Abstract
Nitric oxide (NO) is a very active molecule involved in many and diverse biological pathways where it has proved to be protective against damages provoked by oxidative stress conditions. In this work, we studied the effect of two NO donors, sodium nitroprusside (SNP) and S-nitroso-N-acetylpenicillamine SNP-treated on the response of wheat (Triticum aestivum) to water stress conditions. After 2 and 3 h of drought, detached wheat leaves pretreated with 150 μm SNP retained up to 15% more water than those pretreated with water or NO2−/NO3−. The effect of SNP treatment on water retention was also found in wheat seedlings after 7 d of drought. These results were consistent with a 20% decrease in the transpiration rate of SNP-treated detached wheat leaves for the same analyzed time. In parallel experiments, NO was also able to induce a 35%, 30%, and 65% of stomatal closure in three different species, Tradescantia sp. (monocotyledonous) and two dicotyledonous, Salpichroa organifolia and fava bean (Vicia faba), respectively. In SNP-treated leaves of Tradescantia sp., the stomatal closure was correlated with a 10% increase on RWC. Ion leakage, a cell injury index, was 25% lower in SNP-treated wheat leaves compared with control ones after the recovery period. Carboxy-PTIO (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide), a specific NO scavenger, reverted SNP action by restoring the transpiration rate, stomatal aperture, and the ion leakage to the level found in untreated leaves. Northern-blot analysis showed that SNP-treated wheat leaves display a 2-fold accumulation of a group three late embryogenesis abundant transcript with respect to control leaves both after 2 and 4 h of drought periods. All together, these results suggest that the exogenous application of NO donors might confer an increased tolerance to severe drought stress conditions in plants.
Nitric oxide (NO) is a labile free radical that is produced from l-Arg by NO synthase (NOS) in various mammalian cells. NO was originally identified as an endothelium-derived relaxing factor in rabbits, but now it is recognized to be an intra- and inter-cellular mediator of several animal cell functions (Anbar, 1995; Moilanen and Vapaatalo, 1995). At the beginning, based in its ability to react with redox centers in proteins and membranes, NO was found to be toxic or injurious. Later, in animals, functional studies described NO as a molecular component of different signal transduction pathways. Depending on the concentration and the tissue where it is acting, NO can be considered either toxic or protective as well in animals as in plants (Wink et al., 1993; Beligni and Lamattina, 1999c, 2001).
Although plant NOS (gene, cDNA, or protein) has not been isolated yet, both NOS activity and NO accumulation has been reported in different plants species (Ninneman and Maier, 1996; Barroso et al., 1999). Whereas some authors considered it as a stress-inducing agent (Leshem et al., 1997), others reported its protective role acting as a radical chain reaction breaker under oxidative stress conditions generated by pathogen attack or methylviologen herbicides (Laxalt et al., 1997; Beligni and Lamattina, 1999a, 1999b). New evidences involving NO in signal transduction pathways mediated by some key molecules such as cGMP, cADPR, and Ca2+ recently have been reported in plants (Durner et al., 1998; Durner and Klessig, 1999).
It is known that the most important factors limiting crop productivity are environmental stresses. The most serious among them is the lack of water. This lack of water occurs when the rate of transpiration exceeds water uptake and is a component of several different stresses including drought, salinity, and low temperatures (McCue and Hanson, 1990). Plants have different mechanisms to avoid water deficit. Among them, stomatal conductance is reduced as part of the systemic response triggered by a signal that originates in the root system. One of these responses is the production of abscisic acid (ABA) that, in turn, elevates cytosolic Ca2+ concentration in guard cells leading to stomatal closure. Increased concentrations of cytosolic Ca2+ may be produced either by Ca2+ influx from outside of the cell or by liberation from intracellular stores. This event involves different mechanisms such as voltage-dependant ion channels, inositol-1,4,5-triphosphate (IP3) cascade, and cADP-Rib (cADPR) (Muir and Sanders, 1996; Kopka et al., 1997; Wu et al., 1997; Blatt, 2000).
At the whole-plant level, the effect of stress is usually perceived as a decrease in photosynthesis and growth, and it is associated with alterations in C and N metabolism. At the molecular level, the negative effect is associated with damage produced by the oxidative stress to the cell, due to a deficiency in energy dissipation, as a consequence of the drought-induced photosynthesis limitation (Loggini et al., 1999). Thus, the oxidative damage of important molecules is produced as a result of the imbalance between production of reactive oxygen species (ROS) of reduced O2 (i.e. superoxide radical [·O2−], hydrogen peroxide [H2O2], and the hydroxyl radical [·OH]) and antioxidant defenses (Iturbe-Ormaetxe et al., 1998).
During water deficit and other osmotic stresses, a plant strategy that may confer stress tolerance is the accumulation of compatible, low-molecular-weight osmolytes, such as sugar alcohols, special amino acids, and Gly-betaine. Another strategy is the activation of a large set of plant genes that leads to the accumulation of new proteins in vegetative tissues. That is the case of a gene family, which codifies for late embryogenesis abundant (LEA) proteins. In all angiosperms, LEA proteins are expressed to high levels during embryo maturation and often in dehydrated seedlings. These proteins are hydrophilic and, in some cases, it has been proposed to confer water stress tolerance. Whereas lea mRNAs and the corresponding proteins are not normally found in vegetative or immature seed tissue, the accumulation of some of these products can be induced in response to osmotic stress (Curry and Walker-Simmons, 1993; Ried and Walker-Simmons, 1993; Xu et al., 1996; Swire-Clark and Marcotte, 1999).
