Abstract
Background
Rheumatoid arthritis (RA) and chronic obstructive pulmonary disease (COPD) are prevalent and incapacitating conditions, sharing common pathogenic pathways such as tobacco use and pulmonary inflammation. The influence of respiratory conditions including COPD on RA has been observed, meanwhile RA may constituting one of the risk factors for COPD. It unclear that whether a bidirectional associate between RA and COPD. Our study aims to explore the bidirectional relationship between RA and COPD.
Methods
We systematically searched PubMed, Cochrane Library, and Embase for observational studies from the databases inception to February 20, 2024, utilizing medical subject headings (MeSH) and keywords. We included studies in which RA and COPD were studied as either exposure or outcome variables. Statistical analyses were conducted employing STATA software (version 14.0). The relationship was reported as odds ratios (OR) and corresponding 95% confidence intervals (CI). Publication bias was assessed using funnel plots and Egger’s regression.
Results
Nineteen studies with 1,549,181 participants were included. Risk of bias varied from low to moderate, with evidence levels rated as low or very low. Pooled analysis revealed a significant association between RA and increased COPD risk (OR=1.41, 95%CI 1.13 to 1.76, I2 = 97.8%, P=0.003). Subgroup analyses showed similar COPD risk elevations in both of genders, seropositive/seronegative RA, cohort and case control studies. Additionally, there was a significant RA risk increase among those with COPD (OR=1.36, 95%CI 1.05 to 1.76, I2 = 55.0%, P=0.022), particularly among females and seropositive RA, and cohort studies.
Conclusion
The meta-analysis identifies a significant bidirectional association between RA and COPD, emphasizing mutually increased risk. Recognizing this connection may can inform proactive approaches to disease prevention and management, potentially reducing the public health burden and improving quality of life.
Systematic Review Registration
https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024518323.
Keywords: rheumatoid arthritis, chronic obstructive pulmonary disease, bidirectional association, COPD, RA
1. Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease primarily affecting joints, with an estimated prevalence ranging from 0.5% to 1% of the population (1). Its societal impact encompasses substantial costs, disability, and diminished productivity. Despite advancements in diagnosis, understanding of its pathogenesis, and therapeutic options, RA remains incurable. Thus, early identification of associated risk factors could potentially delay its onset. Previous studies have explored various risk factors including genetic predisposition, familial history, female gender, exposure to tobacco smoke and air pollutants, antibiotic use, obesity, periodontitis, and atopic dermatitis (2–8). Notably, respiratory conditions such as asthma, pneumonia, interstitial lung disease, and chronic obstructive pulmonary disease (COPD) have also been implicated as influencing factors on RA (9–12).
COPD is a major cause of global mortality and disability, characterized by persistent and often progressive airflow obstruction. Individuals with RA have increased mortality associated with COPD, while RA itself serves as a risk factor for developing COPD. This association is evident due to shared risk factors such as cigarette smoking and systemic inflammation. Smoking triggers the production of anti-citrullinated protein antibodies (ACPA), elevating the risk of both RA and COPD (13, 14). Although there were studies on the relationship between COPD and RA, they may share a common pathogenesis, however, there was lack of systematic review on the bidirectional correlation between them. Consequently, we hypothesize a bidirectional association between RA and COPD, and aim to systematically review existing evidence to ascertain whether they are mutual risk factors for each other.
2. Methods
2.1. Data sources and selection criteria
We systematically retrieved articles from the PubMed, Cochrane Library, and Embase for studies published from the databases inception through February 20, 2024, with no language restrictions. The search strategy utilized Medical Subject Headings (MeSH) and keywords, including: “arthritis, rheumatoid”, “rheumatoid arthritis”, “RA”, “pulmonary disease, chronic obstructive”, “COPD”, and their variants. Details of the search strategy were shown in the Supplementary Tables 1 - 3 . Additionally, we manually screened the reference lists of relevant systematic reviews to supplement our search and ensure comprehensive study identification (15, 16).
The observational studies were included based on the following criteria: (1) cohort studies, case-control studies, or cross-sectional studies; (2) investigating the association between RA and the risk of COPD, or the association between COPD and the risk of RA; (3) report relative risk (RR), hazard ratio (HR), or odds ratio (OR) with corresponding 95% confidence interval (CI). The studies were excluded according to exclusion criteria: (1) not the observational studies; (2) did not investigate the association between RA and the risk of COPD, or the association between COPD and the risk of RA; (3) studies lack relevant outcomes, such as did not report relative risk (RR), hazard ratio (HR), or odds ratio (OR) with corresponding 95% confidence interval (CI); (4) the studies was conference abstracts, study protocols, or letters.
2.2. Study selection and data extraction
Two independent reviewers (MJ Wang and HJ Pan) screened titles and abstracts, followed by full-text review of potentially eligible articles. Discrepancies were resolved through discussion with a third arbiter (XL Li). Data extraction was independently conducted by the aforementioned reviewers, following the guidelines on data extraction for systematic reviews and meta-analysis (17). We used predesigned forms for extracting data, including the first author, year of publication, study region, study period, study type, sample size, female/male, age, and diagnosis of RA or COPD. Disagreements were also resolved through discussion with the third reviewer.
2.3. Risk of bias
The Newcastle-Ottawa Scale (NOS) was utilized to assess cohort and case-control study quality based on selection, comparability, and exposure (18). Each study could earn up to nine stars, with four for participant selection and exposure measurement, two for comparability, and three for outcome assessment and follow-up adequacy. Higher star counts reflect higher study quality (0-3: low, 4-6: moderate, 7-9: high, respectively). The Agency for Healthcare Research and Quality (AHRQ) assessed cross-sectional study quality with 11 items, where “yes” scored 1 point and “no” or “unclear” scored 0. Scores of 0-3, 4-7, and 8-11 were defined as low, medium, and high quality, respectively (19).
2.4. Evidence certainty
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was used to evaluate evidence certainty, initially grading observational studies as low quality (20, 21). The quality of evidence can then be graded as high, moderate, low, or very low, based on rating is modified downward (such as study limitations, inconsistency, indirectness, imprecision, and publication bias), and rating is modified upward (large magnitude of effect, dose response, confounders likely minimize the effect) (22, 23).
