Abstract
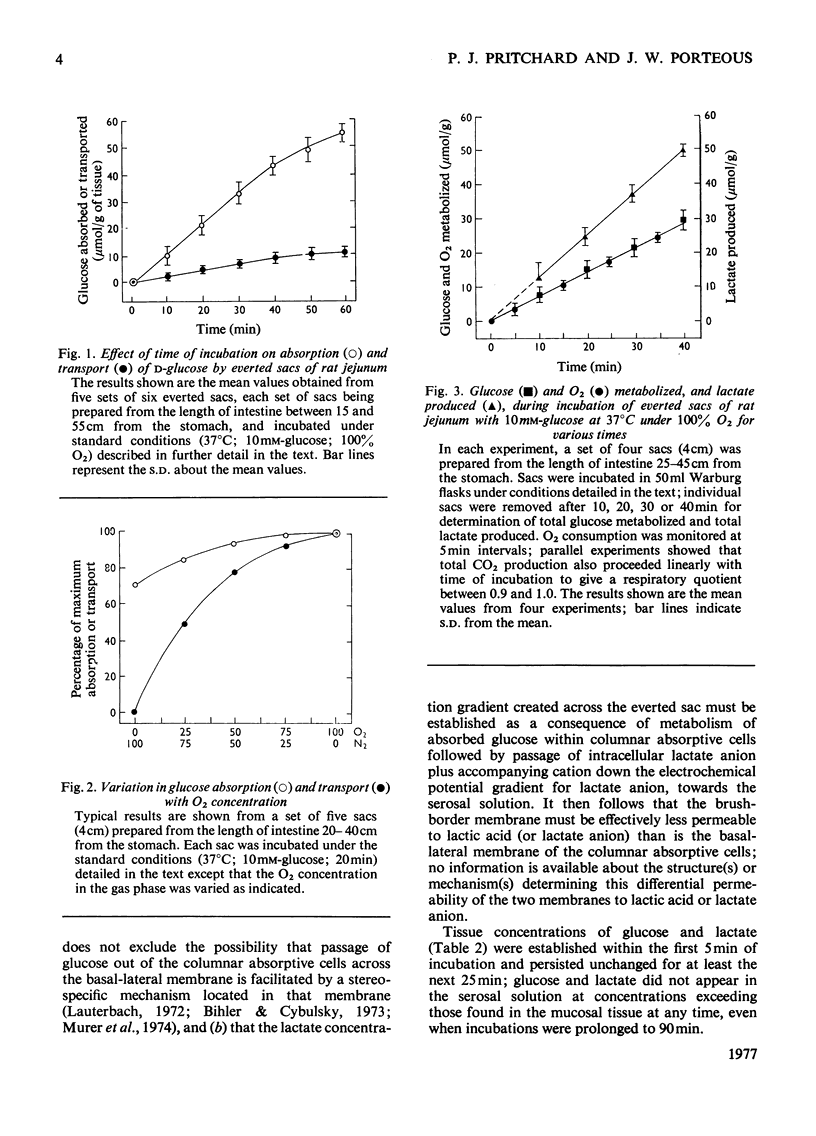
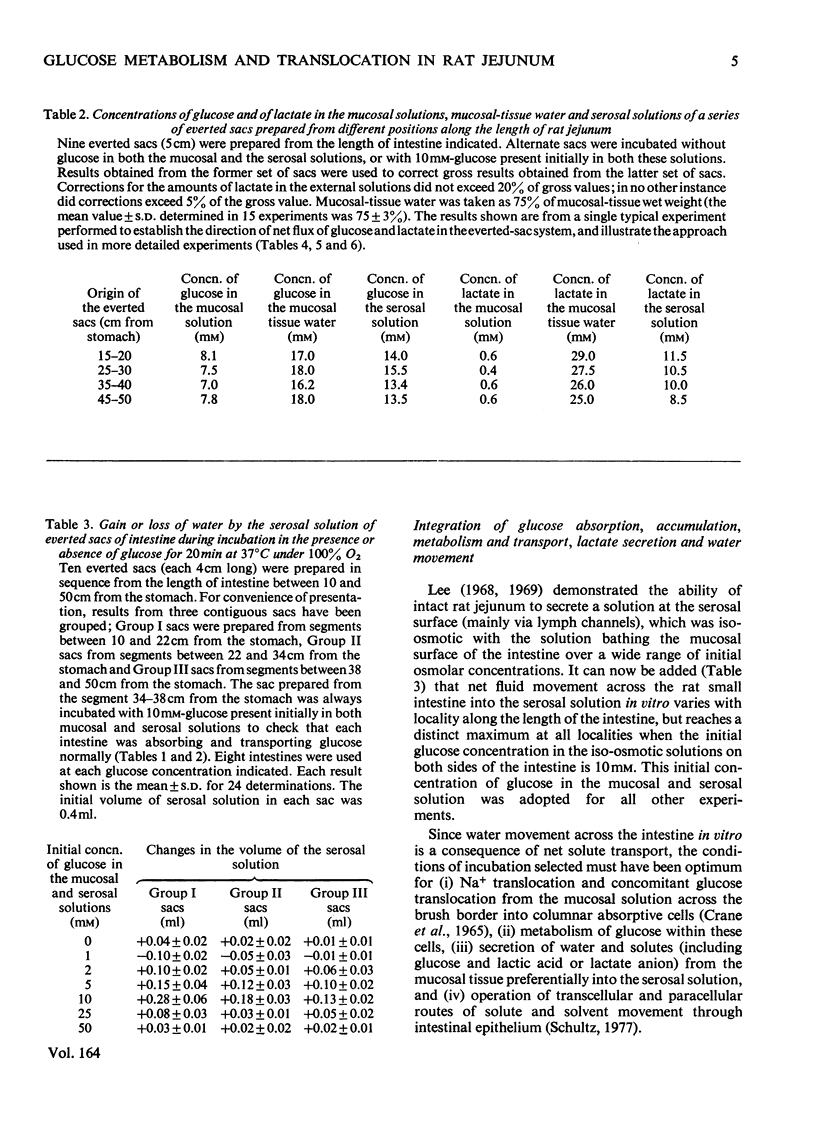
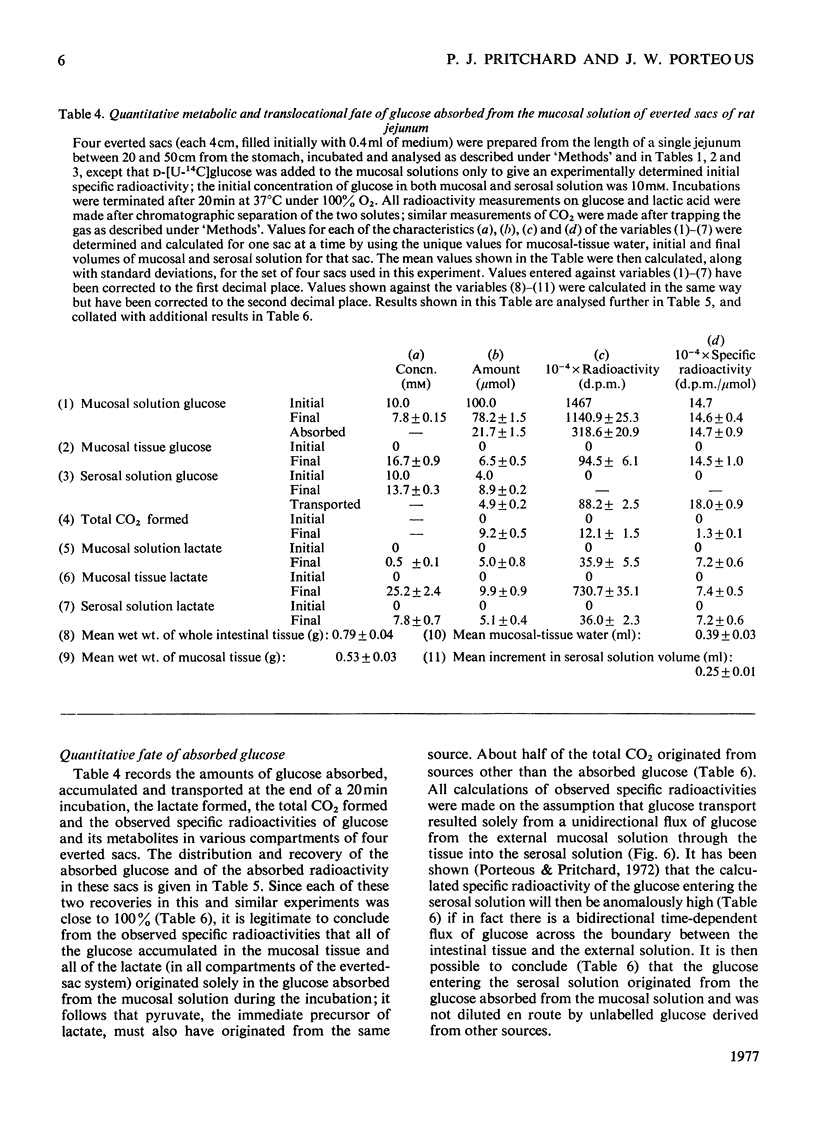
1. Conditions of incubation of everted sacs of rat small intestine were selected to ensure that absorption of d-glucose by mucosal tissue from the incubation medium, intracellular metabolism of the absorbed glucose and transport of glucose through the intact intestinal tissue proceeded linearly with respect to time of incubation within stated time intervals. 2. Under these experimental conditions, steady intracellular concentrations of glucose and lactate were demonstrated. 3. The quantitative translocational and metabolic fate of absorbed glucose was determined under these steady-state conditions. About 25% of glucose absorbed from the external mucosal solution was accumulated (temporarily) within mucosal tissue and about 25% transported through the intact tissue into the external serosal solution; the remainder (about 50%) of the absorbed glucose was metabolized, 90% to lactate and 10% to CO2. Concomitant respiration rates were comparable with those reported for several other preparations of intestine and were stoicheiometrically in excess of the O2 metabolism required to account for the production of CO2 from the absorbed glucose. 4. Water transport through the everted sacs proceeded at an optimum rate under the experimental conditions selected. 5. Some other observations are recorded which influenced the design of the experiments and the interpretation of results; these include the initial physiological state of the animal, the anaesthetic used and the ionic composition of the incubation medium.
