced circulating levels may reflect an increased tissue harbouring that contributes to the local “inflammatory” microenvironment. We observed loss of rhythmicity of the daily oscillation of monocyte subsets in patients, consistently with previous observations in mice,42 even though the underlying mechanisms were still debated.43 A diminished variation of classical monocyte migration could have a double-edged effect on the complications in CS: first, the inability to reach peripheral tissues and participate in the phagocytic response impairs the antimicrobial defence; second, the monocytes “trapped” in visceral fat, through local cytokines release, disturb adipose tissue function and contribute to the metabolic derangement.
Interestingly, monocytes did not regain a complete fluctuation during remission, and the alterations in both classical and non-classical monocyte levels persisted. This is consistent with the recent report of an increased mortality of cardiometabolic origin that persists in patients with CD, even after long-term remission, when compared to the general population.17 In a much shorter time frame, the incomplete recovery of innate immunity we observed supports the persistence of cardiometabolic risk, possibly through inflammation, as recently suggested.44 More interestingly, the near-normal restoration of circadian gene expression rhythmicity is associated with only a partial reversal of the immune phenotype. Given the relatively short life of PBMCs, it is unlikely that such divergence is related to a different time course, rather to the remodelling of other systems (vascular, metabolic) that have a much longer carryover effect.
Regulation of adaptive immunity, and more specifically of T lymphocytes, has been shown to rely both intrinsically and extrinsically on circadian rhythms and more so on GC.45 In our study, patients with CS showed a significant decrease in circulating total T lymphocytes and an inversion of the CD8+/CD4+ ratio, with increased CD8+ and decreased CD4+ compared to controls. Moreover, circadian rhythm of total T lymphocytes and CD8+ lymphocytes was lost in CS, and regained in total lymphocytes only.
There is evidence that shuttling lymphocytes in and out of the bloodstream and their function are tightly circadian46,47 primarily due to variations in the cytokine environment and homing receptors.48 The decreased number of total and CD4+ T lymphocytes we have observed in CS and their impaired migration towards peripheral homing sites could contribute to the increased risk for infectious diseases.
Similarly, we found decreased NK cells in patients with CS that only partially normalised after remission. NK cells are important in antitumoral surveillance and first-line defence against pathogens. The effector subset, CD56dimCD16+ cells, and the cytokine-secreting subset, CD56bright cells, failed to normalise six months after remission. Whether the long-lasting effect of hypercortisolism on NK activity contributes to the increased risk for neoplasms that has been described in patients with CS remains to be established.
Overall, we confirmed the immune alterations known to occur in CS but also provided evidence for a specific, unique signature of subpopulations and an altered redistribution of subsets closely resembling that observed in experimental clock gene disruption.46 The downstream consequences of such alteration can fully explain the increased risk of infectious disease and the rebound autoimmunity after remission of CS. Moreover, they can explain a relevant part of the metabolic and cardiovascular consequences related to CS, including their persistence after the cure, as they can mimic the metabolic impairment associated with circadian shifts and desynchronisation. The additive direct (muscle/hepatic/insulin-signalling) and indirect (circadian/immune misalignment) effects of endogenous hypercortisolism explain its detrimental consequence on overall and cardiometabolic mortality. Knowledge of these alterations can help identify patients more likely to develop worse outcomes and adopt early intervention strategies.
The second finding of our trial is the quantitative and time-specific alterations in the expression of clock genes in PBMCs from patients with CS. The organisation of central and peripheral clocks relies on the integration of vertical (hierarchical) and horizontal signals, with many pathways synchronising the two, as nearly every peripheral tissue exhibits autonomous rhythmic gene expression.49,50 Immune cells have been successfully used to analyse circadian gene expression, with promising results.51 This approach provides a reliable, non-invasive indicator of central clock disruption, or more precisely, its integrated output, as observed in chronic endogenous hypercortisolism, where SCN control over the HPA axis is lost due to tumorous secretion of ACTH or cortisol, depending on the aetiology.52 Briefly, the mean 24-h levels of expression of the core clock genes BMAL1 (ARNTL) and PRF1 were found to increase, as opposed to a down-regulation of PER3 of the Period gene family. The absolute expression level for all examined genes returned to control levels during remission, except for PER1, which increased after the cure.