The aim of this work was to study, at the physiological and molecular level, the ability of NO to promote adaptive responses to cope with water deficit conditions. In this work, we show correlative evidence to explain why, when treated with NO donors, both wheat (Triticum aestivum) seedlings and detached leaves are more tolerant to drought-stress periods.
RESULTS
NO Diminished Water Loss Produced by Drought Stress
In previous works we demonstrate that NO has the capacity to counteract deleterious effects produced by methylviologen herbicides in plants (Beligni and Lamattina, 1999a, 1999b). We also discussed the dual behavior of NO (i.e. toxic or protective) depending on both the presence of large amount of ROS and the concentration of NO (Beligni and Lamattina, 1999c, 2001).
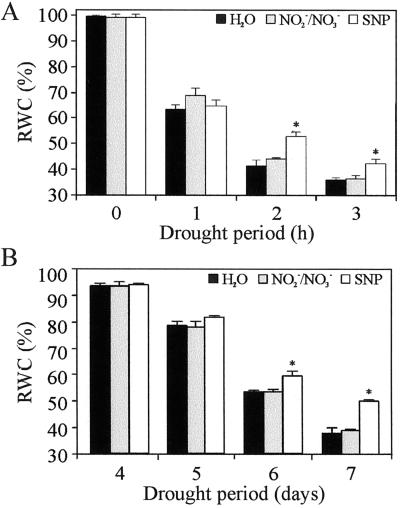
Since water deficit induces the generation of oxidative stress (Iturbe-Ormaetxe et al., 1998), we first tested if NO had any protective effect on detached wheat leaves subjected to severe drought periods. As NO donor we used 150 μm sodium nitroprusside (SNP), which releases 0.5 μm NO, in solution, during the first 48 h (data not shown). With that purpose, we determined relative water content (RWC) of detached wheat leaves subjected to different periods of drought for no longer than 3 h. The experiments were not extended because after 3 h, desiccated leaves cannot regain full turgor when rehydrated. Figure 1A shows that just 1 h of drought is enough to produce a 30% reduction of the RWC. Differences between treatments were significant after 2 h of drought when SNP-treated leaves had an RWC of approximately 53%, whereas in control leaves, treated with either water or NO2−/NO3− solution (as control for NO decomposition), RWC was significantly lower (42%). To enlarge the results of NO effect toward whole plant system, we measured RWC of wheat seedlings treated with water or 1 μm NO2−/NO3− or 150 μm SNP and subjected to a 10-d drought period. Figure 1B shows that SNP-treated seedlings tended to retain more water than control ones after 5 d without watering. This difference was statistically significant after 6 and 7 d of drought. Treatments with SNP did not affect the cycle of wheat, since all plants come into flowering and ear, normally (data not shown).
Figure 1.
Effect of NO on RWC in wheat. A, RWC of detached leaves: fully expanded wheat leaves were detached and treated with water or 1 μm NO2−/NO3− or 150 μm SNP and subjected to different drought periods (from 0–3 h). RWC values are expressed as percentages and represent the mean of 10 leaves per treatment repeated in three independent experiments. B, RWC of wheat seedlings: 10-d wheat seedlings watered once with Hoagland plus water or 1 μm NO2−/NO3− or 150 μm SNP were kept for 10 d without watering. RWC values were determined after 4, 5, 6, and 7 d of drought and represent the mean of five seedlings per treatment. The values are the average of three independent experiments. Bars correspond to se of each treatment. Asterisks show significant differences with a P < 0.05 (non-parametric unpaired t test).
Effect of NO on Transpiration and Stomatal Opening
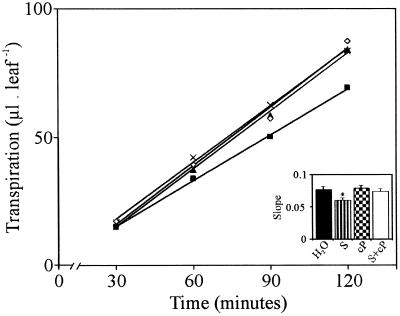
A possible effect of NO on transpiration was tested in detached wheat leaves subjected to 3 h of drought (Fig. 2). Those leaves treated with 150 μm SNP show a significant reduction (P < 0.05) in the amount of water loss compared with those of control treatments (water). To attribute to NO a role in decreasing transpiration, a specific NO scavenger, carboxi-PTIO, was analyzed on the same experimental system. Carboxi-PTIO alone did not have any effect on transpiration compared with control leaves. However, when combined with SNP, c-PTIO was able to prevent the SNP-mediated decrease of the transpiration (Fig. 2). When transpiration rate (TR, represented by the slope of each straight) was statistically tested, results showed a significant reduction of the TR (P < 0.005) in SNP-treated leaves compared with control ones (Fig. 2, inset).
Figure 2.
NO effect on the TR of detached wheat leaves. Fully expanded first leaves of 10-d-old wheat seedlings were detached and pretreated with water (⋄) or 150 μm SNP (▪), or 300 μm c-PTIO (▴) or with 150 μm SNP + 300 μm c-PTIO (×) as described in “Materials and Methods.” Detached leaves were then maintained on a white paper under light for 3 h (drought period). After that, leaves were transferred into tubes containing 200 μL of water. Water loss was measured and expressed as μL leaf−1. The TR was represented by the slope of each straight as transpiration per hour. The values are the average of three independent sets of 10 leaves each. Bars correspond to se of each treatment. Asterisk means significant value with a P < 0.05 (non-parametric unpaired t test).