Full text
PDF


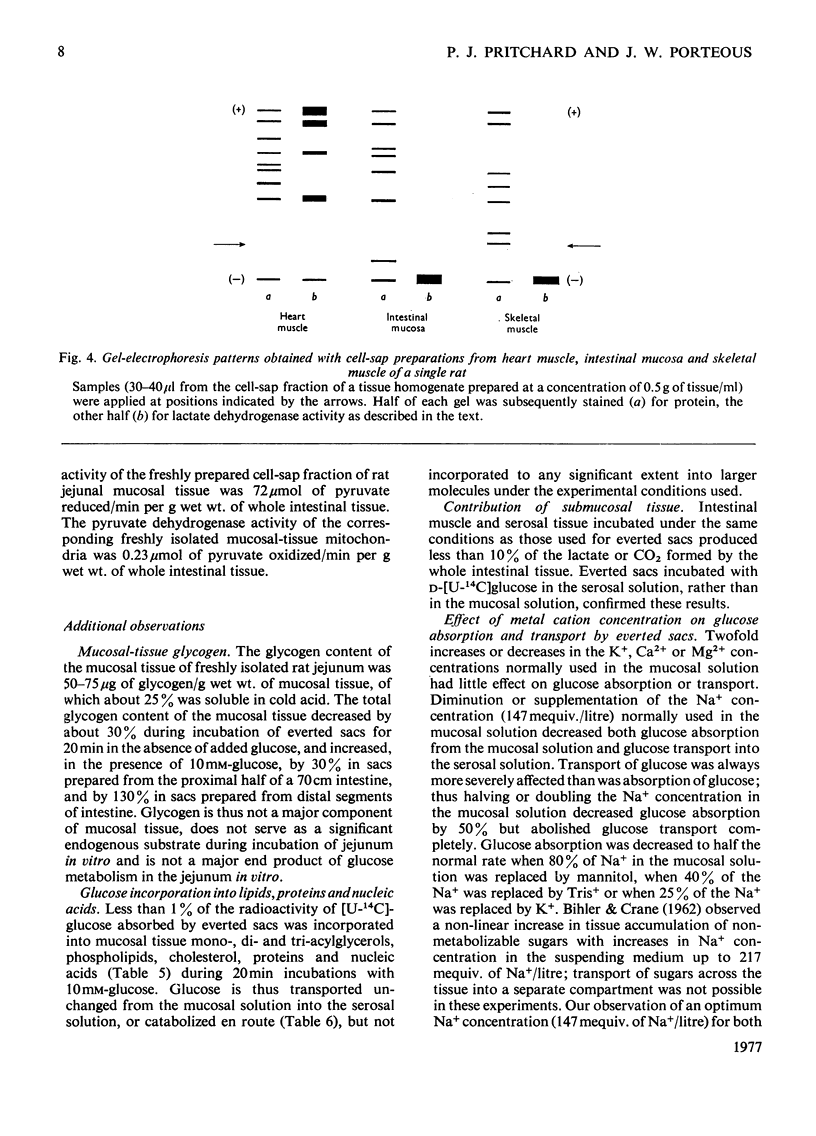

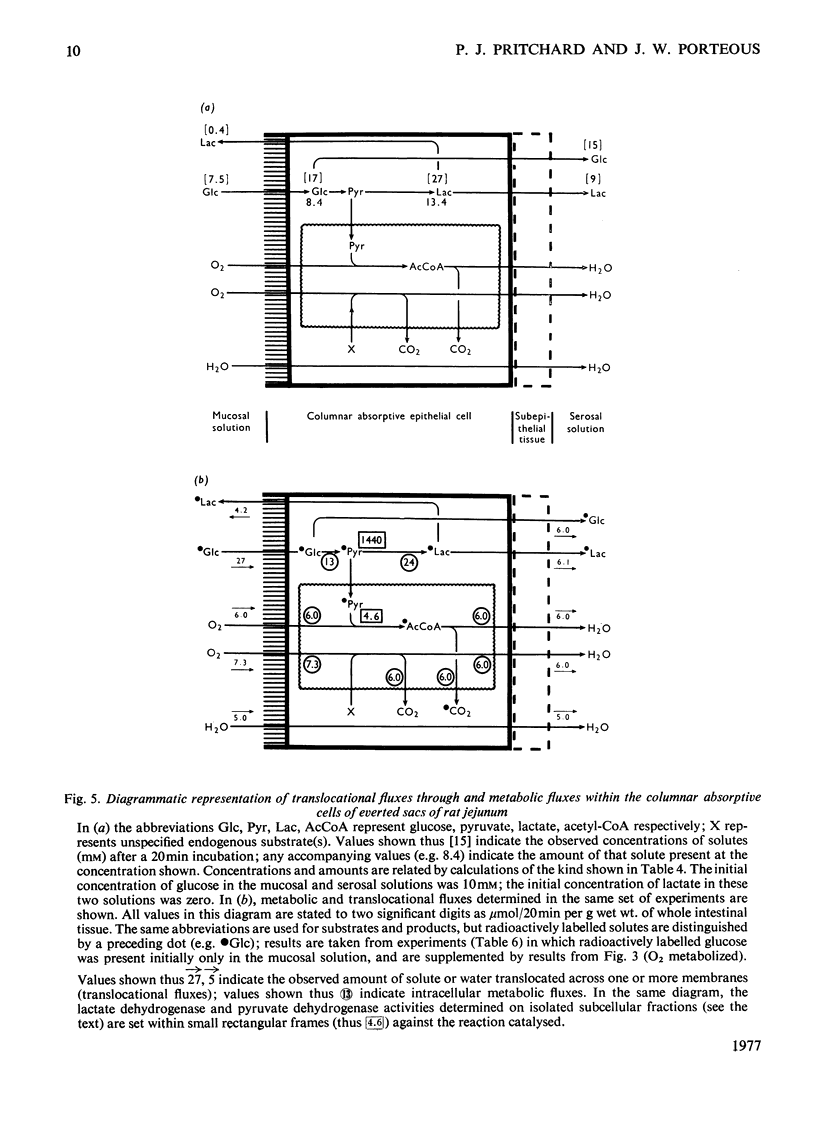

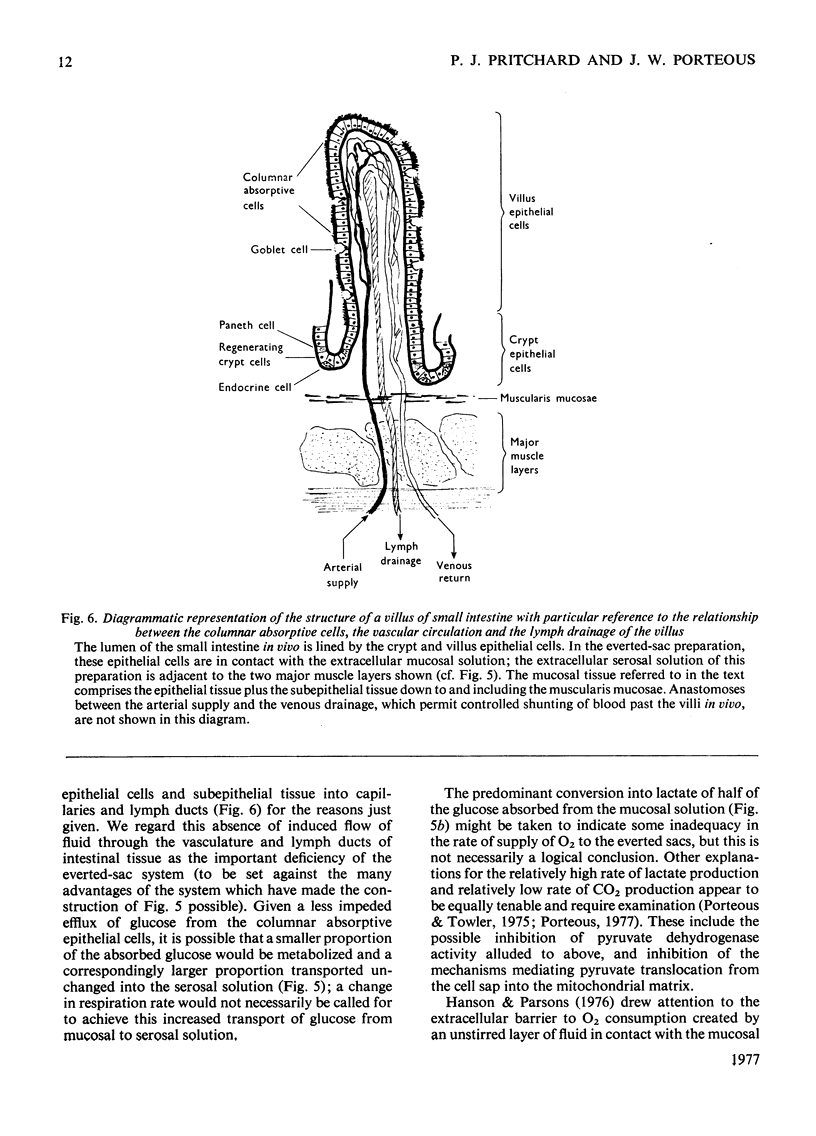


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIHLER I., CRANE R. K. Studies on the mechanism of intestinal absorption of sugars. V. The influence of several cations and anions on the active transport of sugars, in vitro, by various preparations of hamster small intestine. Biochim Biophys Acta. 1962 May 7;59:78–93. doi: 10.1016/0006-3002(62)90699-6. [DOI] [PubMed] [Google Scholar]
- BIHLER I., HAWKINS K. A., CRANE R. K. Studies on the mechanism of intestinal absorption of sugars. VI. The specificity and other properties of Na ion-dependent entrance of sugars into intestinal tissue under anaerobic conditions, in vitro. Biochim Biophys Acta. 1962 May 7;59:94–102. doi: 10.1016/0006-3002(62)90700-x. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihler I., Cybulsky R. Sugar transport at the basal and lateral aspect of the small intestinal cell. Biochim Biophys Acta. 1973 Mar 16;298(2):429–436. doi: 10.1016/0005-2736(73)90370-2. [DOI] [PubMed] [Google Scholar]
- Bronk J. R., Parsons D. S. The polarographic determination of the respiration of the small intestine of the rat. Biochim Biophys Acta. 1965 Oct 18;107(3):397–404. doi: 10.1016/0304-4165(65)90183-2. [DOI] [PubMed] [Google Scholar]
