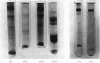
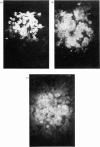
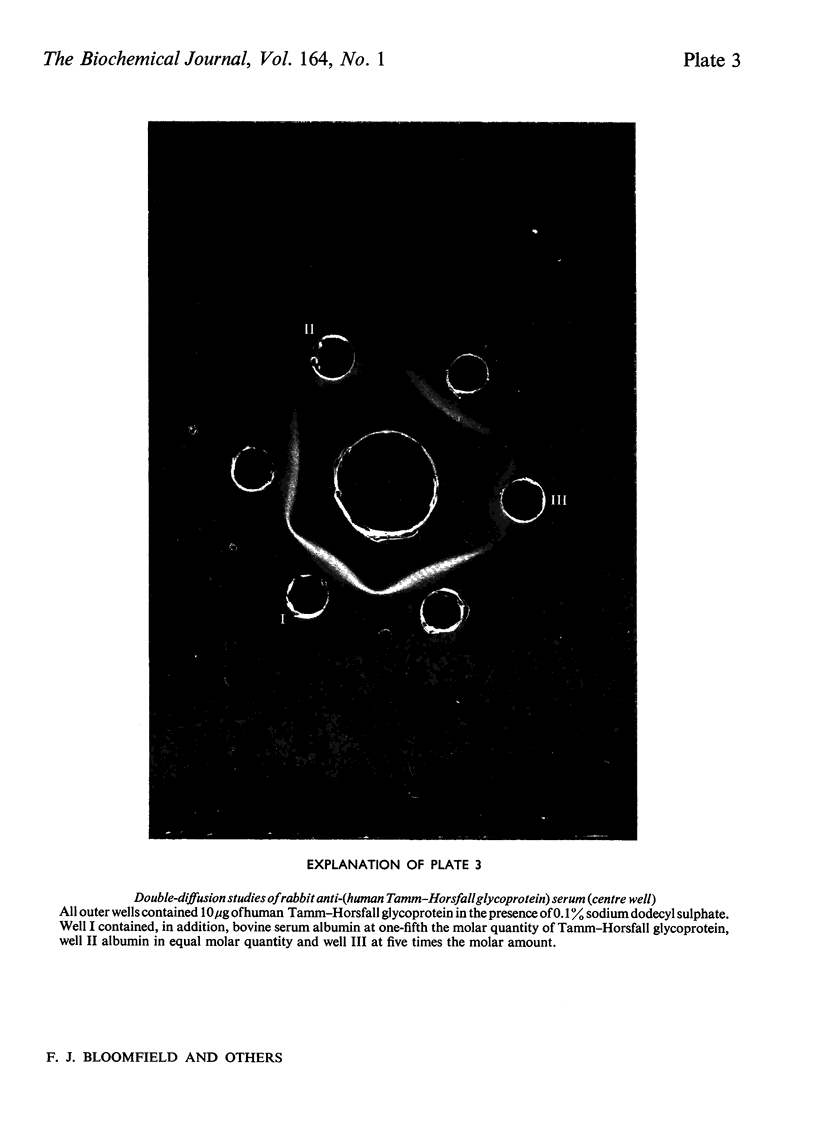
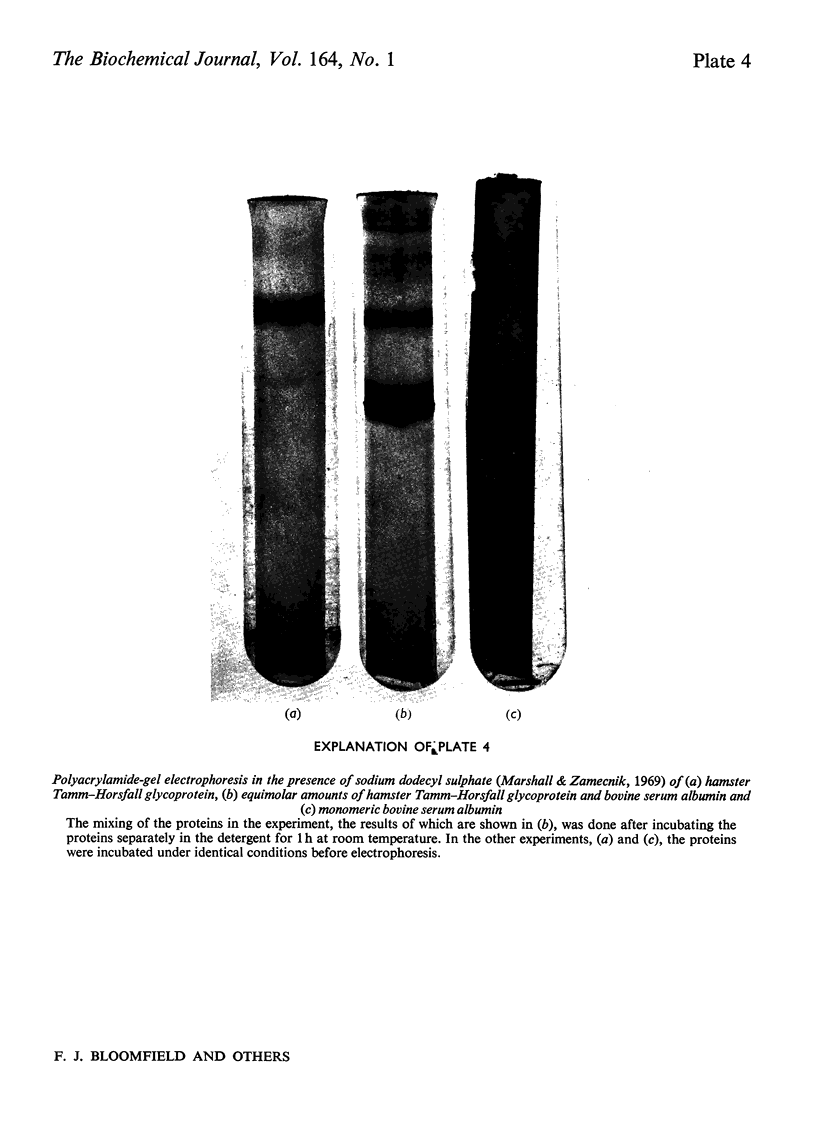
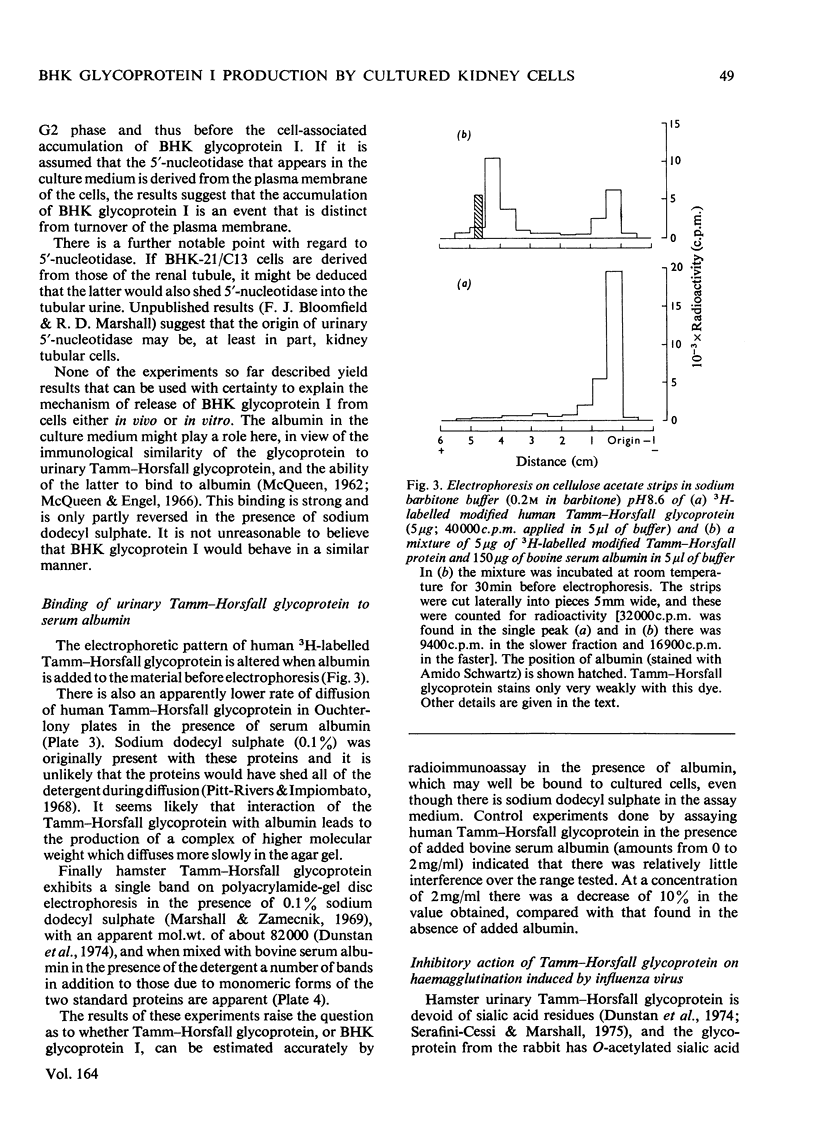
Abstract
Cultured baby-hamster kidney cells (BHK-21/C13), which are adapted to grow in suspension (strain 2P), roduce a glycoprotein, termed BHK glycoprotein I, which cross-reacts immunologically with hamster urinary (Tamm-Horsfall glycoprotein. BHK glycoprotein I was isolated in an electrophoretically (sodium dodecyl sulphate/polyacrylamide gel) homogeneous form by application of affinity chromatography to the medium in which cells had been cultured. Insolubilized anti-(Tamm-Horsfall glycoprotein immunoglobulin G) was used as the adsorbent. The amount of BHK glycoprotein I associated with the cultured cells was found by both radioimmunoassay and immunofluorescence to be related to the amount of Ca2+ in the medium and to the particular stage of the cell cycle. 5'-Nucleotidase was also shed by the cells into the culture medium in amounts related to the stage of the cell cycle. The turnover of hamster Tamm-Horsfall glycoprotein in vivo appeared to be considerably more rapid than can be accounted for by cell turnover. Hamster Tamm-Horsfall glycoprotein was shown to be ineffective in inhibiting agglutination of chicken erythrocytes caused by influenza virus.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bürk R. R. One-step growth cycle for BHK21-13 hamster fibroblasts. Exp Cell Res. 1970 Dec;63(2):309–316. doi: 10.1016/0014-4827(70)90218-1. [DOI] [PubMed] [Google Scholar]