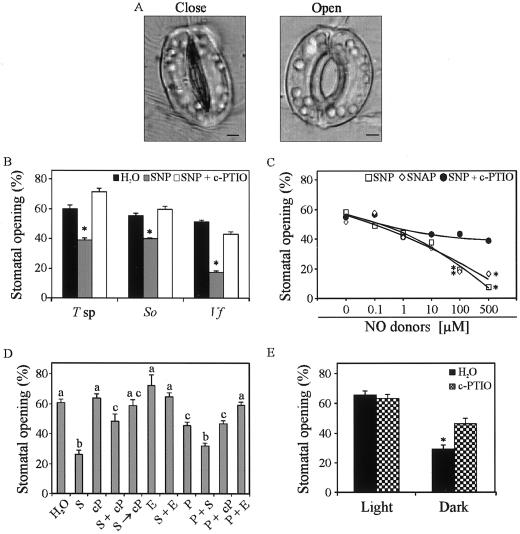
Guard cells modulate leaf transpiration and CO2 uptake by changing stomatal aperture (Kopka et al., 1997). Since we could not find the way to detect stomatal opening in wheat leaves, we decided to use leaf peels of another monocotyledonous species (Tradescantia sp.), as well as two dicotyledonous species (S. organifolia and fava bean [Vicia faba]). Epidermis strips from the different species were treated with water, SNP, or SNP + c-PTIO and observed at 400× under an optical microscope after 2 h of incubation. NO pretreated peels showed a significant reduction in the stomatal opening (35% for Tradescantia sp., 30% for S. organifolia, and 65% for fava bean) compared with control treatments, whereas c-PTIO treatments prevented again the SNP-mediated stomatal closure in the three assayed plant species (Fig. 3B). This result confirmed that NO, and no others derived compounds, was responsible for the induction of stomatal closure. Moreover, complete reversibility of the closure after SNP-treatment was achieved in experiments where c-PTIO was added together with SNP treatment or 1 h after (Fig. 3D; S + cP or S→cP, respectively), whereas cPTIO by itself did not induce stomatal closure (Fig. 3D; cP). When fava bean strips were treated with increasing concentrations of both NO donors, SNP or S-nitroso-N-acetylpenicillamine (SNAP), the percentage of open stomata decreased proportionally to increasing concentrations of the NO-donor, reaching significant differences at concentrations higher than 10 μm of either SNP or SNAP (Fig. 3C). When c-PTIO was added together with SNP treatment (c-PTIO concentration at each point was twice the concentration of SNP), the percentage of open stomata remain constant, independently of SNP concentrations. It is of consequence that the reduction of the percentage of stomatal opening due to NO-treatment was dose-dependent and reversible. Stomatal closure is induced by many different factors, such as osmotic stress, darkness, high CO2 concentrations, and some mechanical stresses (Kearns and Assmann, 1993). Regardless of the stimulus, all responses are accompanied by an increase in intracellular Ca2+ concentrations in the guard cell. Increases of Ca2+ concentration precede stomatal closure (Grabov and Blatt, 1999). Figure 3D shows that when 2 mm EGTA was added to epidermal strips to chelate intracellular Ca2+, NO-induced reduction of stomatal opening was reverted achieving values similar to control treatment. Also, when osmotic stress was imposed by incubating the epidermal strips in a 20% (w/v) polyethylene glycol (PEG) solution, the percentage of open stomata drops down from 60.6% ± 2.2% to 45.5% ± 1.8% (Fig. 3D; H2O and P). This reduction was more important (32.2% ± 2%) when 100 μm SNP was added to the PEG solution (Fig. 3D; P + S). Even in this case, when Ca2+ was chelated, the percentage of stomatal opening reach the normal levels (Fig. 3D, P + E).
Figure 3.
Effect of NO on stomatal opening. A, Open and closed fava bean stomata observed under optical microscope (×400) (bars = 5 μm). B, Epidermal strips of a monocotyledonous Tradescantia sp. (T sp) and two dicotyledonous S. organifolia (So) and fava bean (Vf) leaves were pre-incubated in opening solution and then treated with or without 200 μm SNP and with 200 μm SNP + 400 μm c-PTIO for 2 h. C, Fava bean strips treated for 1 h with increasing concentrations (0, 0.1, 1, 10, 100, or 500 μm) of SNP (□) or SNAP (⋄) or with increasing concentrations of SNP (0, 0.1, 1, 10, 100, or 500 μm) plus increasing concentrations (0, 0.2, 2, 20, 200, or 1,000 μm) of c-PTIO (●; se bars were excluded from the graph to make it clearer). D, Fava bean strips treated with: water, 150 μm SNP (S), 200 μm c-PTIO (cP), 100 μm SNP + 200 μm c-PTIO (S + cP), 100 μm SNP for 1 h and then 200 μm c-PTIO (S→cP), 2 mm EGTA (E), 150 μm SNP + 2 mm EGTA (S + E), 20% (w/v) PEG 8000 (P), 100 μm SNP + 20% (w/v) PEG 8000 (S + P), and PEG 8000 + 200 μm c-PTIO (P + cP) and 20% (w/v) PEG 8000 + 2 mm EGTA (P + E). Bars with different letters are significantly different at P < 0.05. E, Fava bean strips treated with or without 200 μm c-PTIO under light or darkness. Each value represents the mean of at least 90 stomata taken from different leaves, bars (when present) represent the se of each treatment. One way ANOVA was used for comparisons between the means. Asterisks indicates those treatments that were significantly different with respect to control treatments (P < 0.05).
