Abstract
Pulmonary embolism (PE) is a common cardiovascular disease diagnosis in emergency departments that can be associated with significant morbidity and mortality. One of the first steps after diagnosing PE is to risk stratify for adverse outcomes using risk scores such as PE Severity Index and European Society of Cardiology risk scheme. While intermediate- and high-risk PE patients should be admitted to the hospital, there is increasing evidence to support early discharge and home-based anticoagulation therapy for low-risk patients. The Hestia criteria encompass many of the clinicians’ considerations for who may be suitable for early discharge, considering both medical and social factors. Additionally, professional guidelines have provided algorithms on determining which low-risk patients may be suitable. Despite this, low-risk acute PE patients are still often admitted for inpatient treatment. In this review, we present a case-based approach on how to risk stratify and evaluate patients who may be good candidates for early discharge and home therapy.
Keywords: anticoagulation therapy, emergency medicine, pulmonary embolism, risk assessment, venous thromboembolism
1 ∣. INTRODUCTION
Pulmonary embolism (PE) is the third most common cardiovascular disease after coronary artery disease and stroke. With an incidence between 300 000 and 600 000 in the United States and 39 to 115 per 100 000 individuals in Europe, PE is a global disease accounting for hundreds of thousands of deaths each year [1]. While strongly associated with morbidity and mortality, PE is not always life-threatening. In fact, the majority of PE patients who are hemodynamically stable at presentation have low risk for adverse outcomes [2]. Risk prediction models, such as PE Severity Index (PESI), simplified PESI (sPESI), and Hestia criteria help clinicians stratify patients into appropriate risk groups [3-5]. One important application of these risk scores is to identify patients in whom outpatient management may be safe. This approach is supported by robust clinical evidence and guideline recommendations from multiple professional organizations and clinical societies [6-9].
Indeed, there is a preponderance of evidence from multiple clinical trials to support safe outpatient management of acute PE. A recent Cochrane review of 2 trials found no differences between outpatient management and traditional hospitalization with regard to all-cause mortality at 7 to 10 days or 30 to 90 days as well as no difference in major or minor bleeding [1]. Another systematic review included 12 studies (4 randomized and 8 nonrandomized trials) and showed low rates of all 90-day major adverse outcomes in low-risk PE patients managed at home, including <1% all-cause mortality, PE-related mortality, recurrent venous thromboembolism (VTE), and major bleeding [10].
Despite these robust safety data, widespread acceptance of risk assessment tools, and the weight of multiple guideline recommendations, adoption of outpatient management has been modest [1,11]. A number of recent studies indicate that the vast majority of PE patients are still being hospitalized, even when they meet low-risk criteria, and prior efforts to promote outpatient management have produced less change than anticipated [11-14]. The resulting hospitalizations are estimated to contribute more than $500 million to annual healthcare costs in the United States alone, while exposing patients to risks of hospital adverse events and conflicting with their preference to be treated at home [15,16]. This is contrast to many other countries where outpatient management of acute PE is quite common [17-19].
Lack of adoption has prompted investigation of the barriers to outpatient management of low-risk PE [20]. One frequently cited concern on the part of providers is the cost of direct oral anticoagulants (DOACs) and uncertainty regarding patients’ insurance coverage for these potentially expensive prescriptions. The medicolegal climate, particularly in the United States, may also contribute by raising fears of clinical decompensation or adverse outcomes after discharge. Social factors also play a part, with both guidelines and pragmatic trials emphasizing the need for medication adherence [14], access to specialty or primary care follow-up, and insurance coverage to defray the cost of oral anticoagulation. Finally, local and institutional culture may play a significant role in determining the confidence of providers in using outpatient management pathways. Institutions with established protocols and clinical decision support demonstrate higher rates of outpatient management of low-risk PE [21,22]. In this case-based review, we review 3 clinical scenarios of patients with acute PE and discuss how various factors influence management decisions pertaining to outpatient vs hospital-based treatment.
2 ∣. CASE 1
A 58-year-old man presents to the emergency department with acute onset shortness of breath and mild chest discomfort. His symptoms started 3 hours before. Three weeks ago, he injured his foot and ankle while skiing and has been in a walking boot since that time. He has no history of cancer or cardiopulmonary disease. He denies any loss of consciousness or syncope. His vital signs in the emergency department show a temperature of 37.8 °C, heart rate of 88 beats/min, blood pressure of 136/84 mm Hg, respiratory rate of 18, and oxygen saturation of 94% on room air. Due to an elevated D-dimer level, a PE protocol computed tomography (CT) scan is ordered, showing multiple segmental acute PEs in his right upper and middle lobes without evidence of right ventricular strain. His laboratory tests reveal a normal high-sensitivity troponin T and normal brain natriuretic peptide level. The emergency room providers consider what anticoagulant to initiate and whether the patient may be candidate for outpatient management.
2.1 ∣. Approach to PE risk stratification
Once an acute PE is confirmed, the next step typically involves risk stratification for PE-related complications. This can be done using a variety of different systems, most commonly the PESI, sPESI, the Bova score, and/or the European Society of Cardiology (ESC) risk scheme (Tables 1-3). Based on the PESI score, he would be considered low-risk (68 points for age and male sex, class II), and by sPESI as well, he would be considered low-risk (no risk factors). When using the ESC risk scheme, he would again be considered low-risk given that his PESI and sPESI scores both indicate low risk, he has no evidence of right ventricular strain on imaging, and his biomarker tests are normal. Even according to the Bova score, he would be considered low-risk (Stage I) as he has no risk elements present.
TABLE 1.