- CRANE R. K. Studies on the mechanism of the intestinal absorption of sugars. III. Mutual inhibition, in vitro, between some actively transported sugars. Biochim Biophys Acta. 1960 Dec 18;45:477–482. doi: 10.1016/0006-3002(60)91483-9. [DOI] [PubMed] [Google Scholar]
- Crane R. K., Forstner G., Eichholz A. Studies on the mechanism of the intestinal absorption of sugars. X. An effect of Na+ concentration on the apparent Michaelis constants for intestinal sugar transport, in vitro. Biochim Biophys Acta. 1965 Nov 29;109(2):467–477. doi: 10.1016/0926-6585(65)90172-x. [DOI] [PubMed] [Google Scholar]
- Csáky T. Z., Hara Y. Inhibition of active intestinal sugar transport by digitalis. Am J Physiol. 1965 Sep;209(3):467–472. doi: 10.1152/ajplegacy.1965.209.3.467. [DOI] [PubMed] [Google Scholar]
- Esposito G., Faelli A., Capraro V. Metabolism and transport phenomena in isolated intestine of normal and semistarved rats. Arch Int Physiol Biochim. 1967 Sep;75(4):601–608. doi: 10.3109/13813456709112508. [DOI] [PubMed] [Google Scholar]
- Everse J., Kaplan N. O. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- FISHER R. B., PARSONS D. S. Glucose absorption from surviving rat small intestine. J Physiol. 1949 Dec;110(3-4):281–293. doi: 10.1113/jphysiol.1949.sp004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLKOW B., LEWIS D. H., LUNDGREN O., MELLANDER S., WALLENTIN I. THE EFFECT OF THE SYMPATHETIC VASOCONSTRICTOR FIBRES ON THE DISTRIBUTION OF CAPILLARY BLOOD FLOW IN THE INTESTINE. Acta Physiol Scand. 1964 Aug;61:458–466. [PubMed] [Google Scholar]
