Abstract
BACKGROUND:
Osteosarcoma is the most common primary bone malignancy. As a rare cancer, population-based studies remain small with limited information on finer demographic categories. Recent studies have reported important genetic differences based on age and ethnicity, and more detailed studies are needed to better understand potentially important osteosarcoma risk groups.
METHODS:
Incidence and survival rates for 5016 patients with osteosarcoma from the Surveillance, Epidemiology, and End Results (SEER) program (1975–2017) were analyzed by age (0–9, 10–24, 25–59, and >60 years old), race/ethnicity, histologic subtype, stage, and tumor location using SEER*Stat software.
RESULTS:
For cases 0 to 9 years old, incidence of primary osteosarcoma was similar between the sexes, increased significantly throughout the study period (P < .05), and the 5-year relative survival has steadily increased over time. Blacks had the highest incidence in all aged cases combined and a significant increase in incidence throughout the study period (P < .05). Overall, survival rates for all cases have remained relatively unchanged over recent decades, with worse survival observed in males, American Indian/Alaska Native cases, older patients, metastatic disease, axial tumors, and subsequent osteosarcoma cases. For cases 0 to 24 years old, the incidence of subsequent osteosarcoma increased 3-fold since the 2000s.
CONCLUSION:
Important differences in osteosarcoma incidence and survival, particularly for the youngest children, ethnic minorities, and subsequent osteosarcoma, are identified. A genetic risk factor may be associated with observed ancestry-specific incidence differences and illustrates the importance of analyzing osteosarcoma by specific age groups and ethnicities to better understand their unique epidemiology and underlying biology.
Keywords: epidemiology, incidence, osteosarcoma, SEER
INTRODUCTION
Osteosarcoma is the most common malignant primary bone tumor with peak incidence during adolescence.1 Osteosarcoma risk factors include prior therapeutic radiation,2 tall stature,3,4 high birth-weight,3,5 and several cancer predisposition syndromes including Li-Fraumeni syndrome6 and hereditary retinoblastoma.7 Emerging evidence has also identified a remarkably high number of deleterious germline genetic variants in osteosarcoma cases, particularly in the youngest cases.8,9 Approximately one-fourth of osteosarcoma cases were recently reported to have a germline pathogenic variant in an established cancer-susceptibility gene, and cases aged <10 years had the highest frequency.8
Numerous population-based epidemiologic studies have examined osteosarcoma incidence and survival by specific age groups.1,10–16 These studies have identified several unique epidemiological features that vary by age, including an increased occurrence of axial tumors and metastatic disease and worse 5-year survival in the oldest patients.1,13 However, there is still a paucity of data on the youngest osteosarcoma cases, because they are typically grouped with adolescent and young adult cases due to the rarity of disease. A recent study observed that the youngest cases (<10 years old) had the most substantial improvement in relative survival over the past 3 decades.11 Limited information is also available on racial minorities, despite evidence that people of African ancestry have a higher incidence of osteosarcoma.17
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program covers up to 34% of the US population,18 which creates an opportunity to perform detailed analyses on incidence and survival for rare cancers. Here, we compare osteosarcoma incidence and survival in 4 age groups (0–9, 10–24, 25–59, and >60 years old) by race/ethnicity, sex, decade, pathologic subtype, and tumor location. We also compare the incidence and survival of metastatic disease by age group and perform a detailed analysis of subsequent osteosarcoma.
MATERIALS AND METHODS
Patient data were obtained from the SEER 9 and 18 databases. Frequency, incidence, and survival rates by decade were calculated using the SEER 9 registry (1975–2017) to allow for comparisons across 5 decades. SEER 18 (2000–2017) was used for all other analyses to allow for detailed race designations (White, Black, American Indian/Alaska Native, Asian/Pacific Islander, and Hispanic). SEER*Stat (version 8.3.8)19 was used to calculate frequency, incidence, and survival statistics. Incidence rates (IRs) are per 1,000,000, and age-adjusted using the 2000 US standard population, and 95% confidence intervals (CI) were estimated for the main incidence and survival rates. Osteosarcoma cases were classified according to the adolescents and young adults site recode/World Health Organization 2008 classification. Anatomic site codes (C40.0–40.3 and C41.0–41.4) and histology subtypes (9180–9186 and 9192–9194) were classified according to the International Classification of Disease for Oncology, 3rd edition (ICD-O-3). Primary osteosarcoma was defined using sequence number as “one primary only or 1st of 2 or more primaries,” and osteosarcoma as a subsequent primary was defined using sequence number “2nd of 2 or more primaries” or greater (termed “subsequent osteosarcoma”). To characterize the first primary tumors, case listing sessions were restricted to at least 1 additional record. “Distant” disease was considered metastatic.
Cases were divided into 4 age groups (0–9, 10–24, 25–59, and >60 years old) when numbers were sufficient (n ≥ 10 cases). These age groups were chosen based on previously published suggested underlying genetic or biologic differences in pre-pubertal, pubertal, and elderly osteosarcoma cases, and on the ability to compare to previous published studies.1,10,20 Annual percent change (APC) was calculated using the weighted least squares method, and a P value <.05 was considered significant.
Five-year relative survival (RS) rates were calculated by the Ederer II method using SEER*Stat. RS was chosen for estimating population-level survival patterns.21 Percent relative 5-year survival rates by decade were calculated through 2016 to allow for at least 1 year of follow-up, except for metastatic survival rates that were calculated through 2015 to remain consistent with SEER historic stage A classification. Survival differences between decades of diagnosis were analyzed separately for each age group using the most recent decade as the reference group. Number of total years per decade group varies based on available years in the SEER database. Despite incomplete SEER 9 data from the 1970s, cases from this decade were analyzed separately due to the introduction of adjuvant chemotherapy during this time.22 Statistical differences in 5-year RS were measured by the Z test23 and P values <.05 were considered significant. Survival by decade for each age group was also estimated using the Kaplan-Meier method and curves, and the log-rank test was used to assess differences among decades using a Cox proportional hazards regression model (score test), and P values <.05 were considered significant.
RESULTS
The SEER 18 database consisted of 5016 cases of osteosarcoma, including 4336 primary osteosarcoma (86%) and 680 subsequent osteosarcoma (14%) cases. As expected, we observed a peak in primary osteosarcoma incidence in children and adolescents 10 to 24 years old, and a secondary peak in the elderly between 80 to 84 years old (Fig. 1A). Interestingly, the secondary peak only occurred in males (Fig. 1B). The majority of cases, for all ages, occurred in the lower long bones (Supporting Table 1), and histologic subtypes were primarily classified as “not otherwise specified” (NOS) (Supporting Tables 2 and 3). Osteosarcoma IRs were highest in Blacks (for all age cases: Black, IR, 4.1; 95% CI, 3.8–4.4, and the second highest race group, Hispanic, IR, 3.4; 95% CI, 3.2–3.6) (Table 1), and there was a significant increase in incidence only for Blacks throughout the study period (APC, 1.1; 95% CI, 0.3–2.0, P < .05).
Figure 1.