Pulmonary Embolism Severity Index score.
| Predictor variable | Points | Class | PESI score | Risk of 30-d mortality |
|---|---|---|---|---|
| Age | Points equal to age | I | 0-65 | 0-1.6% |
| Male sex | +10 | II | 76-85 | 1.7-3.5% |
| History of cancer | +30 | III | 86-105 | 3.2-7.1% |
| History of heart failure | +10 | IV | 106-125 | 4.0-11.4% |
| History of chronic lung disease | +10 | V | >125 | 10.0-24.5% |
| Heart rate ≥110 beats/min | +20 | |||
| Systolic BP <100 mm Hg | +30 | |||
| Respiratory rate ≥30 | +20 | |||
| Temperature <36 °C | +20 | |||
| Altered mental status (disorientation, lethargy, stupor, and coma) | +60 | |||
| O2 saturation <90% | +20 |
BP, blood pressure.
TABLE 3.
Bova score.
| Predictor variable | Points | Risk stage | Points | PE-related complications | PE-related mortality |
|---|---|---|---|---|---|
| Systolic blood pressure <100 mm Hg | 2 | Stage I, low risk | 0-2 | 4.2% | 1.7% |
| Elevated cardiac troponin | 2 | Stage II, intermediate risk | 3-4 | 10.8% | 5.0% |
| RV dysfunction | 2 | Stage III, high risk | >4 | 29.2% | 15.5% |
| Heart rate ≥100 beats/min | 1 |
PE, pulmonary embolism; RV, right ventricle.
Another approach to determine suitability for outpatient management of low-risk acute PE is for clinicians to review the Hestia criteria (Table 4). In addition to assessing for hemodynamic instability and other objective measures of risk, this set of criteria also includes an assessment for other reasons that hospitalization may be warranted (eg, need for ongoing supplemental oxygen or pain medications), clinical situations in which closer monitoring may be beneficial (eg, pregnancy), or situations where the use of DOAC medications may be limited (eg, advanced kidney or liver disease). It also includes more subjective assessments of appropriateness for outpatient management (eg, medical or social reasons for admission).
TABLE 4.
Hestia criteria.
| Hestia criteria |
|---|
| Is the patient hemodynamically stable? |
| Is thrombolysis or embolectomy necessary? |
| Does the patient have active bleeding or high risk for bleeding? |
| Does the patient require >24 h on supplemental oxygen to maintain SaO2 of >90%? |
| Is the pulmonary embolism diagnosed while on anticoagulation? |
| Does the patient have severe pain needing intravenous pain medication for >24 h? |
| Are there other medical or social reasons for treatment in the hospital for >24 h? |
| Does the patient have a creatinine clearance of <30 mL/min? |
| Does the patient have severe liver impairment? |
| Is the patient pregnant? |
| Does the patient have documented history of heparin-induced thrombocytopenia? |
If any criterion is positive, patient is not eligible for outpatient treatment. SaO2, arterial saturation of oxygen.
2.2 ∣. Evidence and guideline recommendations for outpatient management
The use of outpatient management for low-risk PE is an evidence-based, guideline-recommended practice. A recent systematic review of 2 randomized clinical trials comparing outpatient with inpatient management of acute PE demonstrated no difference in all-cause mortality, recurrent PE, and major bleeding between the 2 management strategies [23]. That same meta-analysis also pooled estimates from 3 prospective cohort studies with similar findings of safety and efficacy. Guidelines from the American Society of Hematology, ESC, and American College of Emergency Physicians support the use of outpatient management for eligible patients with low-risk acute PE [6,7,24].
The HOME-PE randomized trial compared the use of the sPESI and Hestia criteria across 26 hospitals in Europe [25]. Eligibility for outpatient management of acute PE was identified in 39.4% of patients using the Hestia criteria and 48.4% of patients using the sPESI approach. Outpatient management was utilized in 38.4% and 36.6% of patients, respectively (P = .41) with very low rates of 30-d severe adverse events (1.3% and 1.1%, respectively).
One recent study from 33 emergency departments in the United States included patients with acute PE and acute deep vein thrombosis who were considered to be at low risk of death by either the sPESI or Hestia criteria [14]. Sites were provided with clinical protocols incorporating risk stratification, provided with education about the use of these protocols, facilitated access to DOAC medications, and encouraged engagement with specialists (eg, pulmonary, hematology, and family medicine) to assist with outpatient follow-up. This multicomponent implementation effort led to 10% of all low-risk patients with acute PE being managed as outpatients, with very low rates of recurrent VTE requiring hospitalization (1.0%; 95% CI, 0.5%-1.7%) and bleeding requiring hospitalization (0.8%; 95% CI, 0.4%-1.5%). Patient-reported medication nonadherence was associated with an increased risk of VTE recurrence (risk ratio, 6.0; 95% CI, 2.3-15.2), highlighting the importance of DOAC access and support to ensure adherence.
2.3 ∣. Strategies to improve adoption of outpatient management
Several important barriers limit broad adoption of outpatient management for low-risk PE. However, these barriers can be directly addressed with specific strategies (Figure 1). For instance, despite the availability of these various risk scores/tools, clinicians frequently rely on their pre-existing heuristics, often developed during their medical training, when determining if hospital admission or outpatient management is appropriate. To remind clinicians to consider outpatient management when medically appropriate, clinical decision tools can be integrated into the electronic health record system. In 2 separate studies, this had significant impact on emergency medicine provider behavior, increasing the use of outpatient management for patients with low-risk PE in both cases [21,22].
FIGURE 1.