We also tested if a NO scavenger could be able to interfere with a natural stomatal closure response during a well described stimulus like darkness. Figure 3E provides evidence that, under darkness conditions, the percentage of stomatal closure was partially prevented in V. faba (50%, P < 0.05) when endogenous NO was scavenged by 200 μm c-PTIO compared with control treatments.
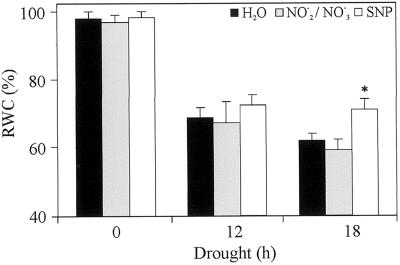
Since results on stomatal closure were not obtained from wheat leaves, we tested the effect of NO on drought stress tolerance in one of the species used for stomatal closure assays. With that aim, we measured RWC on Tradescantia sp. detached leaves treated with water or 1 μm NO2−/NO3− or 150 μm SNP and subjected to different drought periods. Tradescantia detached leaves were more resistant to desiccation than wheat leaves. Consequently, the drought periods assayed for Tradescantia sp. were longer than those used for wheat. Figure 4 shows that after 18 h of drought, SNP-treated leaves retain approximately 12% more water than control ones.
Figure 4.
Effect of NO on the RWC of Tradescantia sp. leaves. Fully expanded detached leaves were floated overnight on water or 1 μm NO2−/NO3− or 150 μm SNP and subjected to different drought periods. RWC values are expressed as percentages and represent the mean of 10 leaves per treatment, repeated in three independent experiments. Bars correspond to se of each treatment. Asterisks show significant differences with a P < 0.05 (non-parametric unpaired t-test).
Effect of NO on Cell Membrane Injury
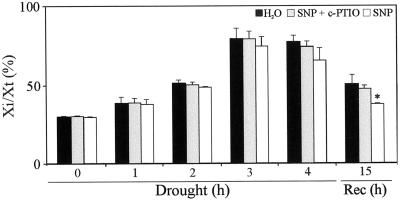
One of the described damages provoked by water-deficit stress is the membrane injury and the liberation of ions from the cell to the extracellular space (Halliwel and Gutteridge, 1984). This is a consequence of an oxidative burst leading to lipid peroxidation, membrane permeabilization and cell death (Scandalios, 1993). As shown in Figure 5, ion leakage expressed as a percentage of total conductivity (Xi/Xt) increased from 30% at the starting point of the experiment to 75% after 3 h of drought. Pretreatment of leaves with 150 μm SNP showed a slight decrease of the ion leakage after 3 and 4 h of drought, which was not statistically significant. However, ion leakage resulted 25% lower after 4 h of drought and 15 h of recovery in SNP-pretreated leaves compared with control ones (Fig. 5, Rec.). When c-PTIO was added together with SNP, Xi/Xt increased again up to levels of water treatment, arresting NO effect.
Figure 5.
Effect of NO on ion leakage in leaves subjected to drought stress. Ten-day-old wheat seedlings were treated with water or 300 μm c-PTIO + 150 μm SNP or 150 μm SNP. Twenty-four hours later, first leaves were detached and subjected to different drought periods (from 1–4 h) or to a 4-h drought period and 15 h of rehydration (Rec). Ion leakage was represented as the percentage of ions leaked at each time compared with the total ion leakage. Results are the average of three independent events. Asterisk shows significant differences with a P < 0.05 (non-parametric unpaired t-test).
Changes in the Expression of LEA Transcript
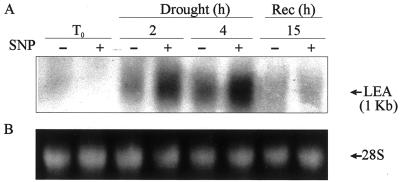
Several molecules accumulated during water-deficit stress are believed to confer tolerance to des-iccation. Among them, the group 3 LEA proteins have been described in desiccation-tolerant seedlings of wheat (Ried and Walker-Simmons, 1993). Therefore, wheat seedlings were pretreated with SNP, and 24 h later the first leaves were assayed to analyze the accumulation level of the group 3 LEA transcript, as a molecular marker of the plant response to drought stress. One of the transcript of approximately 1.0 kb hybridize to pMA1949 cDNA (Fig. 6). After longer exposures (15 d) another transcript of 2.4 kb could also be detected (data not shown). The size of the 1.0-kb transcript is consistent with the molecular masses (27–30 kD) of the four mayor proteins detected with group 3 antibodies in wheat (Ried and Walker-Simmons, 1993). Figure 6 shows that the 1.0-kb transcript was not accumulated in leaves from SNP-treated seedlings just after it had been detached (T0). However, after 2 h of drought period, SNP-pretreated leaves exhibited a 2-fold accumulation of the LEA transcript compared with control ones. Furthermore, this difference was even higher after a 4-h drought period (Fig. 6). It is interesting that after 4 h of drought and 15 h of rehydration by watering leaves to saturation, no differences in group 3 LEA transcripts were detected between leaves from SNP-pretreated seedlings and control ones, being the level similar to those of T0 (Fig. 6, Rec).
Figure 6.