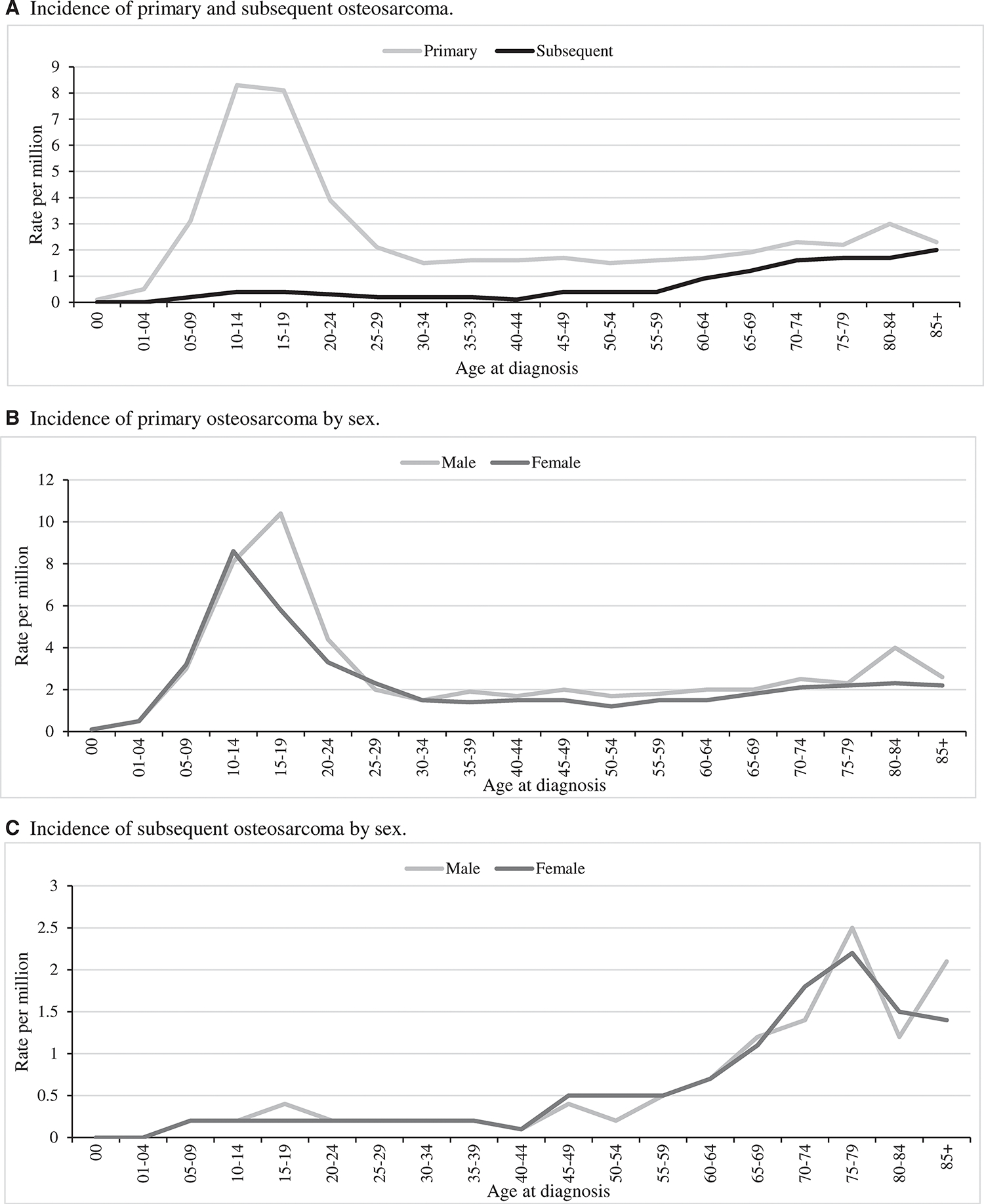
Incidence of osteosarcoma based on the Surveillance, Epidemiology, and End Results 18 database, 2000 to 2017. Incidence rates are age-adjusted calculated using the 2000 US standard population.
TABLE 1.
Osteosarcoma Incidence by Age Group, Race/Ethnicity, and Sex Based on the Surveillance, Epidemiology, and End Results 18 Database, 2000–2017
| Age Group, Race/Ethnicity Designation | Both Sexes |
Male |
Female |
|||
|---|---|---|---|---|---|---|
| IR (95% CI) | No. | IR (95% CI) | No. | IR (95% CI) | No. | |
|
| ||||||
| 0–9 y | ||||||
| White | 1.7 (1.5–2.0) | 165 | 1.7 (1.4–2.1) | 84 | 1.8 (1.4–2.2) | 81 |
| Black | 2.3 (1.7–2.9) | 63 | 2.3 (1.6–3.2) | 32 | 2.3 (1.5–3.2) | 31 |
| American Indian/Alaska Native | 1.0 (0.1–3.7)a | 2 | 0 | 0 | 2.1 (1.3–3.3)a | 2 |
| Asian or Pacific Islander | 2 (1.4–2.8) | 35 | 2.1 (1.3–3.3) | 19 | 1.9 (1.1–3.0) | 16 |
| Hispanic | 1.8 (1.5–2.2) | 113 | 1.7 (1.3–2.2) | 53 | 2 (1.5–2.6) | 60 |
| All races | 1.9 (1.7–2.1) | 378 | 1.8 (1.6–2.1) | 188 | 1.9 (1.6–2.2) | 190 |
| 10–24 y | ||||||
| White | 6.8 (6.4–7.2) | 1055 | 7.6 (7.0–8.2) | 607 | 5.9 (5.4–6.5) | 448 |
| Black | 7.9 (7.1–8.8) | 352 | 8.8 (7.6–10.1) | 197 | 7.1 (6.0–8.3) | 155 |
| American Indian/Alaska Native | 5.5 (3.3–8.7) | 18 | 6.7 (3.3–12.0) | 11 | 4.3 (1.7–8.9)a | 7 |
| Asian or Pacific Islander | 6.0 (5.2–7.0) | 171 | 6.7 (5.5–8.2) | 98 | 5.3 (4.2–6.7) | 73 |
| Hispanic | 7.9 (7.4–8.5) | 704 | 9.2 (8.4–10.1) | 423 | 6.5 (5.8–7.3) | 281 |
| All races | 7.2 (6.9–7.5) | 2300 | 8.1 (7.7–8.6) | 1336 | 6.2 (5.8–6.6) | 964 |
| 25–59 y | ||||||
| White | 1.9 (1.8–2) | 788 | 1.9 (1.7–2.1) | 406 | 1.9 (1.7–2.1) | 382 |
| Black | 2.7 (2.4–3.1) | 231 | 2.9 (2.3–3.4) | 114 | 2.6 (2.1–3.1) | 117 |
| American Indian/Alaska Native | 2.1 (1.1–3.6) | 13 | 1.7 (0.6–4.0)a | 5 | 2.4 (1.0–4.8)a | 8 |
| Asian or Pacific Islander | 1.5 (1.2–1.8) | 110 | 1.7 (1.3–2.1) | 59 | 1.3 (1.0–1.7) | 51 |
| Hispanic | 1.8 (1.5–2.0) | 269 | 1.9 (1.6–2.2) | 147 | 1.6 (1.3–1.9) | 122 |
| All races | 1.9 (1.8–2.0) | 1411 | 2 (1.8–2.1) | 731 | 1.8 (1.7–2.0) | 680 |
| >60 y | ||||||
| White | 3.4 (3.1–3.7) | 631 | 4.1 (3.7–4.6) | 328 | 2.9 (2.6–3.3) | 303 |
| Black | 5.0 (4.1–6.0) | 111 | 4.8 (3.4–6.6) | 45 | 5.1 (3.9–6.5) | 66 |
| American Indian/Alaska Native | 5.0 (2.0–10.6)a | 7 | 11.6 (3.9–25.9)a | 6 | 0.8 (0–6.1)a | 1 |
| Asian or Pacific Islander | 3.0 (2.3–3.8) | 65 | 2.3 (1.4–3.5) | 21 | 3.5 (2.5–4.7) | 44 |
| Hispanic | 3.5 (2.8–4.3) | 93 | 4.8 (3.5–6.4) | 54 | 2.6 (1.8–3.6) | 39 |
| All races | 3.5 (3.3–3.8) | 907 | 4.1 (3.8–4.5) | 454 | 3.1 (2.9–3.4) | 453 |
| All ages | ||||||
| White | 3.1 (3.0–3.3) | 2639 | 3.4 (3.3–3.6) | 1425 | 2.9 (2.7–3.1) | 1214 |
| Black | 4.1 (3.8–4.4) | 757 | 4.3 (3.9–4.8) | 388 | 3.9 (3.5–4.3) | 369 |
| American Indian/Alaska Native | 3.2 (2.2–4.4) | 40 | 4.2(2.4–6.6) | 22 | 2.5 (1.5–4.0) | 18 |
| Asian or Pacific Islander | 2.8 (2.5–3.0) | 381 | 2.9 (2.5–3.3) | 197 | 2.6 (2.2–3.0) | 184 |
| Hispanic | 3.4 (3.2–3.6) | 1179 | 3.9 (3.6–4.2) | 677 | 2.9 (2.6–3.2) | 502 |
| All races | 3.3 (3.2–3.4) | 4996 | 3.6 (3.5–3.8) | 2709 | 3.0 (2.9–3.1) | 2287 |
Abbreviations: CI, confidence interval; IR, incidence rate.