2.5. Data analysis
For statistical analysis, adjusted odds ratios (OR) and corresponding 95% confidence intervals (CI) were used to assess the relationship between RA and COPD risk, and vice versa. Heterogeneity was evaluated using χ2-test and I2-values, with a fixed-effects model applied for P>0.1 and I2<50%, and a random-effects model for I2>50%. Sensitivity analysis ensured result robustness. Egger’s regression and funnel plot examination were used to assess publication bias. Subgroup analyses were conducted based on gender, serum indicators, and study design. All analyses were performed using STATA statistical software version 14.0.
The meta-analysis adhered to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (24). Furthermore, the protocol was preregistered on the International Prospective Register of Systematic Reviews (PROSPERO) platform, with the approval number CRD42024518323.
3. Results
3.1. Literature search and study characteristics
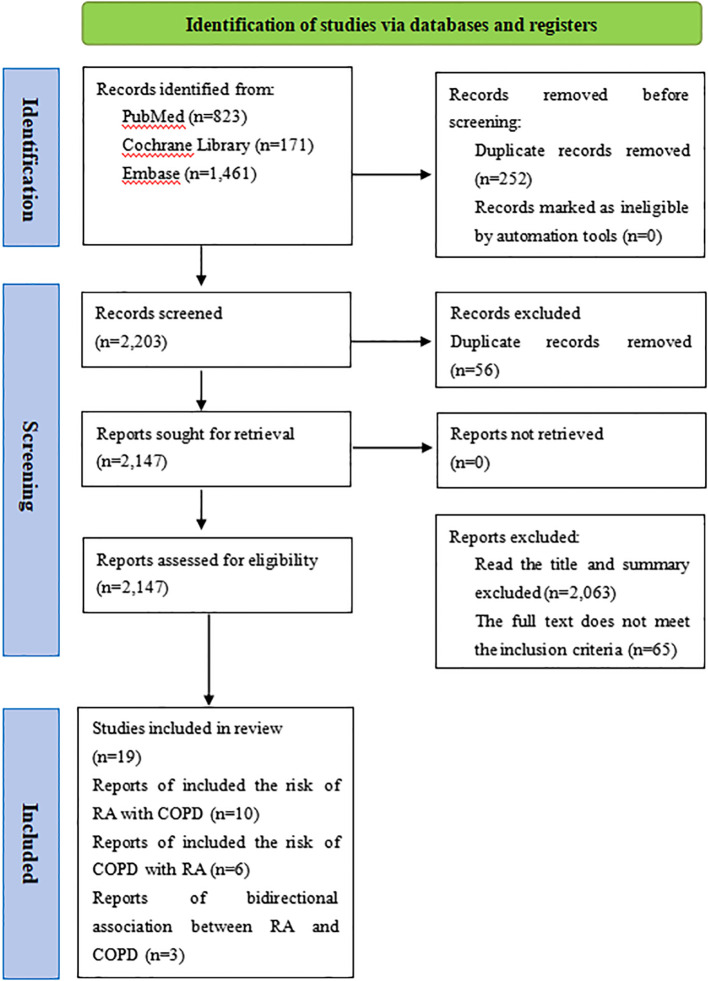
A total of 2,455 records were retrieved through the search. Following title and abstract screening, 84 articles were deemed potentially relevant. Nineteen studies were finally included in the study ( Figure l ).
Figure 1.
Studies screening process.
The meta-analysis included 19 studies with 1,549,181 participants, published up to February 20, 2024 (10, 25–42). Among these, 10 studies analyzed COPD as the outcome with RA as the exposure (26–28, 30, 31, 33, 35, 36, 40, 42) ( Table 1 ), while 6 examined RA as the outcome with COPD as the exposure (10, 25, 29, 32, 37, 39) ( Table 1 ), 3 investigated bidirectional association between RA and COPD (34, 38, 41) ( Table 1 ). These studies included eight cohort, six case-control, and five cross-sectional studies, mainly from North America, Europe, and Asia. Most studies adhered to well-defined diagnostic criteria for RA/COPD, with minor discrepancies in adjusted confounders ( Supplementary Table 4 ).
Table 1.
Characteristics of the included studies.
| Author | Year | Country | Study period | Study type | Study size | Female/Male | Age (years) | Diagnosis of RA/COPD |
|---|---|---|---|---|---|---|---|---|
| Chung C (42) | 2024 | Korea | January 2010 and December 2017 | Cohort study | Case group: 46030, Control group: 230150 |
Case group: 35424/10606, Control group: 177120/53030 | (57.51 ± 9.71) | RA: ICD-10 |
| Kim JG (40) | 2023 | Korea | 2017 to 2019 | Cross-sectional study | Case group: 334, Control group: 13384 |
Case group: 269/65, Control group: 7522/5862 |
Case group: (65.7 ± 11.0) Control group: (59.5 ± 11.95) |
COPD: GOLD guidelines |
| Yang K (41) | 2023 | The UK | As of March 2023 | Cohort study | Case group: 4755, Control group: 391525 |
Case group: 3171/1584, Control group: 204299/187226 | < 50, 50-59 and ≥60 | RA and COPD: ICD-10 |
| Kronzer VL (39) | 2022 | US | 2010 to June 2020 | Case-control study | Case group: 741, Control group: 2223 | Case group: 563/178, Control group: 1689/534 |
Case group: 55 (45,63) Control group: 56 (47,63) |
RA: The 2010 ACR/EULAR criteria |
| Jung JH (36) | 2021 | Korea | 2008 to 2016 | Cross-sectional study | Case group: 318, Control group: 27977 | Case group: 260/58, Control group: 15618/12359 |
Female Case group: (62.2 ± 11.26) Control group: (54.9 ± 12.43) Male Case group: (61.4 ± 10.29) Control group: (55.6 ± 12.82) |
COPD: (FEV1/FVC) <0.7, chronic cough or sputum for more than 3 months, and/or smoking history of ≥10 pack-years |
| Kronzer VL (37) | 2021 | Sweden | 2006 to 2016 | Case-control study | Case group: 1631, Control group: 3283 | Case group: 1152/479, Control group: 2315/968 | Case group: 57 (46,64) Control group: 57 (46,65) |
RA: ACR/EULAR 1987 or 2010 criteria |
| Zaccardelli A (38) | 2021 | US | The NHS in 1976 and the NHSII in 1989 | Cohort study | Case group: 283, Control group: 842 | Case group: 283/0, Control group: 842/0 |
Case group: (51.5 ± 7.6)-(51.5 ± 8.0) Control group: (51.2 ± 7.8)-(51.5 ± 7.9) |
RA: The 1987 ACR or 2010 ACR/EULAR criteria |