Accumulation pattern of a group 3 LEA transcript in NO-treated wheat seedlings. Ten-day-old wheat seedlings were treated with 150 μm SNP or water. Twenty-four hours later, the first leaves were detached and subjected to drought and recovery as described in “Materials and Methods.” A, Total mRNA was isolated from detached leaves, fractionated by electrophoresis, transferred to nylon membranes, and hybridized with a [32P]-labeled cDNA probe of the pMA1949 clone (LEA). B, Ethidium bromide-stained 28S rRNA band is shown as loading control. Samples were collected before drought (T0), after 2 and 4 h of drought period (Drought), and after 4 h of drought plus 15 h of recovery (Rec).
DISCUSSION
In this work we demonstrate that NO, when applied exogenously, participates by conferring water-deficit tolerance to both detached wheat leaves and wheat seedlings subjected to drought stress conditions. As discussed by Bray (1997), resistance to water deficit occurs when a plant withstand the imposed stress, and may arise from either tolerance or a mechanism that permits avoidance of the situation. In this case, detached wheat leaves pretreated with the NO releaser SNP withstand the imposed stress by maintaining up to 15% more water than control ones pretreated with water or NO2−/NO3−. In addition, at the whole plant level, the RWC of SNP-treated wheat seedlings was significantly higher than in control ones after 6 and 7 d of drought (Fig. 1, A and B).
During drought stress processes, stomata are induced to close as leaves sense water deficit, especially after the leaf water potential drops below some threshold level. The production of ABA triggers the increase of cytosolic Ca2+ concentration in guard cells both via IP3 signal transduction cascade and also via cADPR. These processes are correlated with a reduction in stomatal aperture (Muir and Sanders, 1996; Grill and Himmelbach, 1998; Leckie et al., 1998).
In animals, NO has been reported to increase intracellular Ca2+ concentrations both in a cGMP-dependent or cGMP-independent way. These [Ca2+]i increases were reported to be a consequence of an uptake from the extracellular space (via L-type membrane channels) or due to Ca2+ liberation from intracellular stores (via cADPR gated Ca2+ channels or IP3 signal transduction cascade) (Ishii et al., 1997; Volk et al., 1997; Berkels et al., 2000). In plants, some evidences show that NO increased cGMP levels, which in turn stimulates the expression of plant defense genes. Some of these genes were also induced by cADPR, which means that this molecule could be second messenger of NO. On the other hand, cADPR activation of two of these genes was inhibited by a Ca2+ channel blocker, which locates Ca2+ downstream of cADPR in the signal transduction pathway (Durner et al., 1998; Klessig et al., 2000). In this work we demonstrate that concentrations lower than 0.2 μm NO, when applied exogenously through an NO donor, decreases the percentage of open stomata in a reversible manner. When Ca2+ was chelated with 2 mm EGTA, NO effect was completely reverted, suggesting that NO could be acting upstream of Ca2+. As expected, when 20% (w/v) PEG was added, the percentage of open stomata was 20% lower than control. This difference was higher (45%) when PEG was added together with SNP. It cannot be concluded yet whether the effects of PEG and NO are acting in an additive way or if both responses can be achieved through different pathways.
In another experiment, c-PTIO alone was added to fava bean epidermal strips and put under darkness. An inhibition of stomatal closure was observed, suggesting that endogenous NO could be playing a physiological role in stomatal movement mechanisms. The overall pore aperture under any given set of environmental conditions is a complex function of the interactions of hormone levels and signal transduction pathways triggered by mechanical and biochemical stimuli. Moreover, new evidences propose that individual stomata behavior is not responding independently and similarly to stimuli (Mott and Buckley, 2000). Thus, a new theory called “patchy stomatal conductance” in which patches of stomata respond differently from those in adjacent regions of the leaf, could be closely related to endogenous production of NO which is a small, highly diffusible and short-lived molecule. NO rapidly crosses biological membranes and can trigger biological responses in a short period of time. Thus, NO might provide some possible explanation to assemble the complex network of cellular components involved in stomatal responses and dynamics. The results presented here bring strong evidences on the capability of NO to modulate stomatal opening by acting within Ca2+ signal transduction pathway.
Oxidative stress was proven to be generated as a consequence of drought in plants, among other environmental constraints. We have previously reported that NO alleviates several consequences of oxidative stress such as chlorosis, DNA fragmentation, and apoptotic cell death (Laxalt et al., 1997; Beligni and Lamattina, 1999a, 1999b). One consequence of the oxidative stress is the ion leakage from the cell to the intercellular compartments (Halliwel and Gutteridge, 1984). In this matter, our results indicated that the percentage of ion leakage was significantly lower in those leaves treated with NO, after a drought period, and subsequent rehydration.
A number of water-deficit-induced gene products are predicted to protect cellular structures from the effects of water loss. These predictions are derived from the deduced amino acids sequences and mainly from the gene expression behavior. Some of these genes, called lea, were first identified as genes that are expressed during the maturation and desiccation phases of seed development (Close, 1996; Xu et al., 1996). However, it has been recognized that these genes are also expressed in vegetative tissues during periods of water loss resulting from water-deficit and low temperature stress (Curry and Walker-Simmons, 1993; Xu et al., 1996). In this work we report that in detached leaves from wheat seedlings treated with the NO releaser SNP, there is an accumulation of the LEA 3 transcript after different drought periods compared with control leaves and that this accumulation was parallel to an observed increase of water retention. These results are consistent with those reported by Xu et al. (1996) where a high level of the HVA1 protein (a barley group 3 LEA protein) in transgenic rice correlates with an increase in plant tolerance to water-deficit and salt stress. It remains to be demonstrated in our work if a proportional accumulation of LEA protein occurs in parallel to RNA increase. These results suggest that NO may also be acting somewhere in between the signal transduction pathway of LEA expression in response to drought stress.