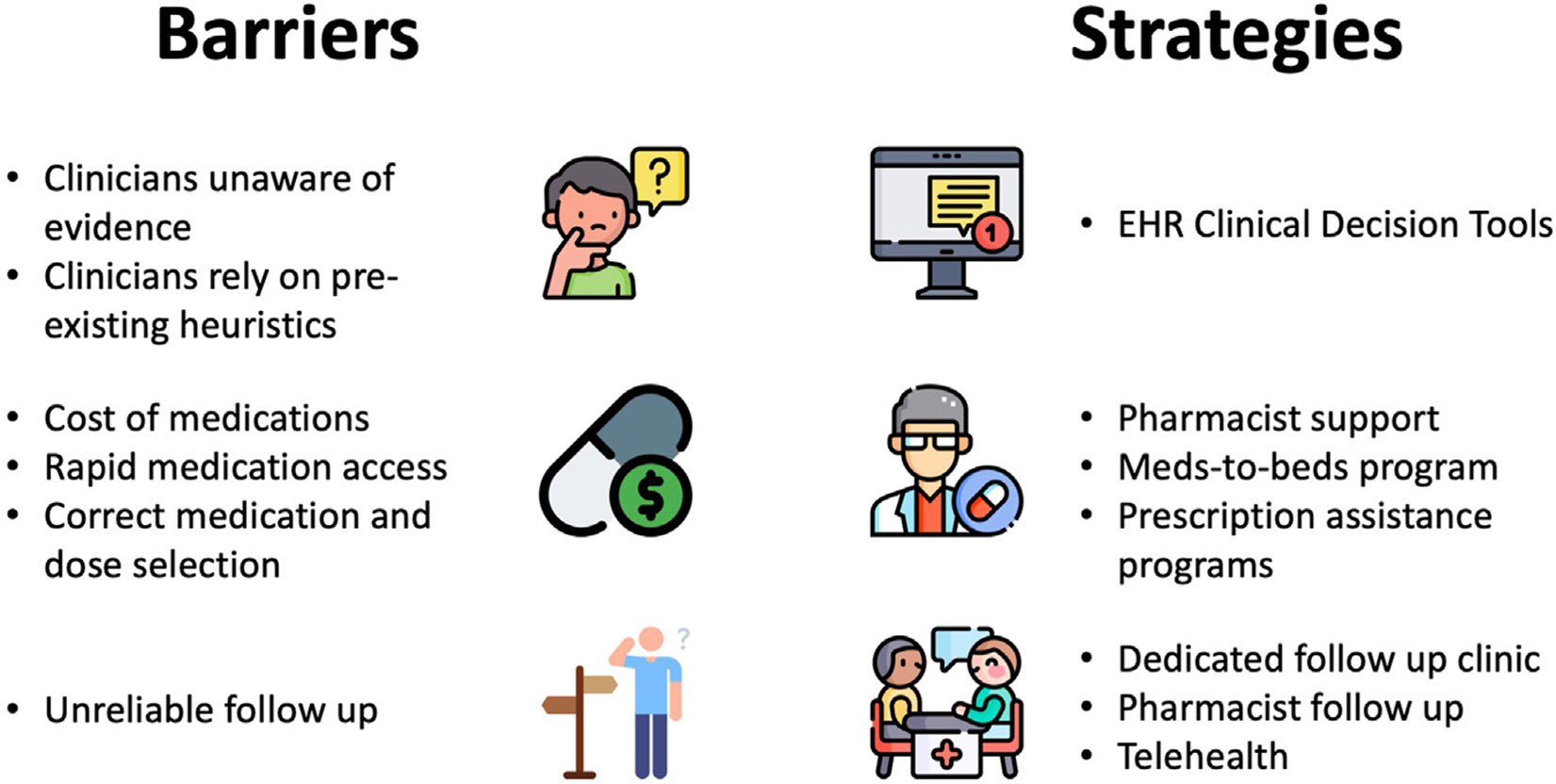
Common barriers and potential strategies for outpatient management of low-risk pulmonary embolism. EHR, electronic health record.
In addition to reminding clinicians about eligibility for outpatient management of low-risk PE, a few other considerations are important before a patient can be safely discharged from the emergency department. These include appropriate selection of anticoagulant medications, reliable access to anticoagulant therapy, and rapid follow-up with a clinician. Almost all patients with acute PE can be safely treated using an oral-only strategy with either apixaban or rivaroxaban [26]. However, there are rare patients with severe renal or liver impairment as well as those with triple-positive anti-phospholipid antibody syndrome (APS) may be better managed with an alternative anticoagulant (eg, vitamin K antagonist). Routine laboratory testing can easily identify those with renal or liver dysfunction, but testing for APS is not typically feasible in the emergency department and is generally discouraged in the acute setting due to a high false-positive rate. Therefore, it is important for clinicians to be aware that the likelihood of APS is exceedingly low in patients with a first thrombotic episode and that the overwhelming majority of low-risk PE patients can be safely treated with DOAC therapy. If clinicians have a very high index of suspicion for APS (eg, patients presenting at a young age with an unprovoked VTE or arterial thrombosis, a history of recurrent pregnancy losses, and/or known systemic lupus erythematosus [27]), they may elect to treat with the combination of heparin (or low-molecular-weight heparin) and a vitamin K antagonist [28].
Ensuring reliable follow-up is critical both to reassuring the emergency medicine provider of care continuity and for assessment of any treatment-related adverse events. This can be accomplished through primary care follow-up or with a dedicated PE clinic staffed by specialist providers. Alternatively, a pharmacist-staffed rapid follow-up clinic can be used to ensure appropriate medication selection, adherence, and assessment for any drug-related side effects or adverse events [29]. Lastly, telehealth is a powerful way to increase rapid follow-up access for patients who may not live geographically close to healthcare facilities.
3 ∣. CASE 2
A 38-year-old woman presents to the emergency department with acute onset chest pain and dyspnea for the past 4 hours. She recently started taking an oral contraceptive pill approximately 4 weeks ago to help with heavy and painful menses. She has a history of obesity (body mass index of 32 kg/m2) and 1 prior pregnancy 4 years ago. Her vital signs in the emergency department are stable (heart rate, 72 beats/min; blood pressure, 118/68 mm Hg; respiratory rate, 23 respirations/min; oxygen saturation, 95% on room air), and her electrocardiogram shows no signs of right ventricular strain. Laboratory tests show a normal high-sensitivity troponin T but an elevated D-dimer level. Renal and liver function test results along with her blood counts are all normal. PE protocol CT scan shows bilateral acute pulmonary emboli in the main and lobar branches associated with a right lower lobe pulmonary infarct. The patient has only mild pain that is not requiring any pain medications currently.