| Ford JA (10) | 2020 | US | June 1, 2014 for NHS and June 1, 2015 for NHSII | Cohort study | Case group: 3573, Control group: 205153 | Case group: 3573/0, Control group: 205153/0 |
Case group: 52.7 Control group: 44.4 |
RA: The 1987 ACR or 2010 ACR/EULAR criteria |
| Kronzer VL (34) | 2019 | US | 2009 | Case-control study | Case group: 821, Control group: 2455 | Case group: 600/221, Control group: 1792/663 | (62 ± 14) | RA: The ACR/EULAR 2010 criteria |
| Mcguire K (35) | 2019 | Canada | January 1996 to March 2010 | Cohort study | Case group: 24625, Control group: 25396 | Case group: 16499/8126, Control group: 17015/8381 | Case group: (57.2 ± 17.1) Control group: (57.3 ± 17.1) |
RA and COPD: ICD-9 |
| Choi IA (30) | 2018 | Korea, International | NA | Cross-sectional study | Case group: 1050, Control group: 3520 | Case group: 872/178, Control group: 3191/329 |
Case group: (56 ± 12) Control group: (56 ± 13) |
NA |
| Dhital R (31) | 2018 | US | 1 January 2013 to 31 December 2013 | Case-control study | Case group: 93750, Control group: 281250 |
Case group: 346785/121965, Control group: 1040354/365895 |
Case group: (67.47 ± 0.06) Control group: (67.47 ± 0.08) | RA: ICD-9 |
| Sheen YH (32) | 2018 | US | January 1, 2002, and December 31, 2007 | Case-control study | Case group: 221, Control group: 218 |
Case group: 156/56, Control group: 154/64 |
Case group: 52.5 (41.7-65.7) Control group: 54.2 (42.6-66.7) |
RA: The 1987 ACR classification criteria |
| Sparks JA (33) | 2018 | US | 1976 to 2014 | Cohort study | Case group: 843, Control group: 8399 |
Case group: 843/0, Control group: 8399/0 |
Case group: (59.8 ± 10.0) Control group: (59.8 ± 10.0) |
NA |
| Jo YS (29) | 2015 | Korea | 2010 to 2012 | Cross-sectional study | Case group: 744, Control group: 3313 |
Case group: 0/744, Control group: 0/3313 |
Case group: (65.02 ± 9.40) Control group: (55.06 ± 10.43) |
COPD: A former or current smoker with spirometry-proven airflow limitation (FEV1/FVC<0.70) |
| Shen TC (28) | 2014 | China, Taiwan | 1998 to 2008 | Cohort study | Case group: 28725, Control group: 114900 |
Case group: 22403/6322, Control group: 89612/25288 | Case group: (53.8 ± 13.9) Control group: (53.2 ± 14.3) |
RA and COPD: ICD-9 |
| Bieber V (26) | 2013 | Israel | NA | Cross-sectional study | Case group: 9039, Control group: 15070 |
Case group: 2001/7038, Control group: 3438/11632 | Case group: (60.1 ± 16.9) Control group: (61.1 ± 18.3) |
RA: CHS physician, COPD: Taken from the CHS Chronic Diseases Registry |
| Nannini C (27) | 2013 | US | January 1, 2006. | Cohort study | Case group: 594, Control group: 596 |
Case group: 435/159, Control group: 438/158 |
Case group: (57.8 ± 15.2) Control group: (58.2 ± 15.3) |
RA: The 1987 ACR classification criteria |
| Bergström U (25) | 2011 | Sweden | Between 1974 and 1992 | Case-control study | Case group:290, Control group: 1160 |
Case group: 139/151, Control group: 556/604 |
Case group: (47 ± 7.1) Control group: (47 ± 7.1) |
RA: The 1987 ACR criteria, COPD: The GOLD criteria |
(RA, rheumatoid arthritis; COPD, chronic obstructive pulmonary disease; NHS, Nurses’ Health Study; GOLD, global initiative for obstructive lung disease; ACR, American College of Rheumatology; EULAR, European League Against Rheumatism; CHS, Clalit Health Services; FEV1/FVC, forced expiratory volume in 1s/forced vital capacity; NA, not applicable).
3.2. Quality assessment
Using the NOS criteria, cohort and case-control studies achieved an average score of 6.79, with all scoring 5 or higher, indicating moderate to high quality ( Supplementary Table 5 ). Meanwhile, cross-sectional studies averaged a score of 4.40 on AHRQ, with two scoring 3, suggesting low to medium quality ( Supplementary Table 6 ).
3.3. Odds of COPD in participants with RA
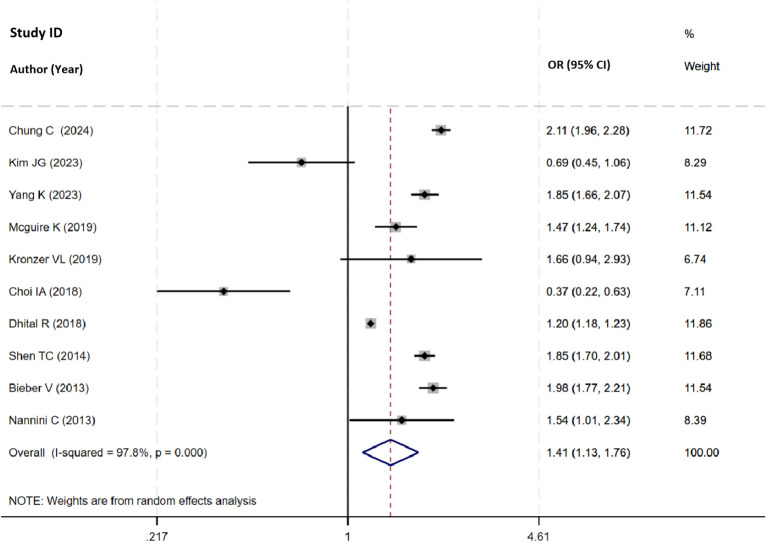
Thirteen studies investigated the risk of COPD associated with RA, ten studies reported the total OR among these. The pooled analysis confirmed a significant association between RA and increased risk of COPD (OR=1.41, 95% CI 1.13 to 1.76, I2 = 97.8%, P=0.003, N=10, Figure 2 ), supported by robust sensitivity analysis ( Supplementary Table 7 , Supplementary Figure 1 ).