Besides quantitative alterations, the most typical feature observed in patients with CS was a loss of rhythmicity of circadian gene expression. The amplitude of oscillation of all the genes of the PER family (PER1, PER2, PER3) was reduced; similarly flattened appeared TIMELESS. Proof of the GC dependency of this time-specific effect is the regain of amplitude of the PER gene during remission compared to controls.
Consistent with our findings, a recent study on 14 patients with ACTH-dependent CS due to pituitary adenoma showed a loss of rhythmicity of CLOCK, ARNTL/BMAL1, PER1, PER2, and PER3, assessed by cosinor rhythmometry.21 However, human cosinor-based rhythmometry has yielded heterogeneous findings. The expression of ARNTL/BMAL1 has been described as rhythmic in some,53,54 but not all studies.55 Rather than a biological discrepancy, this is more likely a sign of the difficulties of performing circadian clinical trials in humans, for the invasiveness of multiple sampling, biases linked to restrictions imposed by protocols, interindividual variability (inner clock) and high background noise.18
In a chrono-pharmacology trial on patients with adrenal insufficiency,56 who fail to produce endogenous cortisol, we showed that a change in the time of administration of exogenous glucocorticoids to better mimic cortisol circadian profile was effective in entraining clock gene and restoring their level of expression, as compared to conventional therapies.20 Single-point analyses, however, require large cohorts and can be affected by many confounding factors. Multiple gene analyses have been proposed as a sample-reduction strategy in tissue biopsies and humans,51 but results might not be as robust as in repeated sampling.18
Other algorithms have been proposed to assess circadian rhythm from multiple-time gene expression, taking into account the noise of human datasets.18,51 Along with analysing rhythmicity with our cosinor mixed-model analysis, we selected the widely used JTK_CYCLE30 for its reliable performance compared to other algorithms.
Accordingly, in our study, the JTK_CYCLE algorithm showed a higher sensitivity than the cosinor-based rhythmometry only for a few genes, namely CLOCK, CAMK2D, and PRF1. Both methods confirmed that all oscillating genes in control subjects lost their rhythmicity during the CS active phase and most regained rhythm during CS remission. The use of circadian algorithms, such as JTK_CYCLE, has been associated with an increased risk for false discoveries when comparing different conditions, particularly if not supplemented with prior analysis and direct comparisons of circadian parameters such as mesor, amplitude or phase57; however, the consistency across methods reassured the validity of our findings.
An added value of our trial is the comprehensive analysis of the immune circadian rhythm of healthy controls in real-life settings. The controls enrolled in this trial were not forced to adopt ad hoc procedures for optimal circadian entrainment; they were just asked to follow their regular lifestyle and avoid drugs known to interfere with clock gene expression.26 Studies on daily rhythms are often small because of the difficulties and costs of sampling, mainly recruiting healthy young males. We enrolled a mixed population of adults, males and females, matched to the CS cohort, a wide age range, selected among subjects attending the outpatient clinic (as previously described26). Despite the heterogeneity in enrolled subjects, our analysis revealed how robust the immune circadian rhythm is, especially in the relative composition of the subsets of immune cells circulating throughout the day, both in controls and cured patients. Unfortunately, due to the disrupted circadian rhythm in most patients with CS, an important aspect of circadian studies such as the phase-effect could not be appropriately evaluated. Still, it will likely contribute to the syndrome's clinical complications.
The diagnosis of CS is still a significant challenge for endocrinologists, requiring multiple testing and the combination of dynamic or invasive procedures to obtain reliable sensitivity and specificity.25 We decided to perform an exploratory analysis to identify potential disease-related biomarkers among our large dataset, enriched with time-course variables. We employed machine learning (ML) techniques as they increasingly become part of medical research supporting diagnosis and clinical management. ML can simultaneously deal with a large number of variables that are artificially collapsed into multidimensional “vectors,” representing a subject/patient with a lower number of “characteristics” or “features” without altering the objective complexity of the significantly higher number of actual variables. This allows comprehensive analyses that identify differences among groups or clusters of variables associated with a specific trait or disease.