In this paper, we expand upon our previous data concerning the putative physiological roles of NO in plants. Now, we are presenting results on the potential ability of NO to enhance plant fitness to withstand an environmental constraint like drought, probably through multiple factors that directly or indirectly result in a better housekeeping to make use of the available water.
MATERIALS AND METHODS
Plant Material
Wheat (Triticum aestivum L. var Oasis) seeds were obtained from EEA-INTA (Balcarce, Argentina). Seedlings were grown in pots containing vermiculite or soil:vermiculite (3:1, v/v). When wheat seedlings were growing in vermiculite, they were watered with Hoagland solution. Pots were kept at 25°C with a 14-h photoperiod, a photosynthetically active radiation (PAR) of 200 μE m−2 s−1 and 40% to 50% relative humidity. Fava bean (Vicia faba [Leguminosae]), Tradescantia sp. (Commelinaceae), and Salpichroa organifolia (Solaneceae) plants were grown in soil:vermiculite (3:1, v/v) at the same temperature and light conditions described above.
Chemicals and Treatments
SNP (Merck, Darmstadt, Germany) and SNAP (Molecular Probes, Eugene, OR) were used as NO donors, and carboxy-PTIO (c-PTIO) (Molecular Probes) were used as NO scavenger. The amount of NO released from 100 and 200 μm donor solutions in our experimental conditions was determined by the Griess reagent colorimetric kit (Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer's instructions and resulted within the range described in the literature from nanomolar to low micromolar amounts (Ferrer and Ros Barceló, 1999).
Ethylene glycol-bis N,N,N′,N′-tetraacetic acid (EGTA) and PEG 8000 were from Sigma (St. Louis).
Whole Plant Assays
Wheat seedlings grown in soil:vermiculite (3:1) were watered to saturation with Hoagland solution or Hoagland plus 1 μm NO2−/NO3− or Hoagland plus 150 μm SNP for 10 d at 25°C and 14-h photoperiod. Then, they were maintained for 10 d without watering under the same light and temperature conditions.
Detached Leaf Assays
Experiments were done with the first, fully expanded leaves from 10-d-old wheat seedlings. Pretreatments were done in two different ways, obtaining similar results: (a) by putting detached leaves from untreated wheat seedlings in tubes containing water, 150 μm SNP, 300 μm c-PTIO, or 150 μm SNP plus 300 μm c-PTIO and submitting them to three pulses of 15 s of vacuum (with a gap of 1 min between each pulse). After vacuum pulses, leaves were transferred to water containing tubes for 2 h under light at 25°C; (b) 10-d-old wheat seedlings were treated with NO donors, and 24 h later the first leaves were detached and used for assays. Drought stress was performed by placing different sets of detached leaves over a white paper under light (200 μE m−2 s−1) for 1 to 10 h depending on the experiment. After drought periods, different set of leaves were maintained under light in tubes containing distilled water to evaluate their rehydration capacity.
Tradescantia sp. Detached Leaves
Fully expanded Tradescantia sp. leaves were detached and floated for 10 h in Petri dishes containing water or 1 μm NO2−/NO3−or 150 μm SNP under light (200 μE m−2 s−1). Drought treatment was done by putting leaves over a white paper under light at 25°C for different times of stress.
RWC
RWC was determined for wheat seedlings, detached leaves, and for detached Tradescantia sp. leaves. For each experiment, RWC measurements were determined after different periods of drought, according to the formula:
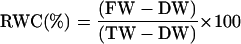 |
Fresh weight (FW) was measured at the end of the drought period, and dry weight (DW) was obtained after drying the samples at 75°C for at least 24 h. Turgor weight (TW) was determined by subjecting leaves to rehydration for 2 h, after drought treatments.
Transpiration Assay
These assays were done according to the method described by Wilkinson et al. (1998) with some modifications. Fully expanded first leaf from 10-d wheat seedlings where cut and treated as described before for detached leaves. Later, leaves were kept for 2 h in water and then transferred to ependorff tubes containing 200 μL of distilled water. Leaves were maintained under light (200 μE m−2 s−1) at 25°C and weighed individually every 30 min for 2 h.
Stomatal Aperture
Stomatal aperture experiments were performed with epidermis strips taken from fully developed leaves of one monocotyledonous plant, Tradescantia sp. (Commelinaceae), and two dicotyledonous plants, fava bean (Leguminaceae) and S. organifolia (Solaneceae).
Strips were cut with a scalpel and pre-incubated for 45 min with 10 mm KCl (fava bean) or 2 h with 50 mm PIPES, 50 mm KCl, 1 mm MgCl2 (Tradescantia sp. and S. organifolia) for promoting stomatal opening (at 25°C, under a PAR of 200 μE m−2 s−1). After pre-incubation, strips were maintained in the opening buffer (control) or treated with different solutions according to the experiment. Chemicals were added to the opening solution in the same plastic pots on which the epidermal strips were floated. After 2 h (Tradescantia sp. and S. organifolia) or 1 h (fava bean) of incubation, stomatal opening was measured considering open those with a pore width >2.5 μm or close those with a pore width <2.5 μm. Measurements were done at 400× with an optical microscope, and pore width was calculated using the Matrox Inspector 2.2 (Matrox Electronic System, Dorval, Canada) image analysis software. Each data value represents the mean of at least 90 stomata from at least three different epidermis sections of different leaves. Aperture values are presented as mean ± se.