- Greenway C. V., Lawson A. E. The effects of adrenaline and noradrenaline on venous return and regional blood flows in the anaesthetized cat with special reference to intestinal blood flow. J Physiol. 1966 Oct;186(3):579–595. doi: 10.1113/jphysiol.1966.sp008057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHORST H. J. Enzymatische Bestimmung von L(+)-Milchsäure. Biochem Z. 1957;328(7):509–521. [PubMed] [Google Scholar]
- Hanson P. J., Parsons D. S. The utilization of glucose and production of lactate by in vitro preparations of rat small intestine: effects of vascular perfusion. J Physiol. 1976 Mar;255(3):775–795. doi: 10.1113/jphysiol.1976.sp011307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrikson R. L., Hartley B. S. Purification and properties of methionyl-transfer-ribonucleic acid synthetase from Escherichia coli. Biochem J. 1967 Oct;105(1):17–24. doi: 10.1042/bj1050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins H., Von Brand T. Separation of lactic acid and some Krebs cycle acids by thin-layer chromatography. Anal Biochem. 1966 Apr;15(1):122–126. doi: 10.1016/0003-2697(66)90254-5. [DOI] [PubMed] [Google Scholar]
- KOHN J. Small-scale membrane filter electrophoresis and immuno-electrophoresis. Clin Chim Acta. 1958 Sep;3(5):450–454. doi: 10.1016/0009-8981(58)90038-x. [DOI] [PubMed] [Google Scholar]
- LIS E. W., TINOCO J., OKEY R. A micromethod for fractionation of lipids by silicic acid chromatography. Anal Biochem. 1961 Apr;2:100–106. doi: 10.1016/0003-2697(61)90058-6. [DOI] [PubMed] [Google Scholar]