3.1 ∣. Clinician perception of risk and clinical evidence
While this patient has clear provoking risk factors for PE in her use of an estrogen-containing oral contraceptive pill and obesity, her objective risk for PE-repeated complications is quite low according to each of the various risk scores/schemes. As such, she would be a good candidate for outpatient management of her acute PE as long as she has reliable access to anticoagulant therapy and follow-up. However, many clinicians may opt to admit her given some “concerning” findings on her CT scan, including the presence of bilateral PEs and a pulmonary infarct.
We recently explored the outcomes among 817 patients presenting to the emergency department with an acute PE, of whom 331 (40.5%) were low-risk according to the PESI score (class I or II) [13]. All patients were assessed for 1 or more concerning findings on CT, including (1) bilateral embolism (saddle or main pulmonary artery), (2) right ventricle (RV)–to–left ventricle (LV) size ratio of >1.0, (3) RV enlargement, (4) septal abnormality suggested of RV pressure over-load, or (5) pulmonary infarct (Figures 2 and 3). Among the 331 patients with a low-risk PESI score, 151 (45.6%) had one or more concerning findings on imaging. However, these low-risk patients had no difference in 7-day mortality (none in either group), need for intensive care unit admission (1.1% and 1.3%, respectively), and hospital length of stay (median, <2 days for both groups) irrespective of the presence of concerning imaging findings. For each of these measures, while the outcomes were similar between the low-risk groups with and without concerning imaging findings, they were notably better than for patients at high risk by PESI (classes III-V).
FIGURE 2.
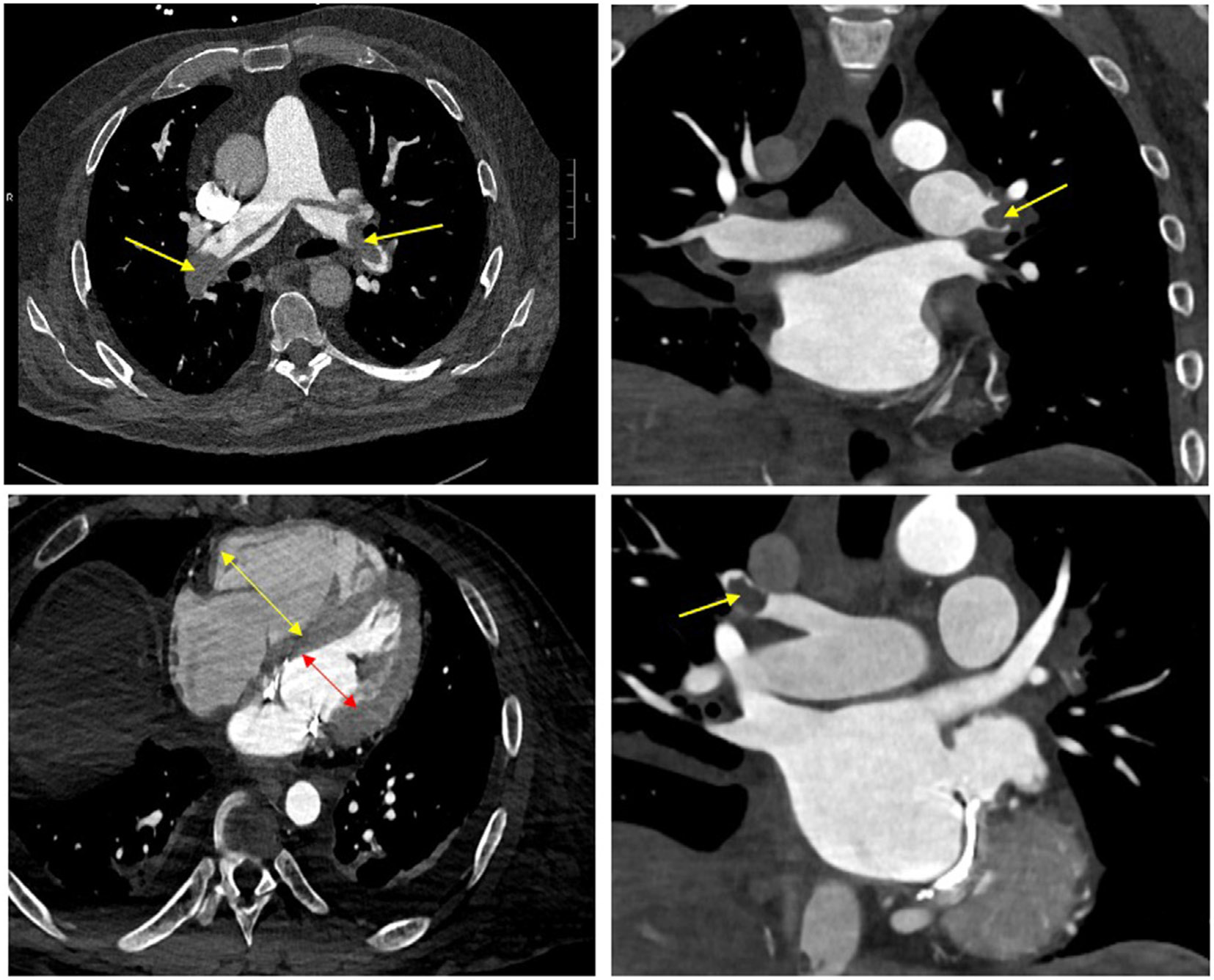
Computed tomography imaging for various cases of acute pulmonary embolism (PE). Top left: computed tomography demonstrating acute saddle PE with bilateral main pulmonary artery involvement; top right: acute PE in left main pulmonary artery with extension into lobar branches; bottom left: increased right ventricle (yellow arrow)–to–left ventricle (red arrow) ratio of >1.0; bottom right: acute PE in right lobar branch.