Ion Leakage
SNP-pretreated and control (water or SNP + c-PTIO) detached wheat leaves were subjected to different drought periods (from 1–4 h) and to 15 h of water recovery as was previously described. After treatments, leaves were cut into 25-mm2 pieces and placed in Petri dishes with 12 mL of de-ionized water at 20°C to 25°C for 2 h. After the incubation, the conductivity in the bathing solution was determined (Xi) with an HI8733 conductivity meter (Hanna Instruments, Sigma). Then, the samples were heated at 80°C for 2 h in their effusates, and conductivity was read again in the bathing solution (Xt). Electrolyte leakage was expressed as a percentage of the total conductivity after heating at 80°C [(Xi/Xt) × 100] (Scotti Campos and Thu Pham Thi, 1997).
RNA Extraction and Northern-Blot Analysis
Total RNA was extracted with TRIZOL (RNA isolation reagent, Life Technologies/Gibco-BRL, Cleveland) according to the protocol suggested by the company. After the extraction, RNA was resuspended in 5 mm dithiothreitol, quantified, and stored at −80°C for further analysis. Extracted RNAs were separated and analyzed on denaturating 1% (w/v) agarose gels containing formaldehyde. After migration, RNAs were transferred onto a nylon membrane (Amersham, Buckinghamshire, UK) according to the manufacturer's directions and hybridized with the wheat LEA cDNA pMA1949 probe (Curry and Walker-Simmons, 1993). DNA probes were labeled by random priming (DuPont) using [α-32P]dCTP. Hybridization and washing conditions were as described by Laxalt et al. (1996).
Statistical Analysis
Each experiment was repeated at least three times. Values are expressed as means ± se. Data were analyzed using Statistica (Stat Soft Inc, Tulsa, OK) software. All mean comparisons were done using t-test for independent sample. For stomatal opening assays the different measurements were subjected to a one-way analysis of variance (ANOVA). In all cases the confidence coefficient was set at 0.05.
ACKNOWLEDGMENT
We thank Dr. Walker-Simmons (U.S. Department of Agriculture, Agricultural Research Service, Washington State University, Pullman) for his generous supply of the LEA probe.
Footnotes
This work was supported by the Agency Nacional de Promoción Científica Tecnológica (grant nos. PICT 01349–97 and PICT 1–6496–99 to L.L.), by Conicet (grant no. PIP 0898/98 to L.L.), and by institutional grants from Universidad Nacional de Mar del Plata (Argentina). L.L. is a career member from the Consejo Nacional de Investigaciones Científicas y Técnicas and C.G.M. is a research fellow from Agencia Nacional de Promoción Científica y Tecnológica.
LITERATURE CITED
- Anbar M. Nitric oxide: a synchronizing chemical messenger. Experientia. 1995;51:481–490. doi: 10.1007/BF02128740. [DOI] [PubMed] [Google Scholar]
- Barroso JB, Corpas FJ, Carreras A, Scandalios LM, Valderrama R, Palma JM, Lupiñes JA, del Rio LA. Localization of nitric oxide synthase in plant peroxisomes. J Biol Chem. 1999;274:36729–36733. doi: 10.1074/jbc.274.51.36729. [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide protects against cellular damage produced by methylviologen herbicides in potato plants. Nitric Oxide Biol Chem. 1999a;3:199–208. doi: 10.1006/niox.1999.0222. [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta. 1999b;208:337–344. [Google Scholar]
- Beligni MV, Lamattina L. Is nitric oxide toxic or protective? Trends Plant Sci. 1999c;4:299–300. doi: 10.1016/s1360-1385(99)01451-x. [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide in plants: the history is just beginning. Plant Cell Environ. 2001;24:267–278. [Google Scholar]
- Berkels R, Suerhoff S, Roesen R, Klaus W. Nitric oxide causes a cGMP-independent intracellular calcium rise in porcine endothelial cells: a paradox? Microvasc Res. 2000;59:38–44. doi: 10.1006/mvre.1999.2191. [DOI] [PubMed] [Google Scholar]
- Blatt MR. Ca2+ signaling and control of guard-cell volume in stomatal movements. Curr Opin Plant Biol. 2000;3:196–204. [PubMed] [Google Scholar]
- Bray EA. Plant responses to water deficit. Trends Plant Sci. 1997;2:48–54. [Google Scholar]
- Close TJ. Dehydrins: emergence of a biochemical role of a family of plant dehydration protein. Physiol Plant. 1996;97:795–803. [Google Scholar]
- Curry J, Walker-Simmons MK. Unusual sequence of group 3 LEA (II) mRNA inducible by dehydration stress in wheat. Plant Mol Biol. 1993;21:907–912. doi: 10.1007/BF00027121. [DOI] [PubMed] [Google Scholar]
- Durner J, Klessig DF. Nitric oxide as a signal in plants. Curr Opin Plant Biol. 1999;2:369–374. doi: 10.1016/s1369-5266(99)00007-2. [DOI] [PubMed] [Google Scholar]
- Durner J, Wendehenne D, Klessig DF. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer MA, Ros Barceló A. Differential effects of nitric oxide on peroxidase and H2O2 production by the xylem of Zinnia elegans. Plant Cell Environ. 1999;22:891–897. [Google Scholar]
- Grabov A, Blatt MR. A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol. 1999;119:277–287. doi: 10.1104/pp.119.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E, Himmelbach A. ABA signal transduction. Curr Opin Plant Biol. 1998;1:412–418. doi: 10.1016/s1369-5266(98)80265-3. [DOI] [PubMed] [Google Scholar]