Cases with unknown race are excluded (n = 20).
These rates were based on <10 cases.
Five-year RS rates were higher in females (63.9%; 95% CI, 61.5%–66.2%) than males (56.5%; 95% CI, 54.2%–58.7%). Similar RS rates were observed for most races/ethnicities, except American Indian/Alaska Native cases that had the lowest RS of 36.9% (95% CI, 19.6%–54.3%) particularly for the males (18.5%), although based on a small number of cases (Table 2). Parosteal osteosarcoma had the highest observed RS for all aged cases at 91.9% and Paget disease of the bone the lowest at 29.9% (Supporting Table 2).
TABLE 2.
Percent 5-Year Relative Survival of Primary Osteosarcoma by Age Group, Race/Ethnicity, and Sex Based on the Surveillance, Epidemiology, and End Results 18 Database, 2000–2017
| Age Group, Race/Ethnicity Designation | Both Sexes |
Male |
Female |
|||
|---|---|---|---|---|---|---|
| RS (95% CI) | No. | RS (95% CI) | No. | RS (95% CI) | No. | |
|
| ||||||
| 0–9 y | ||||||
| White | 69.3 (60.5–76.5) | 154 | 64.2 (51.0–74.8) | 77 | 74.0 (61.7–82.8) | 77 |
| Black | 68.8 (55.1–79.2) | 60 | 74.5 (53.8–87.0) | 29 | 63.7 (43.8–78.1) | 31 |
| American Indian/Alaska Native | 0a | 2 | N/A | 0 | 0a | 2 |
| Asian or Pacific Islander | 73.4 (53.3–85.9) | 34 | 71.0 (43.5–86.9) | 18 | 75.0 (40.3–91.3) | 16 |
| Hispanic | 77.7 (67.7–84.9) | 108 | 79.5 (65.2–88.5) | 53 | 75.8 (60.2–85.9) | 55 |
| All races | 71.8 (66.4–76.5) | 358 | 71.4 (63.5–77.9) | 177 | 72.2 (64.4–78.6) | 181 |
| 10–24 y | ||||||
| White | 69.4 (66.2–72.4) | 1001 | 64.3 (59.9–68.5) | 571 | 76.1 (71.4–80.1) | 430 |
| Black | 67.1 (61.4–72.2) | 329 | 67.6 (59.7–74.4) | 180 | 66.5 (57.9–73.8) | 149 |
| American Indian/Alaska Native | 46.6 (22.2–67.9) | 18 | 20.2 (3.1–47.8) | 11 | 85.8 (33.1–97.9)a | 7 |
| Asian or Pacific Islander | 64.3 (55.4–71.8) | 164 | 62.8 (50.7–72.7) | 95 | 66.0 (52.2–76.6) | 69 |
| Hispanic | 60.9 (56.7–64.9) | 667 | 58.4 (52.8–63.5) | 401 | 64.4 (57.7–70.4) | 266 |
| All races | 65.9 (63.7–68.1) | 2179 | 62.5 (59.4–65.3) | 1258 | 70.5 (67.2–73.6) | 921 |
| 25–59 y | ||||||
| White | 58.6 (54.4–62.6) | 664 | 53.2 (47.3–58.7) | 347 | 64.6 (58.6–70.0) | 317 |
| Black | 50.5 (42.6–57.9) | 202 | 43.0 (32.1–53.5) | 104 | 58.3 (46.8–68.2) | 98 |
| American Indian/Alaska Native | 45.9 (13.6–73.7) | 10 | 30.4 (1.2–72.5)a | 5 | 57.6 (9.7–87.8)a | 5 |
| Asian or Pacific Islander | 57.5 (45.9–67.6) | 97 | 64.7 (48.9–76.8) | 55 | 48.6 (31.2–63.9) | 42 |
| Hispanic | 56.9 (49.4–63.8) | 246 | 50.2 (40.0–59.5) | 138 | 65.7 (54.3–74.9) | 108 |
| All races | 56.8 (53.7–59.8) | 1219 | 51.8 (47.4–56.0) | 649 | 62.5 (58.0–66.7) | 570 |
| >60 y | ||||||
| White | 33.5 (27.6–39.5) | 362 | 29.4 (21.7–37.5) | 185 | 37.0 (28.5–45.6) | 177 |
| Black | 30.7 (17.9–44.5) | 70 | 33.5 (10.2–59.3) | 24 | 28.2 (14.1–44.3) | 46 |
| American Indian/Alaska Native | 0a | 4 | 0a | 3 | 0* | 1 |
| Asian or Pacific Islander | 40.5 (23.4–57.0) | 40 | 0 | 11 | 48.6 (28.1–66.4) | 29 |
| Hispanic | 30.7 (17.8–44.7) | 58 | 19.8 (7.9–35.6) | 37 | 47.4 (20.6–70.2) | 21 |
| All races | 33.1 (28.3–38.0) | 534 | 27.9 (21.4–34.7) | 260 | 38.0 (31.0–45.0) | 274 |
| All ages | ||||||
| White | 60.2 (57.9–62.5) | 2181 | 55.7 (52.5–58.7) | 1180 | 65.6 (62.3–68.8) | 1001 |
| Black | 58.8 (54.6–62.8) | 661 | 58.9 (52.9–64.4) | 337 | 58.7 (52.6–64.2) | 324 |
| American Indian/Alaska Native | 36.9 (19.6–54.3) | 34 | 18.5 (4.6–39.8) | 19 | 63.2 (31.9–83.2) | 15 |
| Asian or Pacific Islander | 60.5 (54.4–66.1) | 335 | 62.0 (53.5–69.4) | 179 | 58.8 (49.8–66.8) | 156 |
| Hispanic | 60.2 (56.8–63.4) | 1079 | 56.3 (51.9–60.5) | 629 | 65.3 (60.1–70.0) | 450 |
| All races | 59.9 (58.2–61.5) | 4290 | 56.5 (54.2–58.7) | 2344 | 63.9 (61.5–66.2) | 1946 |
Abbreviations: CI, confidence interval; N/A, not applicable; RS, percent relative survival.
Cases with unknown race are excluded (n = 18).
These rates were based on <10 cases.
0 to 9 Years Old
There were 382 osteosarcoma cases in the youngest age group (0–9 years old), with an age-adjusted IR of 1.9 per million. Incidence of young-onset osteosarcoma increased from 1975 to 2017 (APC, 1.1; 95% CI, 0.1–2.1, P < .05) and was highest in the most recent decade (2.3 per million) (Fig. 2A). Observed osteosarcoma incidence was equal between sexes and most common in Blacks (Table 1). There were very few cases with tumors in axial locations (1.4%; Supporting Table 1).
Figure 2.