- CORNELIUS C. E., MIA A. S., ROSENFELD S. RUMINANT UROLITHIASIS. VII. STUDIES ON THE ORIGIN OF TAMM-HORSFALL URINARY MUCOPROTEIN AND ITS PRESENCE IN OVINE CALCULOUS MATRIX. Invest Urol. 1965 Mar;2:453–457. [PubMed] [Google Scholar]
- Capstick P. B., Garland A. J., Masters R. C., Chapman W. G. Some functional and morphological alterations occurring during and after the adaptation of BHK 21 clone 13 cells to suspension culture. Exp Cell Res. 1966 Oct;44(1):119–128. doi: 10.1016/0014-4827(66)90418-6. [DOI] [PubMed] [Google Scholar]
- Cleave A. J., Kent P. W., Peacocke A. R. The binding of hydrogen and calcium ions by Tamm-Horsfall glycoprotein. Biochim Biophys Acta. 1972 Nov 28;285(1):208–233. doi: 10.1016/0005-2795(72)90192-4. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan D. R., Grant A. M., Marshall R. D., Neuberger A. A protein, immunologically similar to Tamm-Horsfall glycoprotein, produced by cultured baby hamster kidney cells. Proc R Soc Lond B Biol Sci. 1974 Jul 30;186(1085):297–316. doi: 10.1098/rspb.1974.0051. [DOI] [PubMed] [Google Scholar]
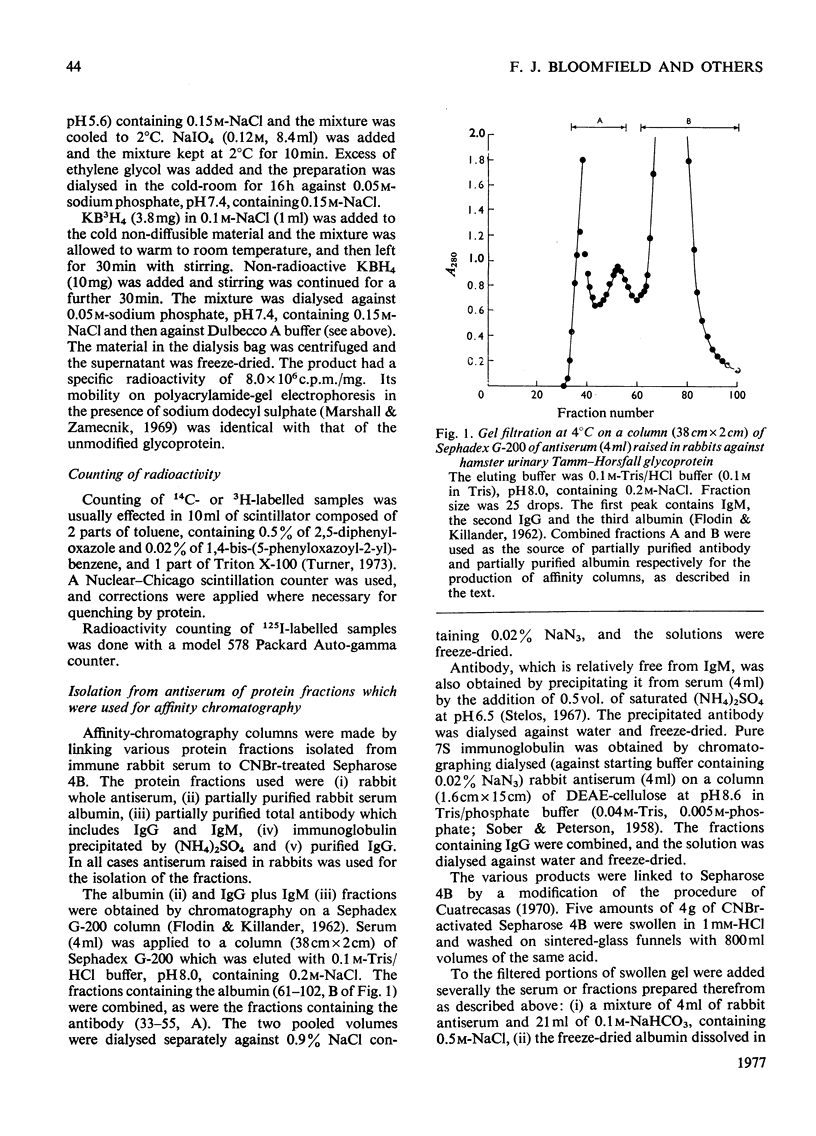