Figure 2.
Forest plot showing the odds ratio of COPD in participants with RA.
3.4. Odds of RA in participants with COPD
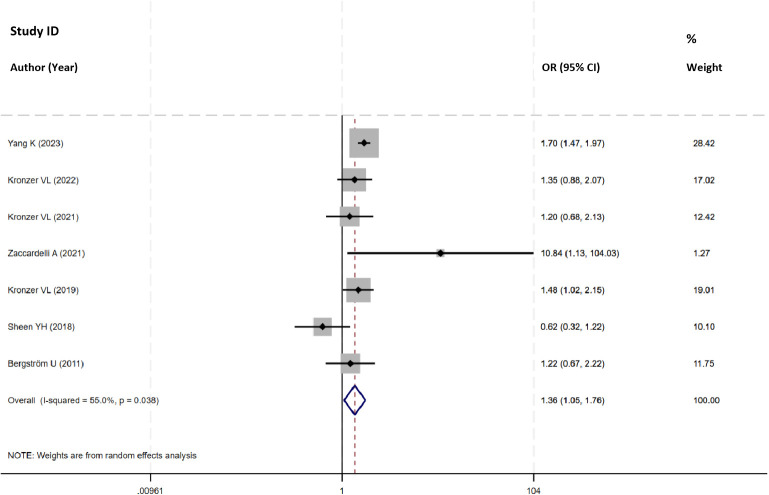
Nine studies explored the risk of RA linked to COPD, and seven of them reported the total OR. After pooling the data, the analysis revealed a notable association between COPD and an increased risk of RA (OR=1.36, 95%CI 1.05 to 1.76, I2 = 55.0%, P=0.022, N=7, Figure 3 ). Despite significant heterogeneity attributed to a 2018 study by Sheen YH, its limited sample size didn’t alter the pooled-effect size, reinforcing result robustness ( Supplementary Table 8 , Supplementary Figure 2 ).
Figure 3.
Forest plot showing the odds ratio of RA in participants with COPD.
3.5. Subgroup analysis
In subgroup analysis, the risk of COPD was significantly related to RA in both genders, with females showing a higher risk (OR=1.74, 95% CI 1.59 to 1.90, I2 = 0.0%, P=0.000) ( Table 2 ). Additionally, there was a significant association between seropositive RA and seronegative RA and the risk of COPD, with seropositive RA carried a notably higher COPD risk than seronegative RA (OR=2.09, 95% CI 1.69 to 2.57, I2 = 51.4%, P=0.000) ( Table 2 ). In the subgroup analyses by study design, the meta-analysis of cohort studies and case-control studies showed a significant association between the risk of COPD and RA (OR=1.77, 95%CI 1.60 to 1.97, I2 = 71.8%, P=0.000; OR=1.43, 95%CI 1.17 to 1.75, I2 = 20.0%, P=0.028), whereas the meta-analysis of cross-sectional studies suggested a slightly significant link between the risk of COPD and RA ( Table 2 ).
Table 2.
Subgroup analysis for the risk of COPD related to RA.
| Subgroups | Included studies | OR (95% CI) |
Heterogeneity | |
|---|---|---|---|---|
| I2 (%) | P value | |||
| Gender | ||||
| Female | 5 | 1.74 (1.59, 1.90) | 0.0 | 0.000 |
| Male | 3 | 1.57 (1.15, 2.14) | 69.5 | 0.004 |
| Serum reactivity | ||||
| Seropositive RA | 3 | 2.09 (1.69, 2.57) | 51.4 | 0.000 |
| Seronegative RA | 3 | 1.58 (1.21, 2.06) | 50.8 | 0.001 |
| Study type | ||||
| Cohort study | 7 | 1.77 (1.60, 1.97) | 71.8 | 0.000 |
| Cross-sectional study | 3 | 0.81 (0.28, 2.36) | 96.4 | 0.706 |
| Case-control study | 2 | 1.43 (1.17, 1.75) | 20.0 | 0.028 |
In the subgroup analysis, the risk of RA was significantly linked to COPD in females (OR=1.96, 95%CI 1.37 to 2.81, I2 = 0.0%, P=0.000), while no significant association was observed in males (OR=0.94, 95% CI 0.56 to 1.59, I2 = 0.0%, P=0.829) ( Table 3 ). There was a significant association between COPD and the risk of both seropositive RA (OR=1.65, 95%CI 1.24 to 2.19, I2 = 4.6%, P=0.001) and seronegative RA(OR=1.70, 95%CI 1.23 to 2.36, I2 = 0.0%, P=0.001) ( Table 3 ). In the subgroup analyses by study design, the meta-analysis of cohort studies revealed a significant association between the risk of RA and COPD (OR=1.74, 95%CI 1.50 to 1.99, I2 = 28.6%, P=0.000), whereas meta-analysis of case-control studies and cross-sectional study suggested a slightly significant link between the risk of RA and COPD ( Table 3 ).
Table 3.
Subgroup analysis for the risk of RA associated with COPD.
| Subgroups | Included studies | OR (95% CI) |
Heterogeneity | |
|---|---|---|---|---|
| I2 (%) | P value | |||
| Gender | ||||
| Female | 2 | 1.96 (1.37, 2.81) | 0.0 | 0.000 |
| Male | 2 | 0.94 (0.56, 1.59) | 0.0 | 0.829 |
| Serum reactivity | ||||
| Seropositive RA | 4 | 1.65 (1.24, 2.19) | 4.6 | 0.001 |
| Seronegative RA | 4 | 1.70 (1.23, 2.36) | 0.0 | 0.001 |
| Study type | ||||
| Case-control study | 5 | 1.24 (0.10, 1.52) | 21.6 | 0.051 |
| Cohort study | 3 | 1.74 (1.50, 1.99) | 28.6 | 0.000 |
| Cross-sectional study | 1 | 0.92 (0.40, 2.12) | – | 0.845 |
3.6. GRADE quality of evidence
The GRADE level of evidence for the risk of COPD related to RA was assessed as very low. Similarly, the GRADE level was very low for the risk of COPD in males, seropositive RA, cohort studies, and cross-sectional studies, and low for the risk of COPD in females, seronegative RA, and case control study. The GRADE level of evidence was also very low for the risk of RA association with COPD. For the risk of RA in females, males, seropositive/seronegative RA, case-control studies, and cohort studies, the GRADE level of evidence was low. GRADE evidence certainty for the outcomes was summarized in Supplementary Table 9 .