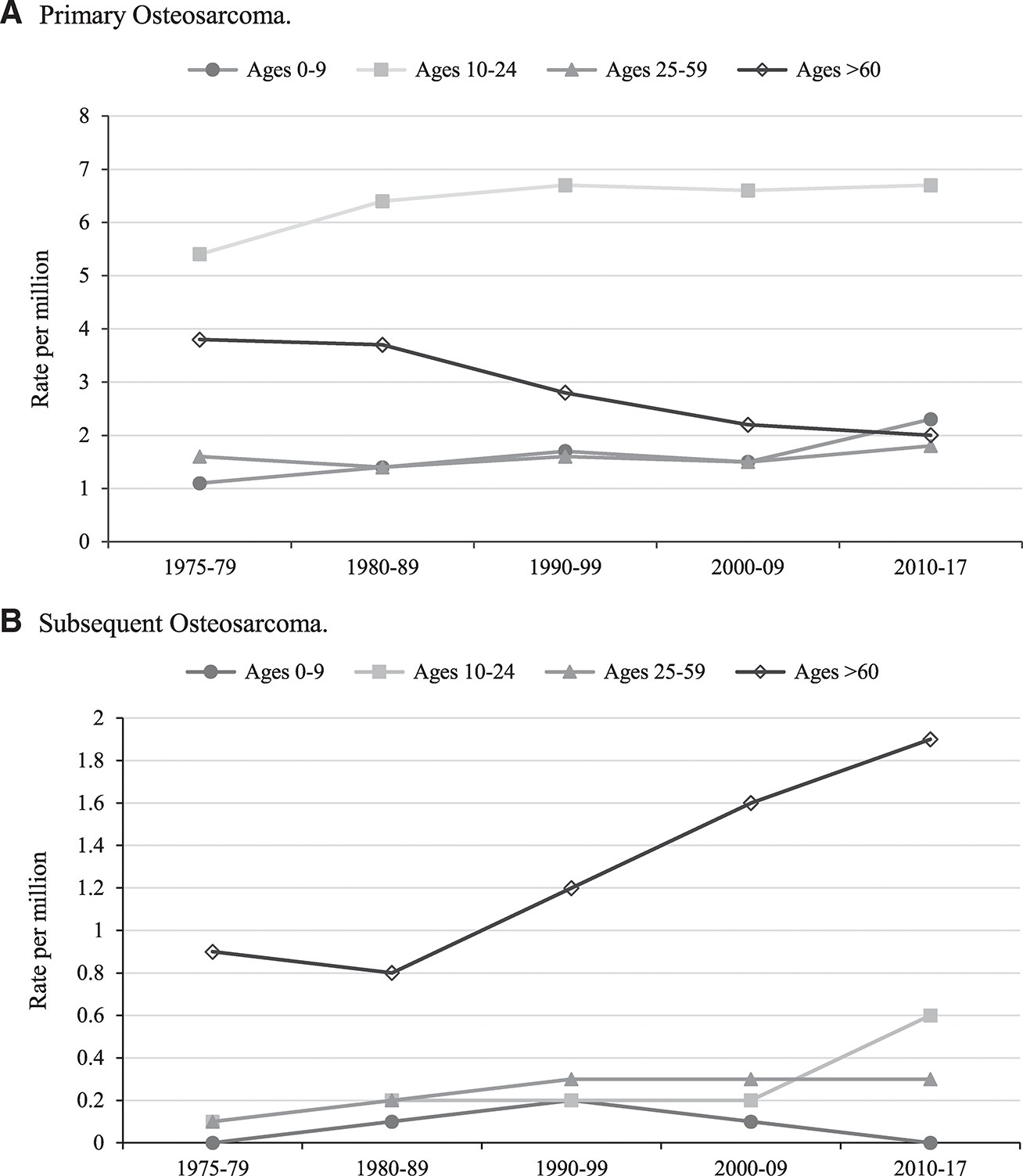
Incidence of osteosarcoma based on the Surveillance, Epidemiology, and End Results (SEER) 9 database, 1975 to 2017, by age group and year of diagnosis. Incidence rates are age-adjusted calculated using the 2000 US standard population. Number of total years per decade group varies based on available years in the SEER database.
The overall 5-year RS for this age group was 71.8%; 95% CI, 66.4–76.5 (Table 2). When evaluating trends by decades, survival steadily increased over the study period. In the most recent decade, RS was 80.4%, which is a statistically significant improvement from both the 1970s (P < .001) and 1980s (P = .002) (Fig. 3A). Cox proportional hazards models showed statistically significant improvements in survival from the 1970s (hazard ratio [HR], 6.5; P < .001), the 1980s (HR, 3.5; P = .001), and the 1990s (HR, 2.5; P = .018), compared to 2010 through 2016 (Supporting Table 4). Survival was similar between sexes (Table 2). Observed RS for telangiectatic osteosarcoma was the highest at 85.4% (Supporting Table 2).
Figure 3.
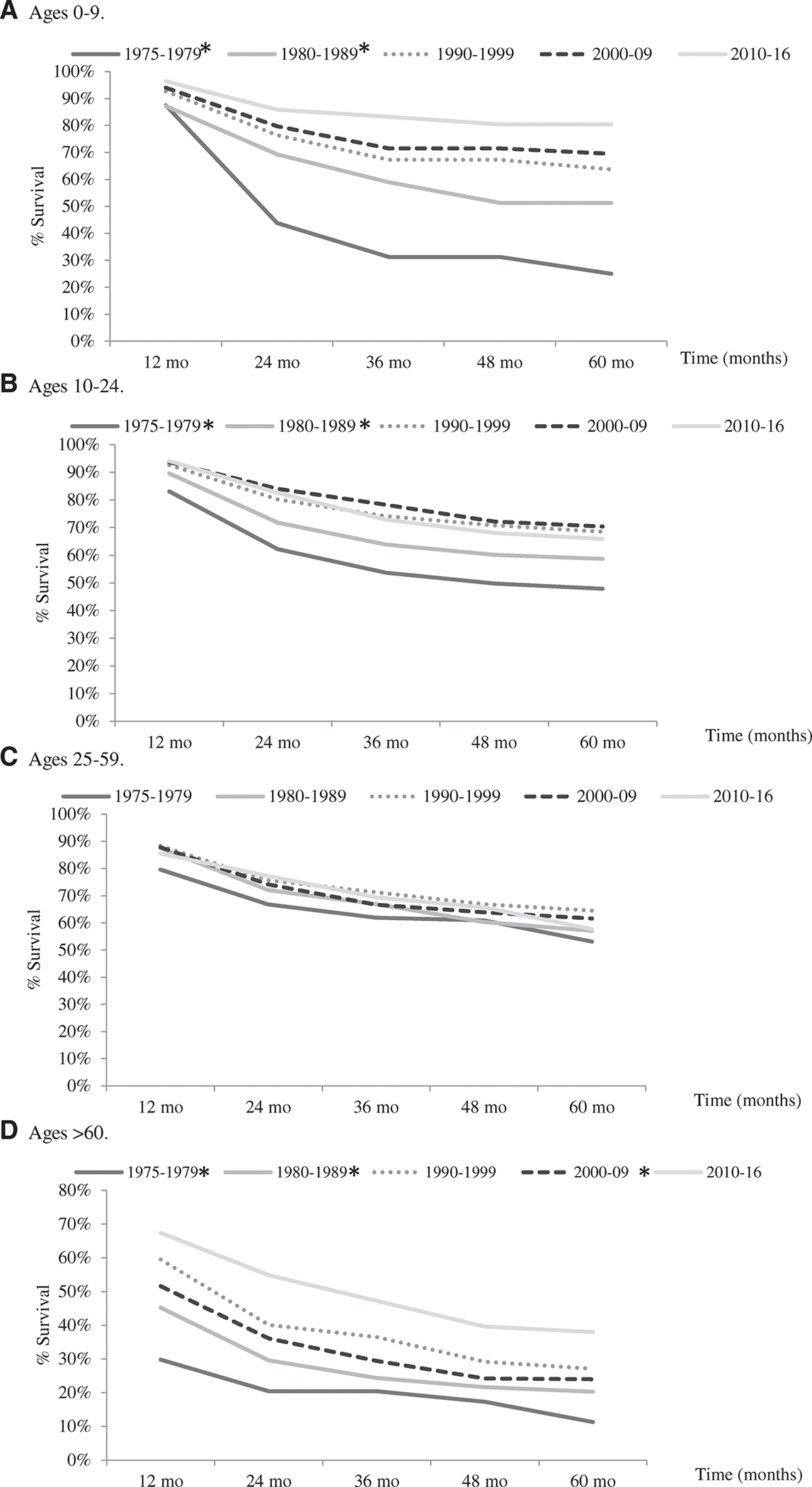
Five-year relative survival of primary osteosarcoma by decade, based on the Surveillance, Epidemiology, and End Results (SEER) 9 database, 1975 to 2016. Five-year relative survival rates within each age group were compared by decade with 2010 to 2016 as the comparison group. Significantly different survival rates (P < .05) using the Z test are marked with (*). Number of total years per decade group varies based on available years in the SEER database.