Dimensionality reduction and classification methods are among the main features of supervised machine learning techniques that best apply to moderately large data (hundreds of variables), such as those included in our study. Our analyses revealed that immune profiling and circadian genes were among the best-performing sets of variables in separating patients with CS from controls, even outperforming other sets of variables conventionally part of the CS diagnostic workup and evaluation. These results, unachievable with conventional statistical methods, confirmed that a major readout of the body consequence of chronic GC exposure is the disruption of the circadian features of immune cells. This is relevant not only in terms of possible new tools for the diagnosis of endogenous hypercortisolism, as a biomarker of GC is still missing, but also as a measure of therapeutic effectiveness (either surgical or pharmacological), as the current approach based on measurement of the 24-h cortisol production has shown all its limitations. Our multidimensional machine learning approach can provide valuable data for developing Cushing's scores to overcome the loco-regional and ethnicity limitations that have emerged in the various clinical scores tested so far.58,59 Another interesting result from our analyses is that ACTH-dependent and ACTH-independent CS did not behave differently in terms of immune profiling and circadian rhythm disruption. This finding supports the generalisability of our model across different aetiologies of CS and potentially to other forms of GC excess.
Finally, the significance of our data, produced in the ideal, but also rare, model of endogenous hypercortisolism, is amplified when considering the ample use of exogenous GC for various reasons. Experimental studies on human intake of GCs either lack long-term exposure (as unethical) or are affected by the underlying conditions for which patients require GC therapy. Nevertheless, GCs are often taken chronically by patients, recreating the complex phenotype we have described in our study.
Despite the effort to provide a thorough, multi-level analysis of our CS cohort, our study has several limitations, the main one being the number of patients that could be cured in remission, that includes patients with adrenal insufficiency and is significantly smaller than those studied in the active phase. We opted for stringent remission criteria, which is not always achievable within the time frame of a trial for a rare disease. We could have extended the study duration (more than six months). However, this would have meant trading off the biases related to the intercurrent illnesses, confounding factors related to multi-treatment modalities for CS, seasonal effects, and sustainability. Circadian sampling is also time-consuming for patients, and some refused to perform a second 24-h sampling at a relatively short interval (6 months), especially after they had been cured. For the non-cured patients, several safe and effective medical treatments are now available to normalise total cortisol exposure that we could not exempt to offer them. For the trial, however, we decided to exclude patients receiving CS medical treatments since we wanted to assess the actual effect of a complete remission and because available drugs cannot be given with the intent to restore circadian rhythm (unless provided in an off-label schedule). Further studies are warranted to expand the size of patients and controls and extend our analytical strategies to patients under medical treatment. Another potential limitation is the restricted number of clock-related genes we analysed, and the lack of characterisation of each subject's chronotype. However, our primary aim was not to phish genes modulated by GC excess rather to describe, as a proof of principle, the potential role of known clock genes in CS assessment. We also acknowledge the lack of external validation for our ML analyses. A 90/10% masking strategy was applied to train the algorithm through various iterations, but given the rarity of CS and the large number of required procedures, further studies and efforts will be necessary to provide this additional step. Lastly, a potential confounding factor could be the effect of seasonality on circadian rhythms. Homogenous enrolment of patients and controls throughout the study timeframe was performed to reduce this interference.
Our data reveal a unique immune signature, defined by specific changes in the subset of circulating PBMCs, that triggered by endogenous hypercortisolism reversed to near normal in remission, predominantly, but not exclusively sustained by specific alteration in the amplitude of expression of circadian genes within immune cells. Our study could provide the basis for developing strategies to mitigate undesired effects of GC therapies. Finally, our trial supports the use of circulating immune cells as a display of the internal circadian time, demonstrating that therapeutic interventions can help reset the clock and guide innovative chrono-pharmacological strategies.