FIGURE 3.
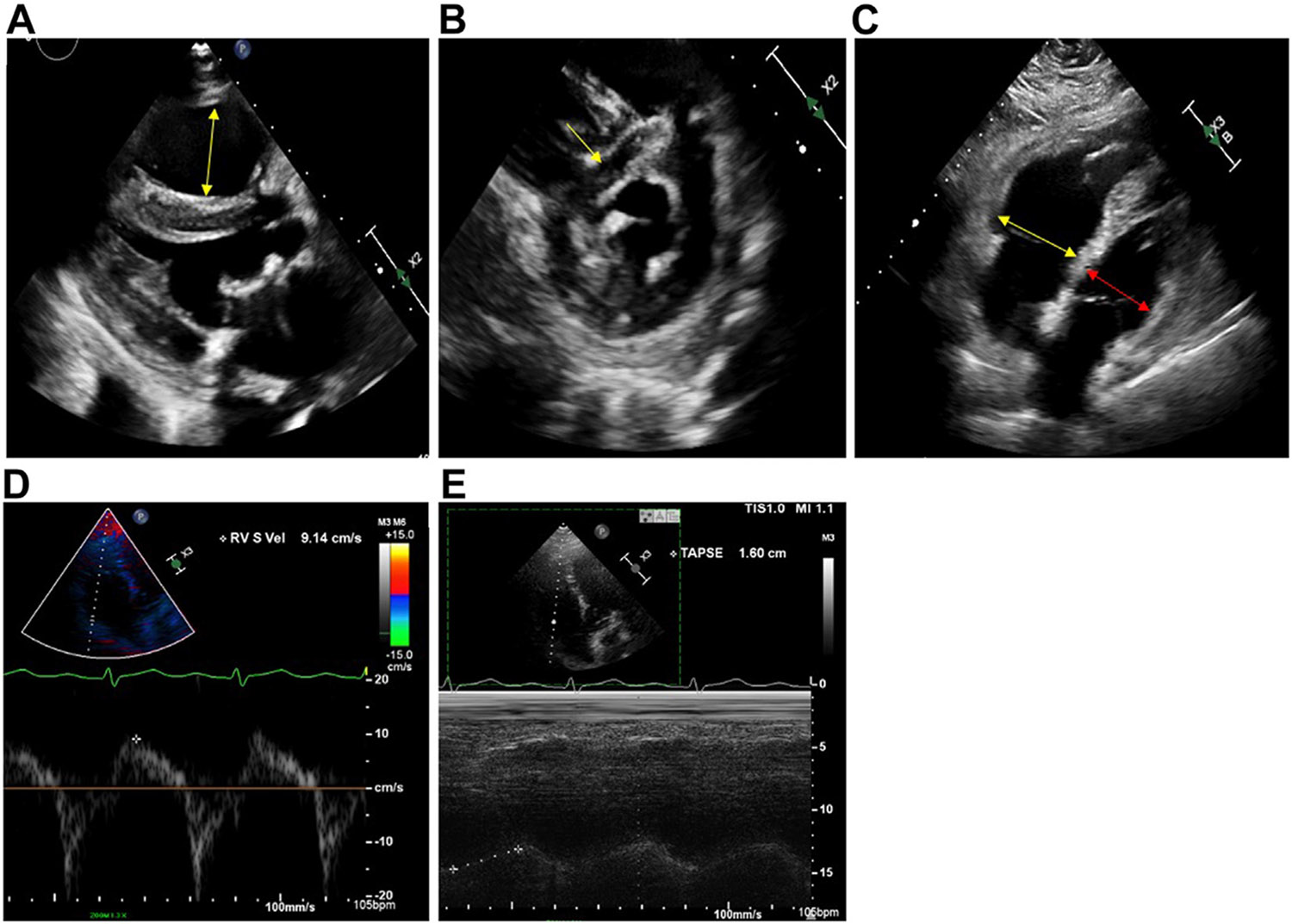
Echocardiographic findings of right ventricle (RV) dysfunction. (A) Enlarged RV, parasternal long axis; (B) flattened interventricular septum, parasternal short axis; (C) dilated RV/left ventricle ratio of >1.0 from apical 4-chamber view; (D) decreased RV S’ tissue Doppler velocity <9.5 cm/s; (E) decreased tricuspid annular plane systolic excursion (TAPSE) measured with M-Mode of <16 mm.
While these findings require confirmation in other populations and settings, they provide some evidence to support the use of outpatient management for patients based more strongly on the risk of complications (as estimated using risk scores) rather than only by imaging findings. Of note, many of these concerning imaging findings should not be considered present or absent but rather considered across a spectrum of severity. For example, an RV/LV ratio of 1.1 and 1.5 are both >1.0, but patients with an RV/LV of 1.5 are at higher risk of complications than patients with an RV/LV of 1.1 [30,31]. Furthermore, other rare situations, including thrombotic occlusion of the inferior vena cava and clot in transit, may portend higher risk of complications.
4 ∣. CASE 3
A 54-year-old woman presents to the emergency department with acute onset shortness of breath over the last 6 hours. She reports having had a viral illness 2 weeks ago but was otherwise in her usual state of health. She has a history of breast cancer that was treated with lumpectomy and adjuvant chemotherapy and radiation therapy with no evidence of active disease. Her vital signs on presentation show a heart rate of 116 beats/min, blood pressure of 145/79 mm Hg, respiratory rate of 24 respirations/min, and oxygen saturation of 92% on room air. Laboratory testing reveals a mildly positive high-sensitivity troponin T of 21 pg/mL (normal <19 pg/mL for acute coronary syndrome). Electrocardiogram shows sinus tachycardia without any ST-T wave abnormalities. A PE protocol CT is performed, which shows bilateral acute pulmonary emboli in the left main pulmonary artery with lobar involvement and RV/LV ratio increased at 1.2. Subsequently, a bedside cardiac ultrasound is performed, which shows LV septal flattening in systole. Formal echocardiogram demonstrates dilated RV with low tricuspid annular plane systolic excursion and S’ tissue Doppler velocities suggestive of RV dysfunction.