- Halliwel B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Yuyama H, Matsuura N, Abe SI, Tanaka Y, Nakayama K. Regulation of the intracellular free calcium concentration of rat pancreatic β cells via the nitric oxide-cyclic GMP system. In: Moncada S, Toda N, Higgs EA, editors. The Proceedings of the Fifth International Meeting on the Biology of Nitric Oxide, Kyoto, Japan. London: Portland Press; 1997. p. 178. [Google Scholar]
- Iturbe-Ormaetxe I, Escudero PR, Arrese-Igor C, Becana M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998;116:173–181. [Google Scholar]
- Kearns EV, Assmann SM. The guard cell-environmental connection. Plant Physiol. 1993;102:711–715. doi: 10.1104/pp.102.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, Zhou JM, Shah J, Zhang S, Kachroo P. Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Scio. 2001;97:8849–8855. doi: 10.1073/pnas.97.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopka J, Provart JN, Müller-Röer B. Potato guard cells respond to drying soil by a complex change in the expression of genes related to carbon metabolism and turgor regulation. Plant J. 1997;11:871–888. doi: 10.1046/j.1365-313x.1997.11040871.x. [DOI] [PubMed] [Google Scholar]
- Laxalt AM, Beligni M, Lamattina L. Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophthora infestans. Eur J Plant Pathol. 1997;73:643–651. [Google Scholar]
- Laxalt AM, Cassia RO, Sanllorenti PM, Madrid EA, Andreu AB, Daleo GR, Conde RD, Lamattina L. Accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase RNA under biological stress conditions and elicitor treatments in potato. Plant Mol Biol. 1996;30:961–972. doi: 10.1007/BF00020807. [DOI] [PubMed] [Google Scholar]
- Leckie CP, McAinsh MR, Allen GJ, Sanders D, Hetherington AM. Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:15837–15842. doi: 10.1073/pnas.95.26.15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem YY, Haramaty E, Iluz D, Malik Z, Sofer Y, Roitman L, Leshem Y. Effect of stress nitric oxide (NO): interaction between chlorophyll fluorescence, galactolipid fluidity and lipoxygenase activity. Plant Physiol Biochem. 1997;35:573–579. [Google Scholar]
- Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F. Antioxidant defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol. 1999;119:1091–1099. doi: 10.1104/pp.119.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue KF, Hanson AD. Drought and salt tolerance: towards understanding and application. Trends Biotechnol. 1990;8:358–363. [Google Scholar]
- Moilanen E, Vapaatalo H. Nitric oxide in inflammation and immune response. Ann Med. 1995;27:359–367. doi: 10.3109/07853899509002589. [DOI] [PubMed] [Google Scholar]
- Mott KA, Buckley TN. Patchy stomatal conductance in emergent collective behavior of stomata. Trends Plant Sci. 2000;5:258–262. doi: 10.1016/s1360-1385(00)01648-4. [DOI] [PubMed] [Google Scholar]
- Muir SR, Sanders D. Pharmacology of Ca2+ release from red beet microsomes suggests the presence of ryanodine receptor homologs in higher plants. FEBS Lett. 1996;395:39–42. doi: 10.1016/0014-5793(96)01000-9. [DOI] [PubMed] [Google Scholar]
- Ninneman H, Maier J. Indications for the occurrence of nitric oxide synthases in fungi and plants and the involvement in photoconidiation of Neurospora crassa. Photochem Photobiol. 1996;64:393–398. doi: 10.1111/j.1751-1097.1996.tb02477.x. [DOI] [PubMed] [Google Scholar]
- Ried JL, Walker-Simmons MK. Group 3 late embryogenesis abundant proteins in desiccation-tolerant seedlings of wheat (Triticum aestivum L.) Plant Physiol. 1993;102:125–131. doi: 10.1104/pp.102.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. Oxygen stress and superoxide dismutases. Plant Physiol. 1993;101:7–12. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti Campos P, Thu Pham Thi A. Effect of abscisic acid pretreatment on membrane leakage and lipid composition of Vigna unguiculata leaf discs subjected to osmotic stress. Plant Sci. 1997;130:11–18. [Google Scholar]
- Swire-Clark GA, Marcotte WR., Jr The wheat LEA protein Em functions as an osmoprotective molecule in Saccharomyces cerevisiae. Plant Mol Biol. 1999;39:117–128. doi: 10.1023/a:1006106906345. [DOI] [PubMed] [Google Scholar]
- Volk T, Mading K, Hensel M, Kox WJ. Nitric oxide induces transient Ca2+ changes in endothelial cells independent of cGMP. J Cell Physiol. 1997;172:296–305. doi: 10.1002/(SICI)1097-4652(199709)172:3<296::AID-JCP3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Corlett JE, Oger L, Davies WJ. Effects of xylem pH on transpiration from wild–type and flacca tomato leaves. Plant Physiol. 1998;117:703–709. doi: 10.1104/pp.117.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink DA, Hanbauer L, Krishna MC, DeGraff W. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Natl Acad Sci USA. 1993;90:9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Kuzma J, Maréchal E, Graeff R, Lee HC, Foster R, Chua N-H. Abscisic acid signaling through cyclic ADP-ribose in plants. Science. 1997;282:226–230. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]
- Xu D, Duan X, Wang B, Hong B, Ho TD, Wu R. Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol. 1996;110:249–257. doi: 10.1104/pp.110.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]