- FLODIN P., KILLANDER J. Fractionation of human-serum proteins by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:402–410. doi: 10.1016/0006-3002(62)90104-x. [DOI] [PubMed] [Google Scholar]
- Fletcher A. P., McLaughlin J. E., Ratcliffe W. A., Woods D. A. The chemical composition and electron microscopic appearance of a protein derived from urinary casts. Biochim Biophys Acta. 1970 Aug 21;214(2):299–308. doi: 10.1016/0005-2795(70)90007-3. [DOI] [PubMed] [Google Scholar]
- Franks L. M., Wilson P. D. "Spontaneous" neoplastic transformation in vitro: the ultrastructure of the tissue culture cell. Eur J Cancer. 1970 Dec;6(6):517–523. doi: 10.1016/0014-2964(70)90072-1. [DOI] [PubMed] [Google Scholar]
- Friedman T. Immunofluorescent localization of Tamm-Horsfall mucoprotein. Experientia. 1966 Sep 15;22(9):624–625. doi: 10.1007/BF01895292. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Simons K. Isolation of plasma membrane fragments from BHK21 cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(2):176–182. doi: 10.1111/j.1699-0463.1970.tb04284.x. [DOI] [PubMed] [Google Scholar]
- Grant A. M., Neuberger A. The turnover rate of rabbit urinary Tamm-Horsfall glycoprotein. Biochem J. 1973 Nov;136(3):659–668. doi: 10.1042/bj1360659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson L. A., Fasth A., Jodal U. Autoantibodies to Tamm-Horsfall protein, a tool for diagnosing the level of urinary-tract infection. Lancet. 1976 Jan 31;1(7953):226–228. doi: 10.1016/s0140-6736(76)91342-8. [DOI] [PubMed] [Google Scholar]
