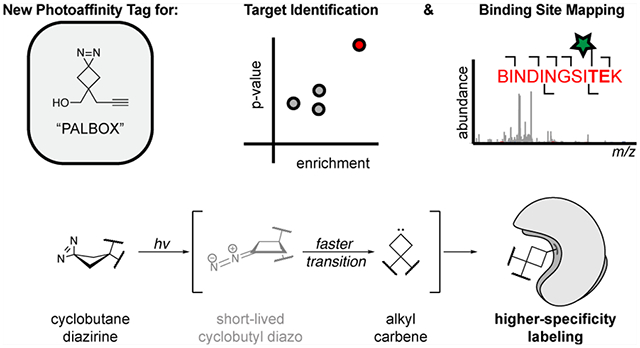
Abstract
Alkyl diazirines are frequently used in photoaffinity labeling to map small molecule–protein interactions in target identification studies. However, the alkyl diazirines can preferentially label acidic amino acids and acidic protein surfaces in a pH-dependent manner, presumably via a reactive alkyl diazo intermediate. Here, we explore the use of ring strain to alter these reactivity preferences and report the development of a cyclobutane diazirine photoaffinity tag with reduced pH-dependent reactivity, termed PALBOX. We show that PALBOX possesses differential reactivity profiles as compared to other diazirine tags in vitro and is readily incorporated into small molecules to profile their binding interactions in cells. Using a set of small molecule fragments and ligands, we show that photoaffinity probes equipped with PALBOX can label the known protein targets in cells with reduced labeling of known alkyl diazirine off-targets. Finally, we demonstrate that ligands equipped with PALBOX can accurately map small molecule–protein binding sites. Thus, PALBOX is a versatile diazirine-based photoaffinity tag for use in the development of chemical probes for photoaffinity labeling experiments, including the study of small molecule–protein interactions.
Graphical Abstract
INTRODUCTION
Photoaffinity labeling (PAL) is an effective method for studying biomolecular interactions since its original development by Singh and Westheimer in 1965.1 In a typical experiment, a molecule of interest is derivatized into a photoaffinity probe which forms a reactive intermediate on photolysis that traps noncovalent binding partners in close proximity. When PAL is paired with enrichment methods and powerful analytical technologies like mass spectrometry, the resulting chemoproteomics workflows enable a wide range of studies, including maps of small molecule–protein interactions for ligandability or target identification studies,2–8 and can further yield structural insights to the binding interface.
Although PAL provides one of the few methods to directly interrogate small molecule targets in cells, the design of the PAL probe itself can influence the protein profile resulting from the experiment. Many photoactivatable chemistries have historically been implemented in PAL, including aryl azides,9 benzophenones,10 and aryl trifluoromethyl11 or alkyl diazirines,12 and new PAL chemistries continue to be developed including 2,5-tetrazoles13,14 and α-keto amides.15 For small molecule target identification studies using PAL, these chemistries ideally react in a relatively unbiased manner such that the labeling event is proportional to the strength of the binding interaction but not the reactivity of nearby biomolecules. The development of PAL probes has been accelerated by multifunctional tags which bear a photoactivatable functional group, a bioorthogonal handle for enrichment, and a site for ready functionalization to small molecules of interest.16–18 Recent efforts have also produced smaller and more compact PAL tags for probe development, including the minimalist tag,18 aryl difluoroalkyne diazirine,19 alkyl difluoroalkyne diazirine,20 and terminal 3H diazirine tags.21
The miniaturization of PAL tags utilizes the alkyl diazirine to afford the reactive intermediate for labeling reactive partners. Diazirines can generate reactive carbenes upon photolysis, which can broadly label biological substrates. However, diazirines also isomerize to linear diazo intermediates during photolysis, and recent studies from our lab22 and others23,24 have shown that these diazo isomers can selectively label acidic amino acids on protein substrates and contribute to a large proportion of diazirine labeling in cells. The pH-dependent labeling from the diazo intermediate can bias diazirine probe labeling toward acidic protein surfaces or proteins with carboxylic acids with elevated pKas (e.g., membrane proteins), which can rationalize the labeling of some proteins observed in the alkyl diazirine “backgroundome”.25 The carbenes generated from alkyl diazirines also experience nonproductive alkene formation from 1,2 H-migration, which may be mitigated via deuteration and the kinetic isotope effect.22 To address these limitations in alkyl diazirine labeling chemistry, but maintain the advantage of the small tag size, we were inspired by the use of ring strain to increase reaction conversion during biological labeling.26 For example, the use of strained cyclo-octynes in bioorthogonal chemistry affords the reactivity necessary for copper-free click chemistry in cells.27 In line with this concept, we hypothesized that the ring strain introduced by a diazirine appended to a cyclobutane would accelerate the conversion of the diazo isomer to a carbene, thus reducing the pH-dependent labeling bias through the diazo intermediate (Figure 1a).