10 to 24 Years Old
There were a total of 2312 osteosarcoma cases in the 10- to 24-year-old age group, which represented nearly 50% of all osteosarcoma cases in the SEER 18 database. The incidence of primary adolescent osteosarcoma increased throughout the study period (APC, 0.5; 95% CI, 0.1–0.9; P < .05) and was highest in the most recent decade, 6.7 per million (Fig. 2A). Osteosarcoma occurred more frequently in males than females for each race/ethnicity category with an overall male to female ratio of 1.3:1 (males, IR, 8.1; 95% CI, 7.7–8.6 vs females, IR, 6.2; 95% CI, 5.8–6.6) (Table 1). The incidence peak was earlier in females (10–14 years old) than males (15–19 years old) (Fig. 1B) with some disparity by race/ethnicity. Observed osteosarcoma incidence was most common in Blacks and Hispanics with the highest incidence in Hispanic males (Table 1). When evaluating incidence by tumor location and race/ethnicity, osteosarcoma of the mandible was 4 times more common in Blacks (data not shown).
The overall 5-year RS rate was 65.9%; 95% CI, 63.7%–68.1% (Table 2). By decade, RS was 65.9% in the most recent decade, a statistically significant increase (P < .05) as compared to the 1970s (P < .001) and the 1980s (P = .013); however, survival has decreased slightly since the 1990s and 2000s (Fig. 3B). Cox proportional hazards models showed similar statistically significant improvements in survival from the 1970s (HR, 2.3; P < .001) and the 1980s (HR, 1.6; P < .001) only, compared to 2010 to 2016 (Supporting Table 4). RS was lower for males compared to females overall, and this was observed across all races except for Blacks (females, 66.5% vs males, 67.6%) (Table 2). Observed RS was lowest for American Indian/Alaska Natives (46.6%) and for osteosarcoma in axial locations, particularly in the pelvis (32.9%) (Table 2; Supporting Table 5).
25 to 59 Years Old
Individuals in the 25- to 59-year-old age group had an osteosarcoma IR of 1.9 per million (95% CI, 1.8–2.0; n = 1411) (Table 1). Incidence was relatively unchanged throughout the study period (APC, 0.5; P> .05) (Fig. 2A), only slightly higher in males than females (1.1:1), and highest in Blacks (IR, 2.7; 95% CI, 2.4–3.1) (Table 1). Osteosarcoma of the pelvis accounted for a higher proportion of these cases at 11.1% (Supporting Table 1). Osteosarcoma of the mandible was 2 times more common in Blacks than Whites (data not shown).
The overall RS was 56.8% and 95% CI, 53.7–59.8 (Table 2). The observed RS of 57.7% in the most recent decade was slightly lower than the RS in the 1990s and 2000s (Fig. 3C). Cox proportional hazards models revealed statistically significant improvements in survival from the 1970s (HR, 1.8; P < .001) and 1980s (HR, 1.6; P = .005) only, compared to 2010 to 2016 (Supporting Table 4). RS was lower for males, and this was observed in all races and/or ethnicities except for Asians (females, 48.6% vs males, 64.7%) (Table 2). Osteosarcoma of the pelvis had the poorest observed RS (27.5%) (Supporting Table 5).
>60 Years Old
The incidence of osteosarcoma in this oldest age group was 2 per million in the most recent decade (n = 908) (Fig. 2A), and rates have significantly decreased throughout the study period (APC, −2.1; 95% CI, −2.7 to −1.5; P < .05). Osteosarcoma occurred more frequently in males than females overall (1.3:1) and by race/ethnicity, except Asian females had a higher observed IR than Asian males (1.5:1) (Table 1). The observed incidence of osteosarcoma was highest in Blacks and American Indian/Alaska Natives (Table 1). The anatomic tumor locations in this age group were more diverse, and the pelvis accounted for 17.9% of cases (Supporting Table 1). Osteosarcoma of the mandible was 2 times more common in Blacks than Whites. Osteosarcoma with Paget disease was more common in this older age group (Supporting Table 2).
The overall RS was 33.1% (95% CI, 28.3–38.0) (Table 2). By decade, RS was 38.0% in the most recent decade which has significantly increased compared to the 1970s (P < .001), 1980s (P = .001), and 2000s (P = .008) (Fig. 3D). Cox proportional hazards models showed statistically significant improvements in survival from the 1970s (HR, 2.8, P < .001), 1980s (HR, 2.2, P < .001), 1990s (HR, 1.8, P = .001), and 2000s (HR, 1.6, P = .01), compared to 2010 to 2016 (Supporting Table 4). Observed survival was lower for males, with the exception of Blacks (28.2% females vs 33.5% males), and similar by race/ethnicity (Table 2). Osteosarcoma of the vertebral column (4.3%) and pelvis (10.1%) had the worst observed RS (Supporting Table 5).
Metastatic Osteosarcoma
There were 932 metastatic osteosarcoma cases during the study period. Metastatic disease was more common in the >60-year-old age group (33.3% of cases vs 18.2%–21.7%) (Supporting Table 6). The observed rates of metastatic osteosarcoma were slightly higher for males (24.4%) than females (20.2%), and for Hispanics (24.6%) and Blacks (23.6%) compared to Whites (21.2%) (data not shown). The observed frequency of metastatic disease varied by tumor location and was greatest for osteosarcoma of the pelvis (41.7% of all cases) (Supporting Table 7) compared to other sites for both the young and old cases.
Survival for individuals with metastatic disease decreased with increasing age. The youngest cases (0–24 years old) had the highest overall 5-year RS at 35.5% (95% CI, 31.0%–41.0%), followed by the 25- to 59-year-old age group at 15.4% (95% CI, 10.1%–20.8%), and the poorest survival was in cases >60 years old at 6.4% (95% CI, 2.4%–10.7%) (data not shown). There were significant improvements in 5-year RS from the 1970s and 1980s compared to 2010 through 2015 for the 0- to 24-year-old age group and for all ages combined (Fig. 4A,D). The 5-year RS for the >60-year-old age group showed a statistically significant increase from the 2000s compared to 2010 through 2015 (Fig. 4C). Cox proportional hazards models showed similar significant improvements in survival from the 1970s and 1980s compared to 2010 through 2015 for the 0- to 24-year-old age group, the >60-year-old age group, and all age groups combined (Supporting Table 8).
Figure 4.