Contributors
VH, ES, DG, MAV, and AMI were involved in conceptualisation and methodology of the study. CSi, CP, FC, GA, CSc, RPi were part of the multicentre clinical team and were involved in the investigation. FS, FR, DF, and MAV were engaged in investigating steroid hormone and immune system analyses and validating the results. FB, DAF, SC, and RN were involved in investigating and validating bioinformatic analyses. VH, MAV and AMI contributed to the data analysis investigation and writing of the first draft, while ES, RPo, IB, MM, and EG contributed to the writing (review and editing) of the initial paper. AMI, VH, MAV and DG accessed and verified the underlying data. All authors contributed to the writing and review of the final manuscript. All authors read and approved the final version of the manuscript.
Data sharing statement
Upon reasonable request, deidentified participant data and code used in the analyses can be shared with other researchers. Data will be made available after the ethics committee approves a study proposal and a data access agreement is signed. For further information, please contact the corresponding author (andrea.isidori@uniroma1.it).
Declaration of interests
None of the authors have received funding or have other fees to declare related to this project. SC is a PC member for the Association for Computational Linguistics (ACL).
Acknowledgements
We acknowledge co-funding from Next Generation EU, in the context of the National Recovery and Resilience Plan, Investment PE8—Project Age-It: “Ageing Well in an Ageing Society”. This resource was co-financed by the Next Generation EU [DM 1557 11.10.2022] to DG CUP B53C22004090006, and the PRecisiOn Medicine to Target Frailty of Endocrine-metabolic Origin (PROMETEO) project (NET-2018-12365454) by the Italian Ministry of Health. The views and opinions expressed are only those of the authors and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them. The analyses were supported by the Ministerial Research Project PRIN PNRR Prot. P20223734 P MAV. SC and RN gratefully acknowledge the support of the PNRR MUR project PE0000013-FAIR.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105462.
Appendix A. Supplementary data
References
- 1.Morgan-Taylor M. Regulating light pollution: more than just the night sky. Science. 2023;380(6650):1118–1120. doi: 10.1126/science.adh7723. [DOI] [PubMed] [Google Scholar]
- 2.Schrader L.A., Ronnekleiv-Kelly S.M., Hogenesch J.B., Bradfield C.A., Malecki K.M. Circadian disruption, clock genes, and metabolic health. J Clin Invest. 2024;134(14) doi: 10.1172/JCI170998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potter G.D., Skene D.J., Arendt J., Cade J.E., Grant P.J., Hardie L.J. Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr Rev. 2016;37(6):584–608. doi: 10.1210/er.2016-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straif K., Baan R., Grosse Y., et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 5.Hansen J. Night shift work and risk of breast cancer. Curr Environ Health Rep. 2017;4(3):325–339. doi: 10.1007/s40572-017-0155-y. [DOI] [PubMed] [Google Scholar]
- 6.Miro C., Docimo A., Barrea L., et al. "Time" for obesity-related cancer: the role of the circadian rhythm in cancer pathogenesis and treatment. Semin Cancer Biol. 2023;91:99–109. doi: 10.1016/j.semcancer.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Pezuk P., Mohawk J.A., Wang L.A., Menaker M. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology. 2012;153(10):4775–4783. doi: 10.1210/en.2012-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mavroudis P.D., Scheff J.D., Calvano S.E., Lowry S.F., Androulakis I.P. Entrainment of peripheral clock genes by cortisol. Physiol Genomics. 2012;44(11):607–621. doi: 10.1152/physiolgenomics.00001.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieman L.K., Biller B.M., Findling J.W., et al. The diagnosis of cushing's syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broder M.S., Neary M.P., Chang E., Cherepanov D., Ludlam W.H. Incidence of Cushing's syndrome and Cushing's disease in commercially-insured patients <65 years old in the United States. Pituitary. 2015;18(3):283–289. doi: 10.1007/s11102-014-0569-6. [DOI] [PubMed] [Google Scholar]