3.7. Publication bias
Funnel plots showed no publication bias for bidirectional risks between COPD and RA, confirmed by Egger’s test ( Supplementary Figures 3 , 4 ).
4. Discussion
4.1. Main findings
Our meta-analysis of 19 studies, encompassing 1,549,181 individuals, elucidated the bidirectional association between COPD and RA. The findings indicated a significant increase in the risk of COPD among individuals with RA compared to those without RA, suggesting that RA may serve as an independent risk factor for COPD. Subgroup analyses by gender, seropositive and seronegative RA, and cohort studies consistently supported this association.
Moreover, our analysis indicated a notable increase in the risk of RA among individuals with COPD, suggesting COPD as an independent contributor to RA development. Subgroup analyses, particularly those focusing on gender, seropositive RA, and seronegative RA, further underscored this association.
Overall, these findings highlight a reciprocal relationship between COPD and RA, suggesting that each condition may predispose individuals to an elevated risk of developing the other. This comprehensive assessment adds valuable insights into the complex interplay between these two chronic inflammatory diseases.
4.2. Interpretation of findings
Two earlier reviews, from 2016 and 2019, explored the increased risk of COPD associated with RA (15, 16). The 2019 review included six studies, four of which overlapped with those from 2016 (27, 28, 43, 44), and added two additional articles (33, 35). Additionally, the 2019 review conducted subgroup analyses, further underscored the relationship between RA and heightened COPD risk in North America, Europe, and Asia. Furthermore, the 2019 review also showed a pooled COPD prevalence of 6.2% among RA patients, emphasized by subgroup analyses.
Our meta-analysis, incorporated 13 studies, underscored this bidirectional risks between COPD and RA. Whereas, we eliminated two studies that included in earlier reviews (43, 44), for one study utilized different outcome indicators (43), and another included patients with inflammatory arthritis beyond RA (44). Our study included nine additional studies, providing a comprehensive assessment of the RA-COPD relationship, and also identified nine studies demonstrating a significant association between COPD and RA risk. This underscores the bidirectional nature of their relationship, investigating both the likelihood of COPD in RA participants and the odds of RA in COPD participants. We further found the bidirectional association to be significant in the studies. Furthermore, our article featured subgroup analyses pertaining to gender, serum reactivity, and study type, aspects not previously explored in meta-analyses.
The studies reported RA is more common in female with three- to five-fold higher prevalence of RA in females than males, and the global prevalence ratio of RA is about 1%, with small continuous fluctuations and an apparent growth from south to north, and from countryside to metropolitan areas (1, 45). This may explain the results in the subgroup analysis of gender.
Several potential mechanisms may explain the association between RA and COPD. The genetic susceptibility to RA correlates with increased COPD risk, particularly early-onset (46). Although mendelian randomization studies support this association, causality has not been definitively established (47). Additionally, specific HLA alleles, like shared epitope-positive 04 alleles, are associated with higher airway disease incidence in RA patients, while HLA-DRB11502 shows a negative correlation (48). Further research is required to fully grasp the genetic ties and underlying mechanisms between RA and COPD.
Smoking is a recognized risk factor for both COPD and RA, with a significant impact on RA development, especially in seropositive cases (49). Smoking contributed to the production of RA-specific autoantibodies like ACPA, anti-cyclic citrullinated peptide (anti-CCP) antibodies (50–52). Furthermore, smoking interacts with specific RA genetic risk factors, like the HLA-DRB1 shared epitope (SE), increasing the risk of ACPA-positive RA, particularly, in SE carriers (53, 54). Additionally, smoking contributes to COPD. The harmful chemicals and particles in cigarette smoke trigger abnormal inflammatory responses in the lungs, leading to tissue damage, airway narrowing, and progressive loss of lung function seen in COPD. Overall, smoking not only increases the risk of developing RA by promoting autoimmune processes such as citrullination and anti-CCP antibody production, but also contributes to the development and progression of COPD through its damaging effects on the respiratory system. Avoiding smoking is crucial for reducing the risk of both RA and COPD and improving overall health.
There are shared pathophysiological features between COPD and RA. Elevated levels of rheumatoid factor and anti-CCP antibodies are associated with COPD, indicating a potential overlap in autoimmune processes. Citrullination of autoantigens, a process implicated in RA, lead to the subsequent production of ACPA, which is increased in COPD (13, 55). Interestingly, individuals with ACPA positivity before RA development have a higher risk of COPD, suggesting ACPAs may play a role in COPD pathogenesis (38). Furthermore, the risk of COPD is higher in patients with seropositive RA compared to seronegative RA in this study, indicating a bidirectional association between RA-associated autoimmune processes and COPD inflammatory pathways. Indeed, systemic inflammation in RA can lead to pulmonary dysfunction, including COPD. Immune cells like neutrophils, macrophages, and T-lymphocytes, pivotal in both diseases, contribute to lung tissue damage and inflammation. Moreover, key cytokines such as TNF-α, IL-6, IL-8, IL-17, and IL-32 drive inflammation, tissue destruction, and remodeling in the lungs, aiding COPD progression (56). Autoimmune processes in RA and chronic inflammation in COPD create an environment where cytokines and immune cells interact, damaging both joints and lungs. Understanding these shared pathways is crucial for developing effective therapeutic strategies for patients with both conditions.
The interplay between lung bacterial composition (microbiota), inflammation, and autoimmune processes in RA and COPD highlights their intricate connection. Alterations in the lung microbiome and inflammatory markers occur in both diseases, indicating a significant link between microbial imbalance and systemic inflammation (57, 58). Chronic infection and microbiome irregularities may trigger autoantigen formation, leading to RA-specific markers like ACPA (59). Microbial diversity decline and specific bacterial taxa proliferation correlate with IL-17-mediated immunity, promoting pro-inflammatory cytokine production crucial in both RA and COPD (60). RA patients often endure chronic pulmonary inflammation and recurrent infections, damaging alveolar structures and increasing COPD susceptibility. Addressing lung inflammation and microbiome composition could potentially mitigate COPD progression in RA patients. Understanding these mechanisms better may pave the way for novel therapeutic strategies, emphasizing the importance of preserving lung health in RA to prevent or alleviate COPD.
Moreover, RA may originate at inflamed mucosal sites, including the lungs, potentially linked to upper respiratory viruses (61). Individuals with COPD show elevated inflammatory markers and autoantibodies associated with RA (62). Nonetheless, further research is needed to confirm whether COPD represents a new pathogenic pathway for RA.