4.1 ∣. RISK STRATIFICATION WITH EMERGING RISK FACTORS
Risk stratification using PESI scoring places our patient in the low-risk category (class II—84 points for age, history of cancer, and heart rate of ≥110 beats/min). While PESI does include 11 clinical factors to predict 30-day mortality, it does not capture features such as RV strain or positive cardiac biomarkers. The Bova score was derived to evaluate and risk stratify normotensive patients and included just 4 clinical features—systolic blood pressure, elevated cardiac troponin, RV dysfunction based on certain imaging criteria, and heart rate [32]. Calculating the Bova score for our patient places her at intermediate risk (Bova stage II—3 points for RV dysfunction and elevated troponin level), which predicts 6.8% 30-day PE-related mortality, significantly higher than her PESI risk estimation of 1.7% to 3.5% 30-day mortality.
Elevated cardiac troponins are seen in up to 60% of all acute PE patients and have been demonstrated to have increased risk of all-cause mortality even in hemodynamically stable patients [33,34]. While not particularly sensitive if used in isolation, combining troponin with the clinical scenario and diagnosis of acute PE does improve its diagnostic utility. Similarly, echocardiographic findings may be useful for guidance of risk assessment and triaging for hemodynamically stable patients (Figure 3). Features such as hypokinesis of RV free wall compared with RV apex (McConnell’s sign) may suggest a higher risk of PE-related complications. Other features such as RV pressure overload, noted a systolic septal flattening (D-sign), and RV systolic dysfunction noted as reduced tricuspid annular plane systolic excursion may suggest RV systolic dysfunction. Increased RV dilation suggesting volume/pressure overload can present as RV/LV diameter ≥1.0, evaluated by both CT and echocardiogram. Discovery of intra-cardiac thrombus in transit may also be observed, though this is rare.
4.2 ∣. Guideline recommendations
ESC guidelines recommend assessment of RV by imaging methods or laboratory biomarkers even in the presence of low PESI or negative sPESI, as these factors may assist in the prognostication of patients [32]. In this group of hemodynamically stable patients, further risk stratification to distinguish between low- and intermediate-risk PE can help clinicians decide between early home discharge and hospitalization. ESC guidelines consider any patient with hemodynamically stable acute PE with elevated troponin level or RV dysfunction in the intermediate-risk group and recommend close monitoring for hemodynamic worsening of the RV. These patients are recommended hospitalization, as meta-analysis has shown increased mortality (OR, 4.19; 95% CI, 1.39-12.58) for all-cause death if RV dysfunction was noted [35]. However, the absolute rate of early all-cause death ranges 1.8% to 3.8%, which is lower than is typically seen in patients with intermediate-risk PE. Presence of both RV dysfunction and positive troponin confers increased risk of early hemodynamic instability [36].
Given the presence of RV dysfunction and elevated troponin level in our patient, she is at modestly increased risk of PE-related complications. Therefore, it is reasonable to admit her for observation based on ESC guidelines to observe for hemodynamic worsening despite her PESI score being low-risk. At the same time, if she has a supportive home environment, reliable access to anticoagulation, and rapid follow-up, it would be reasonable to also consider home discharge in this case as well. In hemodynamically stable patients, it is reasonable to consider checking troponin levels and imaging for the RV. The Bova score takes into account these parameters and can be used in further risk stratification for hemodynamically stable patients.
Overall, most patients with low-risk PESI, sPESI, and/or Hestia scores are likely good candidates for outpatient management (Figure 4). However, in select cases, hospitalization to monitor for any clinical worsening is reasonable. Primarily, these include patients with significant RV enlargement or dysfunction seen on imaging and biomarkers.
FIGURE 4.

Algorithm for determining early discharge and home therapy. CTPA, computed tomography pulmonary angiogram; PE, pulmonary embolism; PESI, Pulmonary Embolism Severity Index; RV, right ventricular; sPESI, Simplified Pulmonary Embolism Severity Index.
5 ∣. CONCLUSION
There is an increasing body of evidence supporting outpatient management of low-risk PE. Despite development of multiple validated risk assessment tools and clinical guidelines with clear and concise algorithms, there has been no significant change in rates of early discharge with home-based management of these low-risk PE patients. Developing care pathways and increasing awareness of clinical guideline recommendations may help overcome some of the existing barriers to outpatient management of these patients.
TABLE 2.
Simplified Pulmonary Embolism Severity Index score.
| Predictor variable | Points |
|---|---|
| Age >80 y | 1 |
| History of cancer | 1 |
| History of cardiopulmonary disease | 1 |
| Heart rate ≥110 beats/min | 1 |
| Systolic blood pressure <90 mm Hg | 1 |
| O2 saturation <90% | 1 |
If score is 0, it is classified as low-risk, with 1.1% 30-day mortality. If score is ≥1, it is classified as high-risk, with 8.9% 30-day mortality.
Funding information
National Heart, Lung, and Blood Institute; Grant Number: R01HL163438 (to J.A.K., C.F.G., G.D.B.)
Footnotes
DECLARATION OF COMPETING INTERESTS
G.D.B. – Grant Funding - Boston Scientific; Consulting - Pfizer, Bristol-Myers Squibb, Janssen, Bayer, AstraZeneca, Sanofi, Anthos, Abbott Vascular, Boston Scientific; Board of Directors - Anticoagulation Forum.