- 11.Valassi E. Clinical presentation and etiology of Cushing's syndrome: data from ERCUSYN. J Neuroendocrinol. 2022;34(8) doi: 10.1111/jne.13114. [DOI] [PubMed] [Google Scholar]
- 12.Pivonello R., Isidori A.M., De Martino M.C., Newell-Price J., Biller B.M., Colao A. Complications of Cushing's syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7):611–629. doi: 10.1016/S2213-8587(16)00086-3. [DOI] [PubMed] [Google Scholar]
- 13.Cherenko M., Appelman-Dijkstra N.M., Priego Zurita A.L., et al. Venous thromboembolism in Cushing syndrome: results from an EuRRECa and Endo-ERN survey. Endocr Connect. 2024;13(6) doi: 10.1530/EC-24-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isidori A.M., Graziadio C., Paragliola R.M., et al. The hypertension of Cushing's syndrome: controversies in the pathophysiology and focus on cardiovascular complications. J Hypertens. 2015;33(1):44–60. doi: 10.1097/HJH.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasenmajer V., Sbardella E., Sciarra F., Minnetti M., Isidori A.M., Venneri M.A. The immune system in cushing's syndrome. Trends Endocrinol Metabol. 2020;31(9):655–669. doi: 10.1016/j.tem.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Birtolo M.F., Armignacco R., Benanteur N., et al. Whole blood transcriptomic signature of Cushing's syndrome. Eur J Endocrinol. 2024;191(1):55–63. doi: 10.1093/ejendo/lvae083. [DOI] [PubMed] [Google Scholar]
- 17.Bengtsson D., Ragnarsson O., Berinder K., et al. Increased mortality persists after treatment of cushing's disease: a matched nationwide cohort study. J Endocr Soc. 2022;6(6) doi: 10.1210/jendso/bvac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hesse J., Malhan D., Yalҫin M., Aboumanify O., Basti A., Relogio A. An optimal time for treatment-predicting circadian time by machine learning and mathematical modelling. Cancers. 2020;12(11) doi: 10.3390/cancers12113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang M.C., Ho C.W., Chen C.H., Liu S.C., Chen C.C., Leu S.J. Reduced expression of circadian clock genes in male alcoholic patients. Alcohol Clin Exp Res. 2010;34(11):1899–1904. doi: 10.1111/j.1530-0277.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- 20.Venneri M.A., Hasenmajer V., Fiore D., et al. Circadian rhythm of glucocorticoid administration entrains clock genes in immune cells: a DREAM trial ancillary study. J Clin Endocrinol Metab. 2018;103(8):2998–3009. doi: 10.1210/jc.2018-00346. [DOI] [PubMed] [Google Scholar]
- 21.Soares V.R., Silva Martins C., Martinez E.Z., et al. Peripheral clock system circadian abnormalities in Cushing's disease. Chronobiol Int. 2020;37(6):867–876. doi: 10.1080/07420528.2020.1758126. [DOI] [PubMed] [Google Scholar]
- 22.James F.O., Boivin D.B., Charbonneau S., Belanger V., Cermakian N. Expression of clock genes in human peripheral blood mononuclear cells throughout the sleep/wake and circadian cycles. Chronobiol Int. 2007;24(6):1009–1034. doi: 10.1080/07420520701800736. [DOI] [PubMed] [Google Scholar]
- 23.Crnko S., Schutte H., Doevendans P.A., Sluijter J.P.G., van Laake L.W. Minimally invasive ways of determining circadian rhythms in humans. Physiology. 2021;36(1):7–20. doi: 10.1152/physiol.00018.2020. [DOI] [PubMed] [Google Scholar]