4.3. Implications and limitations
The strength of our meta-analysis lies in included 19 observational studies, robustly evaluating the RA-COPD association. While two prior meta-analyses examined the risk of COPD in RA and its morbidity, our study offers further retrieved on the bidirectional association relationship between the two conditions. We explore not only the relationship of COPD in individuals with RA but also the odds of RA in those with COPD. Understanding the bidirectional relationship better may benefit for novel therapeutic strategies, emphasizing the importance of preserving lung health to prevent or alleviate COPD, or RA. Understanding the potential link between the two diseases may help clinicians pay closer attention to the progression of the disease and prognosis in time.
The results must be interpreted in light of several limitations. Firstly, these results may be biased due to the unrestricted inclusion of study types, cohort studies, case-control studies, and cross-sectional studies each have their own scopes of application. Secondly, subgroup analyses stratified by gender lacked sufficient statistical power due to limited study availability. Thirdly, some articles relied on ICD codes for defining RA and COPD, which may lack the precision of diagnostic criteria or standardized procedures. Additionally, one study diagnosed COPD based on the CHS Chronic Diseases Registry without elaboration, while two studies omitted mention of diagnostic criteria. Lastly, all studies included in the meta-analysis were conducted in North America, Europe, and Asia, with no representation from African countries. Further large-scale population-based studies are needed to elucidate the significance of the RA-COPD association in African populations.
5. Conclusions
In conclusions, this meta-analysis indicates a significant bidirectional association between RA and COPD across the included studies. The risk of COPD was markedly related to RA in both genders, and in both seropositive RA and seronegative RA, meanwhile, there was a significant COPD risk in females RA, and in both seropositive RA and seronegative RA. Early detection of both conditions may be vital for initiating effective treatments and improving long-term outcomes.
Acknowledgments
We would like to express our gratitude to Dr. Qi Wang, and Liang Yao, McMaster University for their kind assistance with guide in methodology and highly qualified English-speaking editor.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Joint project of State Administration of Traditional Chinese Medicine Science and Technology Department, and the Zhejiang Provincial Administration of Traditional Chinese Medicine (No.2023C03040), the Natural Science Foundation of Zhejiang Province, China (No. Y21H270014), and the Zhejiang Province Traditional Chinese medicine science and technology project (No.2020ZZ006).
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author contributions
MW: Data curation, Formal analysis, Writing – original draft. HP: Data curation, Formal analysis, Writing – review & editing. YZ: Methodology, Validation, Writing – original draft. HL: Methodology, Validation, Writing – original draft. LH: Methodology, Software, Writing – review & editing. ZX: Methodology, Software, Writing – review & editing. CW: Conceptualization, Funding acquisition, Investigation, Writing – review & editing. XL: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1494003/full#supplementary-material
References
- 1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. (2016) 388:1984. doi: 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 2. Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. (2010) 69:70–81. doi: 10.1136/ard.2008.096487 [DOI] [PubMed] [Google Scholar]
- 3. Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. (2015) 17:86. doi: 10.1186/s13075-015-0601-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deane KD, Demoruelle MK, Kelmenson LB, Kuhn KA, Norris JM, Holers VM. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract Res Clin Rheumatol. (2017) 31:3–18. doi: 10.1016/j.berh.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sultan AA, Mallen C, Muller S, Hider S, Scott I, Helliwell T, et al. Antibiotic use and the risk of rheumatoid arthritis: a population-based case-control study. BMC Med. (2019) 17:154. doi: 10.1186/s12916-019-1394-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di D, Zhang L, Wu X, Leng R. Long-term exposure to outdoor air pollution and the risk of development of rheumatoid arthritis: A systematic review and meta-analysis. Semin Arthritis Rheum. (2020) 50:266–75. doi: 10.1016/j.semarthrit.2019.10.005 [DOI] [PubMed] [Google Scholar]
- 7. Qiao Y, Wang Z, Li Y, Han Y, Zhou Y, Cao X. Rheumatoid arthritis risk in periodontitis patients: A systematic review and meta-analysis. Joint Bone Spine. (2020) 87:556–64. doi: 10.1016/j.jbspin.2020.04.024 [DOI] [PubMed] [Google Scholar]
- 8. Rittiphairoj T, Charoenngam N, Ponvilawan B, Tornsatitkul S, Wattanachayakul P, Rujirachun P, et al. Atopic dermatitis is a risk factor for rheumatoid arthritis: A systematic review and meta-analysis. Dermatitis. (2021) 32:S15–23. doi: 10.1097/DER.0000000000000781 [DOI] [PubMed] [Google Scholar]
- 9. Charoenngam N, Ponvilawan B, Rittiphairoj T, Tornsatitkul S, Wattanachayakul P, Rujirachun P, et al. Patients with asthma have a higher risk of rheumatoid arthritis: A systematic review and meta-analysis. Semin Arthritis Rheum. (2020) 50:968–76. doi: 10.1016/j.semarthrit.2020.07.015 [DOI] [PubMed] [Google Scholar]
- 10. Ford JA, Liu X, Chu SH, Lu B, Cho MH, Silverman EK, et al. Asthma, chronic obstructive pulmonary disease, and subsequent risk for incident rheumatoid arthritis among women: A prospective cohort study. Arthritis Rheumatol. (2020) 72:704–13. doi: 10.1002/art.41194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akiyama M, Kaneko Y. Pathogenesis, clinical features, and treatment strategy for rheumatoid arthritis-associated interstitial lung disease. Autoimmun Rev. (2022) 21:103056. doi: 10.1016/j.autrev.2022.103056 [DOI] [PubMed] [Google Scholar]