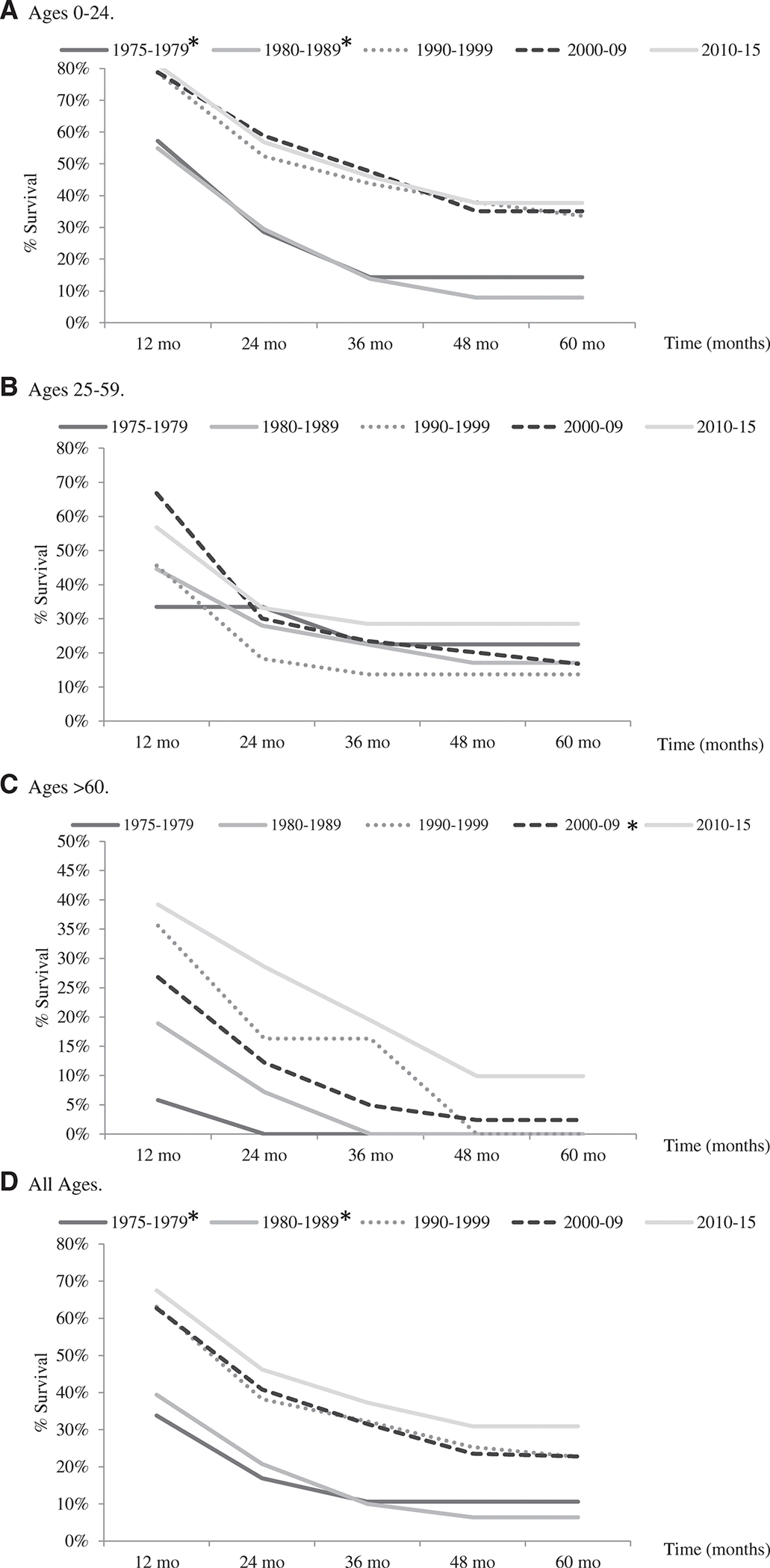
Five-year relative survival of metastatic osteosarcoma by decade, based on the Surveillance, Epidemiology, and End Results (SEER) 9 database, 1975 to 2015. Five-year relative survival rates within each age group were compared by decade with 2010 to 2015 as the comparison group. Significantly different survival rates (P < .05) using the Z test are marked with (*). The 0- to 9-year-old and 10- to 24-year-old age groups were combined to have sufficient number of cases for analysis. Number of total years per decade group varies based on available years in the SEER database. The 25- to 59-year-old age group (B), 1975 to 1979 decade is based on only 9 cases.
Subsequent Osteosarcoma
The incidence of subsequent osteosarcoma has increased significantly from 1975 to 2017 (APC, 2.5; 95% CI, 1.7–3.3; P < .05). The >60-year-old age group had the highest observed incidence of subsequent osteosarcoma, and it has steadily increased over the study period to 1.9 per million in the most recent decade (Fig. 2B). The most common first primaries for subsequent osteosarcomas in the oldest age group were breast and prostate cancers compared to rhabdomyosarcoma and tumors of the central nervous system for the 0- to 24-year-old age group (data not shown). The observed incidence of subsequent osteosarcoma was 0.6 per million for the 10- to 24-year-old age group in the most recent decade, a 3-fold increase since the 2000s (Fig. 2B). There were 17 cases of subsequent osteosarcoma in the 0- to 9-year-old age group, and the primary tumors in these cases included retinoblastoma, choroid plexus carcinoma, leiomyosarcoma, and adrenocortical carcinomas. Males and females had similar IRs of subsequent osteosarcoma for most ages (Fig. 1C), except for elderly cases where males had a higher incidence (>60-year-old age group, 1.4:1). Blacks had the highest observed incidence of subsequent osteosarcoma at 0.6 per million (data not shown).
The lower long bones (28.2% of all cases) were the most common site of subsequent osteosarcoma overall, but tumor location varied by age. Bones of the face or skull were the second most common subsequent osteosarcoma location in both the 0- to 9-year-old (35.3%) and 10- to 24-year-old (15.3%) age groups (Supporting Table 9). The pelvis (35.1%) followed by the lower long bones (21.9%) were the most common subsequent osteosarcoma locations in the >60-year-old age group (Supporting Table 9). By sex in the >60-year-old age group, females had a higher observed percentage of subsequent osteosarcoma located in the chest region as compared to males (11.3% vs 4.4%), males had a higher observed percentage in the mandible (13.2% vs 7.5%), and percentages were similar in the pelvis (Supporting Table 10). The observed 5-year RS rates for subsequent osteosarcoma in the 0- to 9-year-old, 10- to 24-year-old, 25- to 59-year-old, and >60-year-old age groups were 49.4%, 44.4%, 32.1%, and 19.2%, respectively (data not shown).
DISCUSSION
We present a comprehensive and detailed analysis of osteosarcoma incidence and survival and highlight important differences related to age of diagnosis, sex, race/ethnicity, tumor location, and stage. Compared to our prior 2009 SEER osteosarcoma study,1 1534 additional osteosarcoma cases have been added to the SEER registry. Using over 5000 osteosarcoma cases, we confirm several previously reported incidence and survival patterns, including a higher incidence in Blacks and males, higher survival rates in females and the youngest cases, and the poorest outcomes in the elderly, tumors of the axial skeleton, metastatic disease, and subsequent osteosarcoma cases.1,10,11,15–17,24–27 We further identify important novel findings related to the youngest osteosarcoma cases, osteosarcoma as a subsequent malignancy, and incidence and survival trends based on race/ethnicity.
We analyzed 382 cases in the 0- to 9-year-old age group, to our knowledge, the largest number of pre-pubertal osteosarcoma patients with detailed incidence and survival information. Importantly, incidence in these youngest cases has steadily increased over time, and they had unique epidemiological features compared to older cases. Young cases had similar incidence and survival rates between sexes, the highest observed 5-year survival, and the greatest survival improvements over time. Even in large studies, osteosarcoma cases <10 years old in age tend to be very small in number and are typically grouped with adolescent and young adult cases for analyses. However, a recent study suggests that osteosarcoma in young children may have a different genetic etiology than their adolescent counterparts.8 In addition, the youngest cases occur outside of the pubertal growth spurt, which is thought to be important in the pathogenesis of adolescent osteosarcoma. Our data suggest that the youngest pre-pubertal cases likely have a distinct etiology compared to adolescent and adult osteosarcoma and should be evaluated separately when possible.