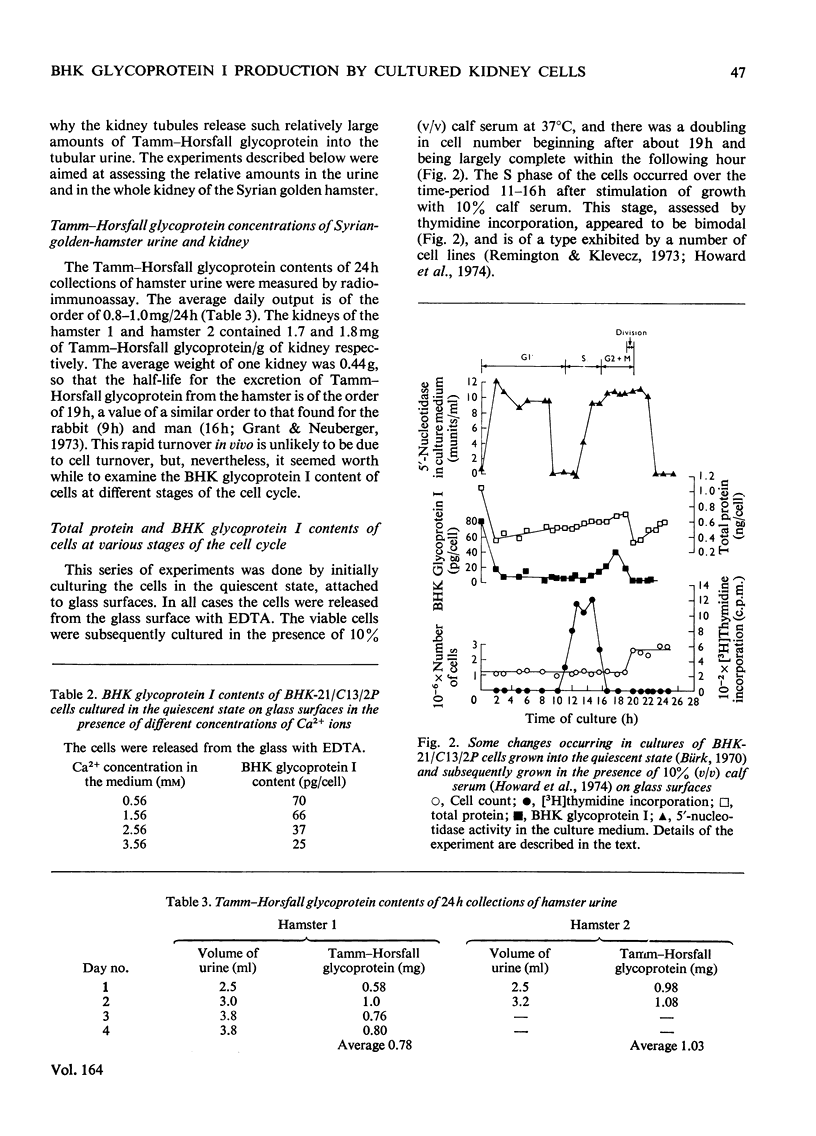
- Howard D. K., Hay J., Melvin W. T., Durham J. P. Changes in DNA and RNA synthesis and associated enzyme activities after the stimulation of serum-depleted BHK21-C13 cells by the addition of serum. Exp Cell Res. 1974 May;86(1):31–42. doi: 10.1016/0014-4827(74)90643-0. [DOI] [PubMed] [Google Scholar]
- Hoyer J. R., Resnick J. S., Michael A. F., Vernier R. L. Ontogeny of Tamm-Horsfall urinary glycoprotein. Lab Invest. 1974 Jun;30(6):757–761. [PubMed] [Google Scholar]
- KEUTEL H. J. LOCALIZATION OF UROMUCID IN HUMAN KIDNEY AND IN SECTIONS OF HUMAN KIDNEY STONE WITH THE FLUORESCENT ANTIBODY TECHNIQUE. J Histochem Cytochem. 1965 Mar;13:155–160. [PubMed] [Google Scholar]
- Khalkhali Z., Marshall R. D. UDP-N-acetyl-D-glucosamine-asparagine sequon N-acetyl-beta-D-glucosaminyl-transferase-activity in human serum. Carbohydr Res. 1976 Jul;49:455–473. doi: 10.1016/s0008-6215(00)83163-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Marshall R. D., Zamecnik P. C. Some physical properties of lysyl and arginyl-transfer RNA synthetases of Escherichia coli B. Biochim Biophys Acta. 1969 Jul 1;181(2):454–464. doi: 10.1016/0005-2795(69)90279-7. [DOI] [PubMed] [Google Scholar]