- 12. Kronzer VL, Hayashi K, Crowson CS, Davis JM, McDermott GC, Cui J, et al. Gene-respiratory disease interactions for rheumatoid arthritis risk. Semin Arthritis Rheum. (2023) 63:152254. doi: 10.1016/j.semarthrit.2023.152254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruiz-Esquide V, Gómara MJ, Peinado VI, Gómez Puerta JA, Barberá JA, Cañete Jde D, et al. Anti-citrullinated peptide antibodies in the serum of heavy smokers without rheumatoid arthritis. A differential effect of chronic obstructive pulmonary disease? Clin Rheumatol. (2012) 31:1047–50. doi: 10.1007/s10067-012-1971-y [DOI] [PubMed] [Google Scholar]
- 14. Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun. (2020) 110:102400. doi: 10.1016/j.jaut.2019.102400 [DOI] [PubMed] [Google Scholar]
- 15. Ungprasert P, Srivali N, Cheungpasitporn W, Davis Iii JM. Risk of incident chronic obstructive pulmonary disease in patients with rheumatoid arthritis: A systematic review and meta-analysis. Joint Bone Spine. (2016) 83:290–4. doi: 10.1016/j.jbspin.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 16. Ma Y, Tong H, Zhang X, Wang M, Yang J, Wu M, et al. Chronic obstructive pulmonary disease in rheumatoid arthritis: a systematic review and meta-analysis. Respir Res. (2019) 20:144. doi: 10.1186/s12931-019-1123-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor KS, Mahtani KR, Aronson JK. Summarising good practice guidelines for data extraction for systematic reviews and meta-analysis. BMJ Evid Based Med. (2021) 26:88–90. doi: 10.1136/bmjebm-2020-111651 [DOI] [PubMed] [Google Scholar]
- 18. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 19. Chou R, Baker WL, Bañez LL, Iyer S, Myers ER, Newberry S, et al. Agency for Healthcare Research and Quality Evidence-based Practice Center methods provide guidance on prioritization and selection of harms in systematic reviews. J Clin Epidemiol. (2018) 98:98–104. doi: 10.1016/j.jclinepi.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 20. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 21. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 22. Li X, Huang L, Tang Y, Hu X, Wen C. Gout and risk of dementia, Alzheimer's disease or vascular dementia: a meta-epidemiology study. Front Aging Neurosci. (2023) 15:1051809. doi: 10.3389/fnagi.2023.1051809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang M, Gu H, Zhai Y, Li X, Huang L, Li H, et al. Vaccination and the risk of systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Res Ther. (2024) 26:60. doi: 10.1186/s13075-024-03296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergström U, Jacobsson LT, Nilsson JÅ, Berglund G, Turesson C. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatol (Oxford). (2011) 50:2005–13. doi: 10.1093/rheumatology/ker258 [DOI] [PubMed] [Google Scholar]
- 26. Bieber V, Cohen AD, Freud T, Agmon-Levin N, Gertel S, Howard A. Autoimmune smoke and fire–coexisting rheumatoid arthritis and chronic obstructive pulmonary disease: a cross-sectional analysis. Immunol Res. (2013) 56:261–6. doi: 10.1007/s12026-013-8395-x [DOI] [PubMed] [Google Scholar]
- 27. Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, et al. Incidence and mortality of obstructive lung disease in rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken). (2013) 65:1243–50. doi: 10.1002/acr.21986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen TC, Lin CL, Chen CH, Tu CY, Hsia TC, Shih CM, et al. Increased risk of chronic obstructive pulmonary disease in patients with rheumatoid arthritis: a population-based cohort study. QJM. (2014) 107:537–43. doi: 10.1093/qjmed/hcu027 [DOI] [PubMed] [Google Scholar]
- 29. Jo YS, Choi SM, Lee J, Park YS, Lee SM, Yim JJ, et al. The relationship between chronic obstructive pulmonary disease and comorbidities: a cross-sectional study using data from KNHANES 2010-2012. Respir Med. (2015) 109:96–104. doi: 10.1016/j.rmed.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 30. Choi IA, Park SH, Cha HS, Park W, Kim HA, Yoo DH, et al. Prevalence of co-morbidities and evaluation of their monitoring in Korean patients with rheumatoid arthritis: comparison with the results of an international, cross-sectional study (COMORA). Int J Rheum Dis. (2018) 21:1414–22. doi: 10.1111/1756-185X [DOI] [PubMed] [Google Scholar]
- 31. Dhital R, Basnet S, Paudel P, Acharya YP, Poudel DR. Prevalence of chronic obstructive pulmonary disease (COPD) among rheumatoid arthritis: results from national inpatient database. J Community Hosp Intern Med Perspect. (2018) 8:211–4. doi: 10.1080/20009666.2018.1485460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sheen YH, Rolfes MC, Wi CI, Crowson CS, Pendegraft RS, King KS, et al. Association of asthma with rheumatoid arthritis: A population-based case-control study. J Allergy Clin Immunol Pract. (2018) 6:219–26. doi: 10.1016/j.jaip.2017.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sparks JA, Lin TC, Camargo CA, Jr, Barbhaiya M, Tedeschi SK, Costenbader KH, et al. Rheumatoid arthritis and risk of chronic obstructive pulmonary disease or asthma among women: A marginal structural model analysis in the Nurses' Health Study. Semin Arthritis Rheum. (2018) 47:639–48. doi: 10.1016/j.semarthrit.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kronzer VL, Crowson CS, Sparks JA, Myasoedova E, Davis JM, 3rd. Comorbidities as risk factors for rheumatoid arthritis and their accrual after diagnosis. Mayo Clin Proc. (2019) 94:2488–98. doi: 10.1016/j.mayocp.2019.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mcguire K, Aviña-Zubieta JA, Esdaile JM, Sadatsafavi M, Sayre EC, Abrahamowicz M, et al. Risk of incident chronic obstructive pulmonary disease in rheumatoid arthritis: A population-based cohort study. Arthritis Care Res (Hoboken). (2019) 71:602–10. doi: 10.1002/acr.23410 [DOI] [PubMed] [Google Scholar]
- 36. Jung JH, Lim JH, Bang CH, Seok H, Song GG, Choi SJ. Prevalence of chronic obstructive pulmonary disease in patients with rheumatoid arthritis: A cross-sectional study. Int J Rheum Dis. (2021) 24:774–80. doi: 10.1111/1756-185X.14129 [DOI] [PubMed] [Google Scholar]
- 37. Kronzer VL, Westerlind H, Alfredsson L, Crowson CS, Nyberg F, Tornling G, et al. Respiratory diseases as risk factors for seropositive and seronegative rheumatoid arthritis and in relation to smoking. Arthritis Rheumatol. (2021) 73:61–8. doi: 10.1002/art.41491 [DOI] [PubMed] [Google Scholar]