- 24.Zong W., Seney M.L., Ketchesin K.D., et al. Experimental design and power calculation in omics circadian rhythmicity detection using the cosinor model. Stat Med. 2023;42(18):3236–3258. doi: 10.1002/sim.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleseriu M., Auchus R., Bancos I., et al. Consensus on diagnosis and management of Cushing's disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9(12):847–875. doi: 10.1016/S2213-8587(21)00235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minnetti M., Hasenmajer V., Sbardella E., et al. Susceptibility and characteristics of infections in patients with glucocorticoid excess or insufficiency: the ICARO tool. Eur J Endocrinol. 2022;187(5):719–731. doi: 10.1530/EJE-22-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman S.A., Grant L.K., Gooley J.J., Rajaratnam S.M.W., Czeisler C.A., Lockley S.W. Endogenous circadian regulation of female reproductive hormones. J Clin Endocrinol Metab. 2019;104(12):6049–6059. doi: 10.1210/jc.2019-00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iannone M., Dima A.P., Sciarra F., Botre F., Isidori A.M. Development and validation of a liquid chromatography-tandem mass spectrometry method for the simultaneous analysis of androgens, estrogens, glucocorticoids and progestagens in human serum. Biomed Chromatogr. 2022;36(5) doi: 10.1002/bmc.5344. [DOI] [PubMed] [Google Scholar]
- 29.Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. doi: 10.1186/1742-4682-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes M.E., Hogenesch J.B., Kornacker K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms. 2010;25(5):372–380. doi: 10.1177/0748730410379711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). 1932. Obes Res. 1994;2(5):486–508. doi: 10.1002/j.1550-8528.1994.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 32.Masri-Iraqi H., Robenshtok E., Tzvetov G., Manistersky Y., Shimon I. Elevated white blood cell counts in Cushing's disease: association with hypercortisolism. Pituitary. 2014;17(5):436–440. doi: 10.1007/s11102-013-0522-0. [DOI] [PubMed] [Google Scholar]
- 33.Fareau G.G., Vassilopoulou-Sellin R. Hypercortisolemia and infection. Infect Dis Clin North Am. 2007;21(3):639–657.viii. doi: 10.1016/j.idc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Kronfol Z., Starkman M., Schteingart D.E., Singh V., Zhang Q., Hill E. Immune regulation in Cushing's syndrome: relationship to hypothalamic-pituitary-adrenal axis hormones. Psychoneuroendocrinology. 1996;21(7):599–608. doi: 10.1016/s0306-4530(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 35.Favero V., Prete A., Mangone A., et al. Inflammation-based scores in benign adrenocortical tumours are linked to the degree of cortisol excess: a retrospective single-centre study. Eur J Endocrinol. 2023;189(5):517–526. doi: 10.1093/ejendo/lvad151. [DOI] [PubMed] [Google Scholar]
- 36.Wurth R., Rescigno M., Flippo C., Stratakis C.A., Tatsi C. Inflammatory biomarkers in the evaluation of pediatric endogenous Cushing syndrome. Eur J Endocrinol. 2022;186(4):503–510. doi: 10.1530/EJE-21-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergquist M., Lindholm C., Strinnholm M., Hedenstierna G., Rylander C. Impairment of neutrophilic glucocorticoid receptor function in patients treated with steroids for septic shock. Intensive Care Med Exp. 2015;3(1):59. doi: 10.1186/s40635-015-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savino W., Mendes-da-Cruz D.A., Lepletier A., Dardenne M. Hormonal control of T-cell development in health and disease. Nat Rev Endocrinol. 2016;12(2):77–89. doi: 10.1038/nrendo.2015.168. [DOI] [PubMed] [Google Scholar]
- 39.Kapellos T.S., Bonaguro L., Gemund I., et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol. 2019;10:2035. doi: 10.3389/fimmu.2019.02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poitou C., Dalmas E., Renovato M., et al. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(10):2322–2330. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 41.Roerink S., Wagenmakers M., Langenhuijsen J.F., et al. Increased adipocyte size, macrophage infiltration, and adverse local adipokine profile in perirenal fat in cushing's syndrome. Obesity. 2017;25(8):1369–1374. doi: 10.1002/oby.21887. [DOI] [PubMed] [Google Scholar]