- McKenzie J. K., McQueen E. G. Immunofluorescent localization of Tamm-Horsfall mucoprotein in human kidney. J Clin Pathol. 1969 May;22(3):334–339. doi: 10.1136/jcp.22.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen E. G., Engel G. B. Factors determining the aggregation of urinary mucoprotein. J Clin Pathol. 1966 Jul;19(4):392–396. doi: 10.1136/jcp.19.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen E. G. The nature of urinary casts. J Clin Pathol. 1962 Jul;15(4):367–373. doi: 10.1136/jcp.15.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger A., Ratcliffe W. A. The acid and enzymic hydrolysis of O-acetylated sialic acid residues from rabbit Tamm-Horsfall glycoprotein. Biochem J. 1972 Sep;129(3):683–693. doi: 10.1042/bj1290683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATEL R., MCKENZIE J. K., MCQUEEN E. G. TAMM-HORSFALL URINARY MUCOPROTEIN AND TUBULAR OBSTRUCTION BY CASTS IN ACUTE RENAL FAILURE. Lancet. 1964 Feb 29;1(7331):457–461. doi: 10.1016/s0140-6736(64)90794-9. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt-Rivers R., Impiombato F. S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968 Oct;109(5):825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. A., Klevecz R. R. Families of replicating units in cultured hamster fibroblasts. Exp Cell Res. 1973 Feb;76(2):410–418. doi: 10.1016/0014-4827(73)90393-5. [DOI] [PubMed] [Google Scholar]
- SOBER H. A., PETERSON E. A. Protein chromatography on ion exchange cellulose. Fed Proc. 1958 Dec;17(4):1116–1126. [PubMed] [Google Scholar]