- LUSTY C. J., SINGER T. P. LIPOYL DEHYDROGENASE. FREE AND COMPLEXED FORMS IN MAMMALIAN MITOCHONDRIA. J Biol Chem. 1964 Nov;239:3733–3742. [PubMed] [Google Scholar]
- Lamers J. M., Hülsmann W. C. Pasteur effect in the in vitro vascularly perfused rat small intestine. Biochim Biophys Acta. 1972 Sep 20;275(3):491–495. doi: 10.1016/0005-2728(72)90234-4. [DOI] [PubMed] [Google Scholar]
- Lauterbach F. Die Permeation von zuckern durch die kontraluminalen Membranen der Mucosazellen des Dünndarmes. Hoppe Seylers Z Physiol Chem. 1972 May;353(5):731–731. [PubMed] [Google Scholar]
- Lee J. S. Isosmotic absorption of fluid from rat jejunum in vitro. Gastroenterology. 1968 Mar;54(3):366–374. [PubMed] [Google Scholar]
- Leese H. J., Bronk J. R. Lactate formation by rat small intestine in vitro. Biochim Biophys Acta. 1975 Sep 8;404(1):40–48. doi: 10.1016/0304-4165(75)90145-2. [DOI] [PubMed] [Google Scholar]
- Leese H. J., Mansford K. R. The effect of insulin and insulin deficiency on the transport and metabolism of glucose by rat small intestine. J Physiol. 1971 Feb;212(3):819–838. doi: 10.1113/jphysiol.1971.sp009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin F. J., Syme G. Thyroid control of small intestinal oxygen consumption and the influence of sodium ions, oxyhen tension, glucose and anaesthesia. J Physiol. 1975 Feb;245(1):271–287. doi: 10.1113/jphysiol.1975.sp010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. R., McNary W. F., Kornguth P. J., LeBlanc R. Histological reevaluation of everted gut technique for studying intestinal absorption. Eur J Pharmacol. 1970 Feb;9(2):211–219. doi: 10.1016/0014-2999(70)90302-x. [DOI] [PubMed] [Google Scholar]