- 38. Zaccardelli A, Liu X, Ford JA, Cui J, Lu B, Chu SH, et al. Elevated anti-citrullinated protein antibodies prior to rheumatoid arthritis diagnosis and risks for chronic obstructive pulmonary disease or asthma. Arthritis Care Res (Hoboken). (2021) 73:498–509. doi: 10.1002/acr.24140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kronzer VL, Huang W, Zaccardelli A, Crowson CS, Davis JM, 3rd, Vassallo R, et al. Association of sinusitis and upper respiratory tract diseases with incident rheumatoid arthritis: A case-control study. J Rheumatol. (2022) 49:358–64. doi: 10.3899/jrheum.210580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim JG, Kang J, Lee JH, Koo HK. Association of rheumatoid arthritis with bronchial asthma and asthma-related comorbidities: A population-based national surveillance study. Front Med (Lausanne). (2023) 10:1006290. doi: 10.3389/fmed.2023.1006290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang K, Wang L, Chen S, Chen R. Longitudinal reciprocal association between rheumatoid arthritis and chronic obstructive pulmonary disease and mediation of inflammation. Rheumatol (Oxford). (2023) 6:kead594. doi: 10.1093/rheumatology/kead594 [DOI] [PubMed] [Google Scholar]
- 42. Chung C, Kim H, Han K, Jung J, Eun Y, Lee H, et al. Rheumatoid arthritis increases the risk of COPD: A nationwide retrospective cohort study. Chest. (2024) 15:S0012–3692(0024)00160-00160. doi: 10.1016/j.chest.2024.02.014 [DOI] [PubMed] [Google Scholar]
- 43. Hemminki K, Liu X JJ, Sundquist K, Sundquist J. Subsequent COPD and lung cancer in patients with autoimmune disease. Eur Respir J. (2011) 37:463–5. doi: 10.1183/09031936.00070410 [DOI] [PubMed] [Google Scholar]
- 44. Ursum J, Nielen MM, Twisk JW, Peters MJ, Schellevis FG, Nurmohamed MT, et al. Increased risk for chronic comorbid disorders in patients with inflammatory arthritis: a population based study. BMC Fam Pract. (2013) 14:199. doi: 10.1186/1471-2296-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Radu AF, Bungau SA. Management of rheumatoid arthritis: an overview. Cells. (2021) 10:2857. doi: 10.3390/cells10112857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cao Z, Li Q, Wu J, Li Y. Causal association of rheumatoid arthritis with obstructive lung disease: Evidence from Mendelian randomization study. Heart Lung. (2023) 62:35–42. doi: 10.1016/j.hrtlng.2023.05.020 [DOI] [PubMed] [Google Scholar]
- 47. Yu X, Cheng X, Lv L, Wang N, Li M, Ji W, et al. The association between chronic obstructive pulmonary disease and autoimmune diseases: a bidirectional Mendelian randomization study. Front Med (Lausanne). (2024) 11:1331111. doi: 10.3389/fmed.2024.1331111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mori S, Koga Y, Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med. (2012) 106:1591–9. doi: 10.1016/j.rmed.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 49. Di Giuseppe D, Orsini N, Alfredsson L, Askling J, Wolk A. Cigarette smoking and smoking cessation in relation to risk of rheumatoid arthritis in women. Arthritis Res Ther. (2013) 15:R56. doi: 10.1186/ar4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. (2006) 119:503.e501–509. doi: 10.1016/j.amjmed.2005.09.053 [DOI] [PubMed] [Google Scholar]
- 51. Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. (2010) 34:J258–265. doi: 10.1016/j.jaut.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 52. Yang DH, Tu CC, Wang SC, Wei CC, Cheng YW. Circulating anti-cyclic citrullinated peptide antibody in patients with rheumatoid arthritis and chronic obstructive pulmonary disease. Rheumatol Int. (2014) 34:971–7. doi: 10.1007/s00296-013-2926-6 [DOI] [PubMed] [Google Scholar]
- 53. Linn-Rasker SP, van der Helm-van Mil AH, van Gaalen FA, Kloppenburg M, de Vries RR, le Cessie S, et al. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann Rheum Dis. (2006) 65:366–71. doi: 10.1136/ard.2005.041079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jiang X, Källberg H, Chen Z, Ärlestig L, Rantapää-Dahlqvist S, Davila S, et al. An Immunochip-based interaction study of contrasting interaction effects with smoking in ACPA-positive versus ACPA-negative rheumatoid arthritis. Rheumatol (Oxford). (2016) 55:149–55. doi: 10.1093/rheumatology/kev285 [DOI] [PubMed] [Google Scholar]
- 55. Kim SK, Park SH, Shin IH, Choe JY. Anti-cyclic citrullinated peptide antibody, smoking, alcohol consumption, and disease duration as risk factors for extraarticular manifestations in Korean patients with rheumatoid arthritis. J Rheumatol. (2008) 35:995–1001. [PubMed] [Google Scholar]
- 56. Agustí A, MacNee W, Donaldson K, Cosio M. Hypothesis: does COPD have an autoimmune component? Thorax. (2003) 58:832–4. doi: 10.1136/thorax.58.10.832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scher JU, Joshua V, Artacho A, Abdollahi-Roodsaz S, Öckinger J, Kullberg S, et al. The lung microbiota in early rheumatoid arthritis and autoimmunity. Microbiome. (2016) 4:60. doi: 10.1186/s40168-016-0206-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Konig MF. The microbiome in autoimmune rheumatic disease. Best Pract Res Clin Rheumatol. (2020) 34:101473. doi: 10.1016/j.berh.2019.101473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mikuls TR, Payne JB, Deane KD, Thiele GM. Autoimmunity of the lung and oral mucosa in a multisystem inflammatory disease: The spark that lights the fire in rheumatoid arthritis? J Allergy Clin Immunol. (2016) 137:28–34. doi: 10.1016/j.jaci.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 60. Mannion JM, McLoughlin RM, Lalor SJ. The airway microbiome-IL-17 axis: a critical regulator of chronic inflammatory disease. Clin Rev Allergy Immunol. (2023) 64:161–78. doi: 10.1007/s12016-022-08928-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Joo YB, Lim YH, Kim KJ, Park KS, Park YJ. Respiratory viral infections and the risk of rheumatoid arthritis. Arthritis Res Ther. (2019) 21:199. doi: 10.1186/s13075-019-1977-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ye C, Yuan L, Wu K, Shen B, Zhu C. Association between systemic immune-inflammation index and chronic obstructive pulmonary disease: a population-based study. BMC Pulm Med. (2023) 23:295. doi: 10.1186/s12890-023-02583-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.