Hispanic males in the 10- to 24-year-old age group had a higher observed IR of osteosarcoma compared to any other age group or sex, which has not been previously reported. Blacks had the highest incidence among all age groups together, confirming that African Americans have a higher incidence and unique risk of osteosarcoma. A high prevalence of osteosarcoma has also been reported in the African countries of Nigeria, Uganda, and Sudan, and it was suggested to be linked to an ancestry-based genetic predisposition.17 African-ancestry cases have also been reported to carry twice as many pathogenic germline TP53 mutations compared to other ancestry cases (9% vs 4% of cases).8 A potential genetic risk factor associated with African-ancestry cases could be related to the higher overall incidence, the 2 times higher incidence of osteosarcoma of the mandible, and the high rate of subsequent osteosarcoma we observed in Blacks. Osteosarcoma of the mandible is less common and thought to be biologically different28 from osteosarcoma at other sites, which further suggests a unique etiology in this population. Historically, African-ancestry individuals are underrepresented in genetic etiology studies, and a concerted effort to improve recruitment is needed to better understand the etiology of osteosarcoma in African-ancestry cases.
Osteosarcoma survival rates for most age groups in our study have remained relatively unchanged over the past 30 years. These plateaued survival rates have been reported in other studies,1,29 but it is important to note the increased survival we observed in the oldest osteosarcoma cases. Although prognosis remains poorest for the >60-year-old age group, these cases had an appreciable increase in osteosarcoma survival over the past decade. The >60-year-old age group is unique given their predilection for osteosarcoma of the axial skeleton, but they are also less likely to receive intense cytotoxic chemotherapy and more likely to be excluded from clinical trials,30 all of which could play a role in survival differences.
Metastatic disease, typically presenting as lung metastasis, is known to be one of the worst prognostic factors.16,24 In our study, metastatic disease was associated with lower survival rates in each age group, and the prognosis for metastatic disease in the >60-year-old age group remained dismal at <10% despite a trend of improved survival over time. Increasing survival rates for metastatic osteosarcoma, and for the >60-year-old age group overall, may be related to more aggressive surgical management of the primary tumor site31,32 and improved multidisciplinary management.33
Survival also varied based on sex and tumor location. We confirmed that females have a survival advantage over males,1,10,34 and recent studies have identified male sex as an independent risk factor for decreased survival.15,16 The etiology underlying a survival difference between sexes is not fully understood but may in part be related to endogenous sex hormones and differences in the pharmacokinetics and response to therapeutic treatments.35,36 Males also had a slightly higher rate of metastatic disease in our study, which may play a role in the survival differences. Survival continues to be worse for osteosarcoma of the pelvis and axial tumor locations over the extremities. Osteosarcoma of the mandible had the lowest observed metastatic rate at 6%, consistent with previous literature.28,37 Parosteal osteosarcoma had the highest survival rate by subtype, however, detailed subtype analyses were difficult due to the large number of NOS classifications. Additional studies with more extensive histologic information are needed.
From a clinical context, osteosarcoma outcomes from cooperative group trials have shown little improvement over recent decades, leading to a standard of care that has remained unchanged for over 40 years.25,38–40 Clinical trials in the palliative setting are currently focused on targeting novel pathways of interest such as small-molecule inhibitors and immunotherapy-based regimens.41 Subsequent randomized controlled trials are needed to see if targeted therapy may be the key to improving survival for osteosarcoma patients.
We identified an increasing incidence of subsequent osteosarcoma over time, particularly for the 10- to 24-year-old and >60-year-old age groups, and a poorer observed survival rate as compared to cases of the same age with primary disease. The incidence of subsequent osteosarcoma in the 10- to 24-year-old age group has increased 3-fold in the most recent decade and should be expected to continue to rise with an increasing number of childhood cancer survivors. Osteosarcoma is the most common radiation associated sarcoma affecting both children and adults,26 and children are more susceptible to radiation-associated sarcomas in a dose-dependent manner.2 Treatment with chemotherapy, particularly alkylating agents, is an additional risk factor.42 In addition, a germline genetic predisposition may also play a role, especially for the youngest children (<10 years old). In our study, primary tumors in these children included cancer predisposition syndrome-associated tumors for Li-Fraumeni as well as retinoblastoma. It is clear that continued long-term follow-up for screening and management of late effects, including subsequent malignancies, is needed to ensure optimal care.43
The incidence of subsequent osteosarcoma in the >60-year-old age group was nearly the same as the incidence of primary osteosarcoma. Women in this age group had a higher percentage of subsequent osteosarcoma occurring in the chest region, likely related to therapeutic radiation for breast cancer. Men had a higher percentage of osteosarcoma of the mandible, which may reflect radiation treatment for head and neck cancer, known to be 2 to 3 times more common in males.44 The increasing rates of subsequent osteosarcoma in elderly patients likely reflects that more patients with early-stage cancer treated with radiation therapy are living longer with more time to develop subsequent cancers. As subsequent osteosarcomas continue to rise, it will be important to follow-up this trend in future studies.
Our study has some limitations worth noting. This was a large retrospective study based on input from specific geographic areas according to the SEER program central cancer registries, which may introduce some selection bias. Additionally, SEER is an extensive and well-curated database, but it lacks detailed clinical information on molecular pathological characteristics, treatment regimens, and histologic response to chemotherapy, all of which could influence prognosis.
Overall, our large, comprehensive population-based analysis has clarified important osteosarcoma incidence and survival patterns at a finer level. Osteosarcoma is a heterogenous disease, and we were able to identify differences among groups that are typically understudied due to limited sample sizes, specifically the youngest children, racial and ethnic minorities, and osteosarcoma as a subsequent malignancy. The epidemiologic differences we identified in these groups may reflect differences in the underlying biology and/or genetic susceptibility, and further studies focusing on these groups are needed to better understand the pathogenesis of osteosarcoma in these cases. A better understanding of osteosarcoma etiology across all ages and racial/ethnic groups could be the basis to improve risk stratification, targeted treatment, and hopefully improve patient outcomes that continue to be unchanged over the past 30 years.
Supplementary Material
LAY SUMMARY:
Osteosarcoma is the most common bone cancer, but still a relatively rare disease, and previous studies have had limited information on finer demographics.
Using a large database, osteosarcoma incidence and survival patterns are thoroughly evaluated and important differences, especially for the youngest children, ethnic minorities, and subsequent osteosarcoma cases, are identified.