- MCDOUGAL D. B., Jr, LITTLE K. D., CRANE R. K. Studies on the me hanism of intestinal absorption of sugars. IV. Localization of galactose concenhrations within the intestinal wall during active transport, in vitro. Biochim Biophys Acta. 1960 Dec 18;45:483–489. doi: 10.1016/0006-3002(60)91484-0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- Murer H., Hopfer U., Kinne-Saffran E., Kinne R. Glucose transport in isolated brush-border and lateral-basal plasma-membrane vesicles from intestinal epithelial cells. Biochim Biophys Acta. 1974 Apr 29;345(2):170–179. doi: 10.1016/0005-2736(74)90256-9. [DOI] [PubMed] [Google Scholar]
- NEWEY H., PARSONS B. J., SMYTH D. H. The site of action of phlorrhizin in inhibiting intestinal absorption of glucose. J Physiol. 1959 Oct;148:83–92. doi: 10.1113/jphysiol.1959.sp006274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTEOUS J. W., CLARK B. THE ISOLATION AND CHARACTERIZATION OF SUBCELLULAR COMPONENTS OF THE EPITHELIAL CELLS OF RABBIT SMALL INTESTINE. Biochem J. 1965 Jul;96:159–171. doi: 10.1042/bj0960159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POULIK M. D. Starch gel electrophoresis in a discontinous system of buffers. Nature. 1957 Dec 28;180(4600):1477–1479. doi: 10.1038/1801477a0. [DOI] [PubMed] [Google Scholar]