- 42.Timmons G.A., O'Siorain J.R., Kennedy O.D., Curtis A.M., Early J.O. Innate rhythms: clocks at the center of monocyte and macrophage function. Front Immunol. 2020;11:1743. doi: 10.3389/fimmu.2020.01743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geiger S.S., Curtis A.M., O'Neill L.A.J., Siegel R.M. Daily variation in macrophage phagocytosis is clock-independent and dispensable for cytokine production. Immunology. 2019;157(2):122–136. doi: 10.1111/imm.13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogel F., Braun L., Zopp S., et al. Low-grade inflammation during the glucocorticoid withdrawal phase in patients with Cushing's syndrome. Eur J Endocrinol. 2023;188(4):375–384. doi: 10.1093/ejendo/lvad041. [DOI] [PubMed] [Google Scholar]
- 45.Trifonova S.T., Zimmer J., Turner J.D., Muller C.P. Diurnal redistribution of human lymphocytes and their temporal associations with salivary cortisol. Chronobiol Int. 2013;30(5):669–681. doi: 10.3109/07420528.2013.775654. [DOI] [PubMed] [Google Scholar]
- 46.Wang C., Lutes L.K., Barnoud C., Scheiermann C. The circadian immune system. Sci Immunol. 2022;7(72) doi: 10.1126/sciimmunol.abm2465. [DOI] [PubMed] [Google Scholar]
- 47.Bollinger T., Leutz A., Leliavski A., et al. Circadian clocks in mouse and human CD4+ T cells. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimba A., Cui G., Tani-Ichi S., et al. Glucocorticoids drive diurnal oscillations in T cell distribution and responses by inducing interleukin-7 receptor and CXCR4. Immunity. 2018;48(2):286–298.e6. doi: 10.1016/j.immuni.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Dibner C., Schibler U., Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 50.Zhang R., Lahens N.F., Ballance H.I., Hughes M.E., Hogenesch J.B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111(45):16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wittenbrink N., Ananthasubramaniam B., Munch M., et al. High-accuracy determination of internal circadian time from a single blood sample. J Clin Invest. 2018;128(9):3826–3839. doi: 10.1172/JCI120874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minnetti M., Hasenmajer V., Pofi R., Venneri M.A., Alexandraki K.I., Isidori A.M. Fixing the broken clock in adrenal disorders: focus on glucocorticoids and chronotherapy. J Endocrinol. 2020;246(2):R13–R31. doi: 10.1530/JOE-20-0066. [DOI] [PubMed] [Google Scholar]
- 53.Charmandari E., Chrousos G.P., Lambrou G.I., et al. Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teboul M., Barrat-Petit M.A., Li X.M., et al. Atypical patterns of circadian clock gene expression in human peripheral blood mononuclear cells. J Mol Med (Berl) 2005;83(9):693–699. doi: 10.1007/s00109-005-0697-6. [DOI] [PubMed] [Google Scholar]
- 55.Kusanagi H., Hida A., Satoh K., et al. Expression profiles of 10 circadian clock genes in human peripheral blood mononuclear cells. Neurosci Res. 2008;61(2):136–142. doi: 10.1016/j.neures.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Isidori A.M., Venneri M.A., Graziadio C., et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): a single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6(3):173–185. doi: 10.1016/S2213-8587(17)30398-4. [DOI] [PubMed] [Google Scholar]
- 57.Pelikan A., Herzel H., Kramer A., Ananthasubramaniam B. Venn diagram analysis overestimates the extent of circadian rhythm reprogramming. FEBS J. 2022;289(21):6605–6621. doi: 10.1111/febs.16095. [DOI] [PubMed] [Google Scholar]
- 58.Parasiliti-Caprino M., Bioletto F., Frigerio T., et al. A new clinical model to estimate the pre-test probability of cushing's syndrome: the cushing score. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.747549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braun L.T., Vogel F., Rubinstein G., et al. Lack of sensitivity of diagnostic Cushing-scores in Germany: a multicenter validation. Eur J Endocrinol. 2023;188(1) doi: 10.1093/ejendo/lvac016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.