REFERENCES
- [1].Yoo HH, Nunes-Nogueira VS, Fortes Villas Boas PJ, Broderick C. Outpatient versus inpatient treatment for acute pulmonary embolism. Cochrane Database Syst Rev. 2022;5:CD010019. 10.1002/14651858.CD010019.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jiménez D, Bikdeli B, Barrios D, Quezada A, Del Toro J, Vidal G, Mahé I, Quere I, Loring M, Yusen RD, Monreal M. RIETE investigators. Epidemiology, patterns of care and mortality for patients with hemodynamically unstable acute symptomatic pulmonary embolism. Int J Cardiol. 2018;269:327–33. [DOI] [PubMed] [Google Scholar]
- [3].Donzé J, Le Gal G, Fine MJ, Roy PM, Sanchez O, Verschuren F, Cornuz J, Meyer G, Perrier A, Righini M, Aujesky D. Prospective validation of the Pulmonary Embolism Severity Index. A clinical prognostic model for pulmonary embolism. Thromb Haemost. 2008;100:943–8. [DOI] [PubMed] [Google Scholar]
- [4].Jiménez D, Aujesky D, Moores L, Gómez V, Lobo JL, Uresandi F, Otero R, Monreal M, Muriel A, Yusen RD, Investigators RIETE. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170:1383–9. [DOI] [PubMed] [Google Scholar]
- [5].Zondag W, Vingerhoets LMA, Durian MF, Dolsma A, Faber LM, Hiddinga BI, Hofstee HM, Hoogerbrugge AD, Hovens MM, Labots G, Vlasveld T, de Vreede MJ, Kroft LJ, Huisman MV, Hestia Study Investigators. Hestia criteria can safely select patients with pulmonary embolism for outpatient treatment irrespective of right ventricular function. J Thromb Haemost. 2013;11:686–92. [DOI] [PubMed] [Google Scholar]
- [6].Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, et al. , ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. [DOI] [PubMed] [Google Scholar]
- [7].Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, Hutten BA, Jaff MR, Manja V, Schulman S, Thurston C, Vedantham S, Verhamme P, Witt DM, Florez ID, Izcovich A, Nieuwlaat R, Ross S, Schünemann HJ, Wiercioch W, et al. Blood Adv. 2020;4:4693–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, Huisman MV, Kearon C, King CS, Knighton AJ, Lake E, Murin S, Vintch JRE, Wells PS, Moores LK. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:e545–608. [DOI] [PubMed] [Google Scholar]
- [9].Howard LSGE, Barden S, Condliffe R, Connolly V, Davies CWH, Donaldson J, Everett B, Free C, Horner D, Hunter L, Kaler J, Nelson-Piercy C, O’Dowd E, Patel R, Preston W, Sheares K, Tait C. British Thoracic Society Guideline for the initial outpatient management of pulmonary embolism (PE). Thorax. 2018;73(Suppl 2):ii1–29. [DOI] [PubMed] [Google Scholar]
- [10].Maughan BC, Frueh L, McDonagh MS, Casciere B, Kline JA. Outpatient treatment of low-risk pulmonary embolism in the era of direct oral anticoagulants: a systematic review. Acad Emerg Med. 2021;28:226–39. [DOI] [PubMed] [Google Scholar]
- [11].Watson NW, Carroll BJ, Krawisz A, Schmaier A, Secemsky EA. Trends in discharge rates for acute pulmonary embolism in U.S. emergency departments. Ann Intern Med. 2024;177:134–43. [DOI] [PubMed] [Google Scholar]
- [12].Westafer LM, Shieh MS, Pekow PS, Stefan MS, Lindenauer PK. Outpatient management of patients following diagnosis of acute pulmonary embolism. Acad Emerg Med. 2021;28:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].O’Hare C, Grace KA, Schaeffer WJ, Hyder SN, Stover M, Liles AL, Khaja MS, Cranford JA, Kocher KE, Barnes GD, Greineder CF. Adverse clinical outcomes among patients with acute low-risk pulmonary embolism and concerning computed tomography imaging findings. JAMA Netw Open. 2023;6:e2311455. 10.1001/jamanetworkopen.2023.11455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kline JA, Adler DH, Alanis N, Bledsoe JR, Courtney DM, d’Etienne JP, Diercks DB, Garrett JS, Jones AE, Mackenzie DC, Madsen T, Matuskowitz AJ, Mumma BE, Nordenholz KE, Pagenhardt J, Runyon MS, Stubblefield WB, Willoughby CB. Monotherapy anti-coagulation to expedite home treatment of patients diagnosed with venous thromboembolism in the emergency department: a pragmatic effectiveness trial. Circ Cardiovasc Qual Outcomes. 2021;14:e007600. 10.1161/CIRCOUTCOMES.120.007600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fanikos J, Rao A, Seger AC, Carter D, Piazza G, Goldhaber SZ. Hospital costs of acute pulmonary embolism. Am J Med. 2013;126:127–32. [DOI] [PubMed] [Google Scholar]
- [16].LaMori JC, Shoheiber O, Mody SH, Bookhart BK. Inpatient resource use and cost burden of deep vein thrombosis and pulmonary embolism in the United States. Clin Ther. 2015;37:62–70. [DOI] [PubMed] [Google Scholar]
- [17].Pradier M, Wang TF, Siegal DM, Le Gal G, Carrier M, Delluc A. Safety of outpatient management of cancer-associated pulmonary embolism: a retrospective study. Haematologica. 2024;109:3063–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gregalio FA, Juana C, Palmili GM, Martínez BJ, Bluro IM, Vázquez FJ, Grande Ratti MF. Comparison of clinical outcomes of venous thromboembolic disease between outpatient and inpatient management. Arch Peru Cardiol Cir Cardiovasc. 2024;5:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guman NAM, Kaptein FHJ, Lohle SB, Mairuhu ATA, Klok FA, Huisman MV, Kamphuisen PW, van Es N. Discharge from the emergency department and outpatient clinic in cancer patients with acute symptomatic and incidental pulmonary embolism: a multi-center retrospective cohort study. Thromb Res. 2024;233:181–8. [DOI] [PubMed] [Google Scholar]