- Robinson J. W., Antonioli J. A., Mirkovitch V. The intestinal response to ischaemia. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1966;255(2):178–191. doi: 10.1007/BF00543211. [DOI] [PubMed] [Google Scholar]
- Robinson J. W. Comparative aspects of the response of the intestine to its ionic environment. Comp Biochem Physiol. 1970 Jun 1;34(3):641–655. doi: 10.1016/0010-406x(70)90290-2. [DOI] [PubMed] [Google Scholar]
- Robinson J. W. The loss of intestinal transport capacity following preincubation in sodium-free media in vitro. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;294(2):182–200. doi: 10.1007/BF00363605. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. II. THE INTERACTION BETWEEN ACTIVE SODIUM AND ACTIVE SUGAR TRANSPORT. J Gen Physiol. 1964 Jul;47:1043–1059. doi: 10.1085/jgp.47.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejwani G. A., Kaur J., Ananthanarayanan M., Ramaiah A. Concentrations of various effectors and substrates of phosphofructokinase in the jejunum of rat and their relation to the lack of Pasteur effect in this tissue. Biochim Biophys Acta. 1974 Nov 25;370(1):120–129. doi: 10.1016/0005-2744(74)90038-2. [DOI] [PubMed] [Google Scholar]
- VESELL E. S. Significance of the heterogeneity of lactic dehydrogenase activity in human tissues. Ann N Y Acad Sci. 1961 Nov 2;94:877–889. doi: 10.1111/j.1749-6632.1961.tb35581.x. [DOI] [PubMed] [Google Scholar]
- VOMHOF D. W., TUCKER T. C. THE SEPARATION OF SIMPLE SUGARS BY CELLULOSE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1965 Feb;17:300–306. doi: 10.1016/s0021-9673(00)99872-8. [DOI] [PubMed] [Google Scholar]