FUNDING SUPPORT
This research was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Vu B, de Vathaire F, Shamsaldin A, et al. Radiation dose, chemotherapy and risk of osteosarcoma after solid tumors during childhood. Int J Cancer. 1998;77:370–377. doi: [DOI] [PubMed] [Google Scholar]
- 3.Mirabello L, Pfeiffer R, Murphy G, et al. Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes Control. 2011;22:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora RS, Kontopantelis E, Alston RD, Eden TO, Geraci M, Birch JM. Relationship between height at diagnosis and bone tumors in young people: a meta-analysis. Cancer Causes Control. 2011;22:681–688. doi: 10.1007/s10552-011-9740-9 [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Yang L, Pu F, et al. High birth weight increases the risk for bone tumor: a systematic review and meta-analysis. Int J Environ Res Public Health. 2015;12:11178–11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mai PL, Best AF, Peters JA, et al. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer. 2016;122:3673–3681. doi: 10.1002/cncr.30248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong F, Boice JD Jr, Abramson DH, et al. Cancer incidence after ret-inoblastoma: radiation dose and sarcoma risk. JAMA. 1997;278:1262–1267. doi: 10.1001/jama.1997.03550150066037 [DOI] [PubMed] [Google Scholar]
- 8.Mirabello L, Zhu B, Koster R, et al. Frequency of pathogenic germline variants in cancer-susceptibility genes in patients with osteosarcoma. JAMA Oncol. 2020;6:724–734. doi: 10.1001/jamaoncol.2020.0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirabello L, Yeager M, Mai PL, et al. Germline TP53 variants and susceptibility to osteosarcoma. J Natl Cancer Inst. 2015;107:djv101. doi: 10.1093/jnci/djv101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Y, Chen D, Hu T, Lv G, Dai Z. Characteristics and prognostic factors of patients with osteosarcoma older than 60 years from the SEER database. Cancer Control. 2019;26:1073274819888893. doi: 10.1177/1073274819888893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Sun H, Li J, et al. Increased survival of patients aged 0–29 years with osteosarcoma: a period analysis, 1984–2013. Cancer Med. 2018;7:3652–3661. doi: 10.1002/cam4.1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG. Osteosarcoma in children 5 years of age or younger at initial diagnosis. Pediatr Blood Cancer. 2010;55:285–289. doi: 10.1002/pbc.22509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirabello L, Troisi R, Savage S. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anfinsen KP, Devesa SS, Bray F, et al. Age-period-cohort analysis of primary bone cancer incidence rates in the United States (1976–2005). Cancer Epidemiol Biomarkers Prev. 2011;20:1770–1777. doi: 10.1158/1055-9965.epi-11-0136 [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Wu B, Zhou Y, et al. Predictors of the survival of primary and secondary older osteosarcoma patients. J Cancer. 2019;10:4614–4622. doi: 10.7150/jca.32627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol. 2015;39:593–599. doi: 10.1016/j.canep.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 17.Sadykova LR, Ntekim AI, Muyangwa-Semenova M, et al. Epidemiology and risk factors of osteosarcoma. Cancer Invest. 2020;38:259–269. doi: 10.1080/07357907.2020.1768401 [DOI] [PubMed] [Google Scholar]
- 18.Surveillance, Epidemiology, and End Results Program. SEER*Stat Database: Incidence–SEER Research Data, 9 Registries, 2019 Sub (1975–2017)–Linked to County Attributes–Time Dependent (1990–2017) Income/Rurality, 1969–2 017 Counties. National Cancer Institute; 2020. [Google Scholar]
- 19.Surveillance Research Program. SEER*Stat Software. Version 8.3.8. National Cancer Institute; 2020. [Google Scholar]
- 20.Mirabello L, Zhu B, Koster R, et al. Frequency of pathogenic germline variants in cancer-susceptibility genes in patients with osteosarcoma. JAMA Oncol. 2020;6:724–734. doi: 10.1001/jamaoncol.2020.0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makkar N, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. A comparison of relative survival and cause-specific survival methods to measure net survival in cancer populations. Cancer Med. 2018;7:4773–4780. doi: 10.1002/cam4.1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misaghi A, Goldin A, Awad M, Kulidjian AA. Osteosarcoma: a comprehensive review. SICOT J. 2018;4:12. doi: 10.1051/sicotj/2017028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown CC. The statistical comparison of relative survival rates. Biometrics. 1983;39:941–948. [PubMed] [Google Scholar]
- 24.Aljubran AH, Griffin A, Pintilie M, Blackstein M. Osteosarcoma in adolescents and adults: survival analysis with and without lung metastases. Ann Oncol. 2009;20:1136–1141. doi: 10.1093/annonc/mdn731 [DOI] [PubMed] [Google Scholar]
- 25.Smeland S, Bielack SS, Whelan J, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019;109:36–50. doi: 10.1016/j.ejca.2018.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mavrogenis AF, Pala E, Guerra G, Ruggieri P. Post-radiation sarcomas. Clinical outcome of 52 patients. J Surg Oncol. 2012;105:570–576. doi: 10.1002/jso.22122 [DOI] [PubMed] [Google Scholar]
- 27.Brady MS, Gaynor JJ, Brennan MF. Radiation-associated sarcoma of bone and soft tissue. Arch Surg. 1992;127:1379–1385. doi: 10.1001/archsurg.1992.01420120013002 [DOI] [PubMed] [Google Scholar]
- 28.Bertin H, Gomez-Brouchet A, Rédini F. Osteosarcoma of the jaws: an overview of the pathophysiological mechanisms. Crit Rev Oncol Hematol. 2020;156:103126. doi: 10.1016/j.critrevonc.2020.103126 [DOI] [PubMed] [Google Scholar]
- 29.Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma - connecting etiology, biology and therapy. Nat Rev Endocrinol. 2017;13:480–491. doi: 10.1038/nrendo.2017.16 [DOI] [PubMed] [Google Scholar]
- 30.Longhi A, Errani C, Gonzales-Arabio D, Ferrari C, Mercuri M. Osteosarcoma in patients older than 65 years. J Clin Oncol. 2008;26:5368–5373. doi: 10.1200/jco.2007.14.9104 [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Guo X, Xu Y, et al. Lung metastases at the initial diagnosis of high-grade osteosarcoma: prevalence, risk factors and prognostic factors. A large population-based cohort study. Sao Paulo Med J. 2019;137:423–429. doi: 10.1590/1516-3180.2018.0381120619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traven SA, Anderson AB, Walton ZJ, Leddy LR. Primary tumor resection in patients with metastatic osteosarcoma. Ann Joint. 2019;4:46. [Google Scholar]
- 33.Federman N, Bernthal N, Eilber FC, Tap WD. The multidisciplinary management of osteosarcoma. Curr Treat Options Oncol. 2009;10:82–93. doi: 10.1007/s11864-009-0087-3 [DOI] [PubMed] [Google Scholar]
- 34.Nathan SS, Healey JH. Demographic determinants of survival in osteosarcoma. Ann Acad Med Singap. 2012;41:390–399. [PubMed] [Google Scholar]
- 35.Anthony M, Berg MJ. Biologic and molecular mechanisms for sex differences in pharmacokinetics, pharmacodynamics, and pharmacogenetics: Part II. J Womens Health Gend Based Med. 2002;11:617–629. doi: 10.1089/152460902760360568 [DOI] [PubMed] [Google Scholar]
- 36.Anthony M, Berg MJ. Biologic and molecular mechanisms for sex differences in pharmacokinetics, pharmacodynamics, and pharmacogenetics: Part I. J Womens Health Gend Based Med. 2002;11:601–615. doi: 10.1089/152460902760360559 [DOI] [PubMed] [Google Scholar]
- 37.Baumhoer D, Brunner P, Eppenberger-Castori S, Smida J, Nathrath M, Jundt G. Osteosarcomas of the jaws differ from their peripheral counterparts and require a distinct treatment approach. Experiences from the DOESAK Registry. Oral Oncol. 2014;50:147–153. doi: 10.1016/j.oraloncology.2013.10.017 [DOI] [PubMed] [Google Scholar]
- 38.Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. 2016;17:1396–1408. doi: 10.1016/s1470-2045(16)30214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bielack SS, Smeland S, Whelan JS, et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alpha-2b versus map alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 Good Response Randomized Controlled Trial. J Clin Oncol. 2015;33:2279–2287. doi: 10.1200/jco.2014.60.0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piperno-Neumann S, Le Deley MC, Rédini F, et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1070–1080. doi: 10.1016/s1470-2045(16)30096-1 [DOI] [PubMed] [Google Scholar]
- 41.Rathore R, Van Tine BA. Pathogenesis and current treatment of osteosarcoma: perspectives for future therapies. J Clin Med. 2021;10:1182. doi: 10.3390/jcm10061182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimatani A, Aono M, Hoshi M, et al. Secondary osteosarcoma in patients previously treated for childhood cancer: three case reports. Mol Clin Oncol. 2019;10:153–158. doi: 10.3892/mco.2018.1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poplack DG, Fordis M, Landier W, Bhatia S, Hudson MM, Horowitz ME. Childhood cancer survivor care: development of the Passport for Care. Nat Rev Clin Oncol. 2014;11:740–750. doi: 10.1038/nrclinonc.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoyanov GS, Kitanova M, Dzhenkov DL, Ghenev P, Sapundzhiev N. Demographics of head and neck cancer patients: a single institution experience. Cureus. 2017;9:e1418. doi: 10.7759/cureus.1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