- [20].Westafer LM, Jessen E, Zampi M, Boccio E, Casey SD, Lindenauer PK, Vinson DR. Barriers and facilitators to the outpatient management of low-risk pulmonary embolism from the emergency department. Ann Emerg Med. 2023;82:381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vinson DR, Mark DG, Chettipally UK, Huang J, Rauchwerger AS, Reed ME, Lin JS, Kene MV, Wang DH, Sax DR, Pleshakov TS, McLachlan ID, Yamin CK, Elms AR, Iskin HR, Vemula R, Yealy DM, Ballard DW, eSPEED Investigators of the KP CREST Network. Increasing safe outpatient management of emergency department patients with pulmonary embolism: a controlled pragmatic trial. Ann Intern Med. 2018;169:855–65. [DOI] [PubMed] [Google Scholar]
- [22].Kabrhel C, Rosovsky R, Baugh C, Connors J, White B, Giordano N, Torrey J, Deadmon E, Parry BA, Hagan S, Zheng H. Multicenter implementation of a novel management protocol increases the outpatient treatment of pulmonary embolism and deep vein thrombosis. Acad Emerg Med. 2019;26:657–69. [DOI] [PubMed] [Google Scholar]
- [23].Khatib R, Ross S, Kennedy SA, Florez ID, Ortel TL, Nieuwlaat R, Neumann I, Witt DM, Schulman S, Manja V, Beyth R, Clark NP, Wiercioch W, Schünemann HJ, Zhang Y. Home vs hospital treatment of low-risk venous thromboembolism: a systematic review and meta-analysis. Blood Adv. 2020;4:500–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].American College of Emergency Physicians Clinical Policies Sub-committee (Writing Committee) on Thromboembolic Disease, Wolf SJ, Hahn SA, Nentwich LM, Raja AS, Silvers SM, Brown MD. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with suspected acute venous thromboembolic disease. Ann Emerg Med. 2018;71:e59–109. [DOI] [PubMed] [Google Scholar]
- [25].Roy PM, Penaloza A, Hugli O, Klok FA, Arnoux A, Elias A, Couturaud F, Joly LM, Lopez R, Faber LM, Daoud-Elias M, Planquette B, Bokobza J, Viglino D, Schmidt J, Juchet H, Mahe I, Mulder F, Bartiaux M, Cren R, et al. , HOME-PE Study Group. Triaging acute pulmonary embolism for home treatment by Hestia or simplified PESI criteria: the HOME-PE randomized trial. Eur Heart J. 2021;42:3146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Renner E, Barnes GD. Antithrombotic management of venous thromboembolism: JACC Focus Seminar. J Am Coll Cardiol. 2020;76:2142–54. [DOI] [PubMed] [Google Scholar]
- [27].Devreese KMJ, de Groot PG, de Laat B, Erkan D, Favaloro EJ, Mackie I, Martinuzzo M, Ortel TL, Pengo V, Rand JH, Tripodi A, Wahl D, Cohen H. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: update of the guidelines for lupus anticoagulant detection and interpretation. J Thromb Haemost. 2020;18:2828–39. [DOI] [PubMed] [Google Scholar]
- [28].Khairani CD, Bejjani A, Piazza G, Jimenez D, Monreal M, Chatterjee S, Pengo V, Woller SC, Cortes-Hernandez J, Connors JM, Kanthi Y, Krumholz HM, Middeldorp S, Falanga A, Cushman M, Goldhaber SZ, Garcia DA, Bikdeli B. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].DiRenzo BM, Beam DM, Kline JA, Deodhar KS, Weber ZA, Davis CM, Walroth TA. Implementation and preliminary clinical outcomes of a pharmacist-managed venous thromboembolism clinic for patients treated with rivaroxaban post emergency department discharge. Acad Emerg Med. 2018;25:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Côté B, Jiménez D, Planquette B, Roche A, Marey J, Pastré J, Meyer G, Sanchez O. Prognostic value of right ventricular dilatation in patients with low-risk pulmonary embolism. Eur Respir J. 2017;50:1701611. 10.1183/13993003.01611-2017 [DOI] [PubMed] [Google Scholar]
- [31].Solverson K, Humphreys C, Liang Z, Prosperi-Porta G, Andruchow JE, Boiteau P, Ferland A, Herget E, Helmersen D, Weatherald J. Rapid prediction of adverse outcomes for acute normotensive pulmonary embolism: derivation of the Calgary Acute Pulmonary Embolism score. ERJ Open Res. 2021;7:00879–2020. 10.1183/23120541.00879-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bova C, Sanchez O, Prandoni P, Lankeit M, Konstantinides S, Vanni S, Jiménez D. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J. 2014;44:694–703. [DOI] [PubMed] [Google Scholar]
- [33].Lankeit M, Friesen D, Aschoff J, Dellas C, Hasenfuss G, Katus H, Konstantinides S, Giannitsis E. Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur Heart J. 2010;31:1836–44. [DOI] [PubMed] [Google Scholar]
- [34].Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007;116:427–33. [DOI] [PubMed] [Google Scholar]
- [35].Barco S, Mahmoudpour SH, Planquette B, Sanchez O, Konstantinides SV, Meyer G. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2019;40:902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B, Couturaud F, Dellas C, Empen K, Franca A, Galiè N, Geibel A, Goldhaber SZ, Jimenez D, Kozak M, Kupatt C, Kucher N, et al. , PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402–11. [DOI] [PubMed] [Google Scholar]