- WAGNER H., HOERHAMMER L., WOLFF P. [Thin layer chromatography of phosphatides and glycolipids]. Biochem Z. 1961;334:175–184. [PubMed] [Google Scholar]
- WARING P. P., ZIPORIN Z. Z. THE SEPARATION OF HEXOSEPHOSPHATES AND TRIOSEPHOSPHATES BY THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Jul;15:168–172. doi: 10.1016/s0021-9673(01)82763-1. [DOI] [PubMed] [Google Scholar]
- WILSON T. H. Concentration gradients of lactate, hydrogen and some other ions across the intestine in vitro. Biochem J. 1954 Mar;56(3):521–527. doi: 10.1042/bj0560521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON T. H. Lactate and hydrogen ion gradients developed across the rat intestine in vitro. Biochim Biophys Acta. 1953 Jul;11(3):448–449. doi: 10.1016/0006-3002(53)90070-5. [DOI] [PubMed] [Google Scholar]
- WILSON T. H. The role of lactic acid production in glucose absorption from the intestine. J Biol Chem. 1956 Oct;222(2):751–763. [PubMed] [Google Scholar]
- WILSON T. H., WISEMAN G. Metabolic activity of the small intestine of the rat and golden hamster (Mesocricetus auratus). J Physiol. 1954 Jan;123(1):126–130. doi: 10.1113/jphysiol.1954.sp005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON T. H., WISEMAN G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol. 1954 Jan;123(1):116–125. doi: 10.1113/jphysiol.1954.sp005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Fat transport and lymph and plasma lipoprotein biosynthesis by isolated intestine. J Lipid Res. 1972 Jan;13(1):92–105. [PubMed] [Google Scholar]