- Schenk E. A., Schwartz R. H., Lewis R. A. Tamm-Horsfall mucoprotein. I. Localization in the kidney. Lab Invest. 1971 Jul;25(1):92–95. [PubMed] [Google Scholar]
- Shodell M. Reversible arrest of mouse 3T6 cells in G2 phase of growth by manipulation of a membrane-mediated G2 function. Nature. 1975 Aug 14;256(5518):578–580. doi: 10.1038/256578a0. [DOI] [PubMed] [Google Scholar]
- Snow C., Allen A. The release of radioactive nucleic acids and mucoproteins by trypsin and ethylenediaminetetra-acetate treatment of baby-hamster cells in tissue culture. Biochem J. 1970 Oct;119(4):707–714. doi: 10.1042/bj1190707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMM I., HORSFALL F. L., Jr A mucoprotein derived from human urine which reacts with influenza, mumps, and Newcastle disease viruses. J Exp Med. 1952 Jan;95(1):71–97. doi: 10.1084/jem.95.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMM I., HORSFALL F. L., Jr Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc Soc Exp Biol Med. 1950 May;74(1):106–108. [PubMed] [Google Scholar]
- Tsantoulas D. C., McFarlane I. G., Portmann B., Eddleston A. L., Williams R. Cell-mediated immunity to human Tamm-Horsfall glycoprotein in autoimmune liver disease with renal tubular acidosis. Br Med J. 1974 Nov 30;4(5943):491–494. doi: 10.1136/bmj.4.5943.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]