Figure 1.
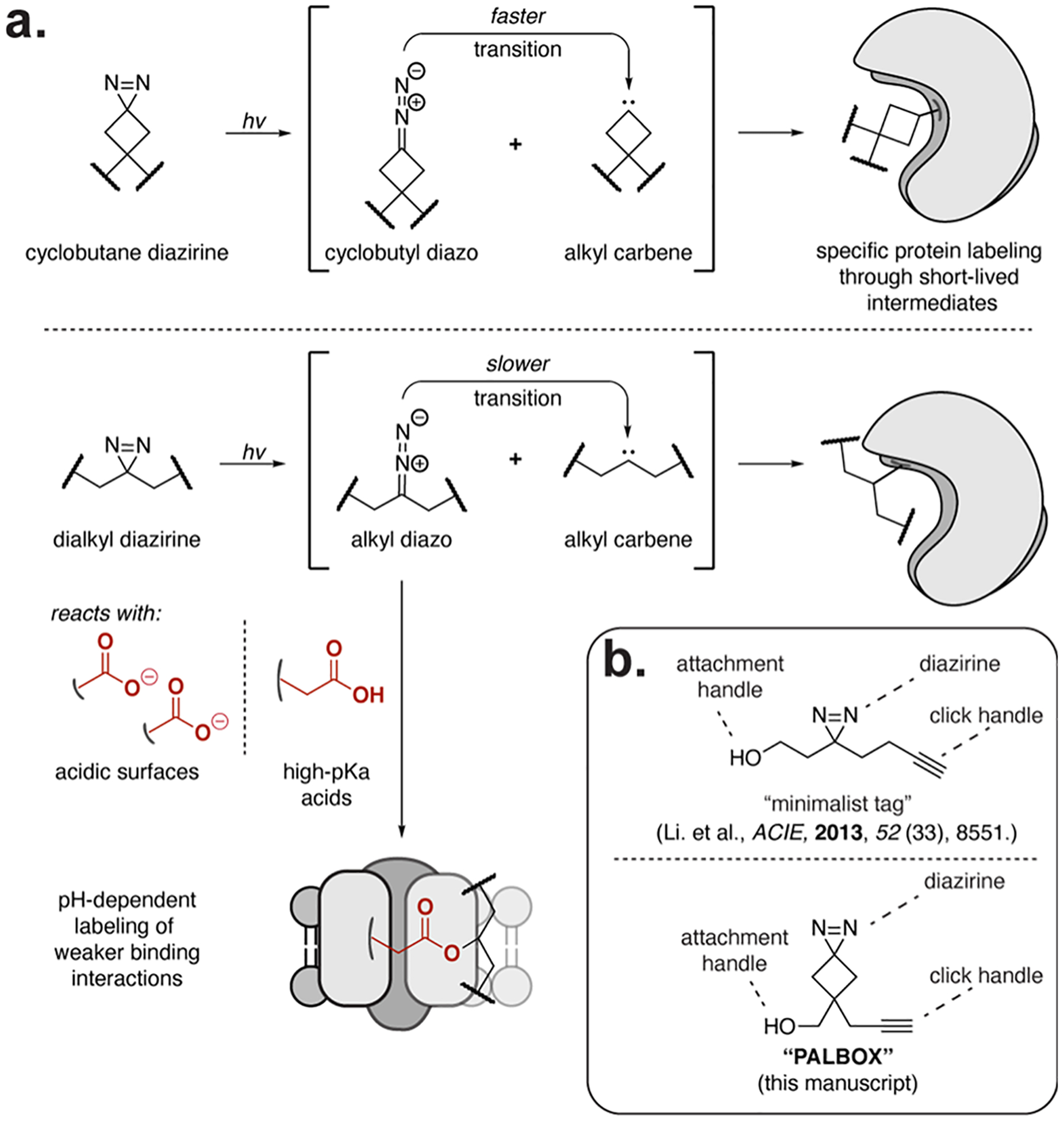
Design of PALBOX, a cyclobutane diazirine photoaffinity tag. (a) Diazirines produce diazo and carbene intermediates upon irradiation that label nearby biological substrates. The alkyl diazo isomer can transition to the carbene or independently produce pH-dependent labeling of weaker binding interactions. (b) PALBOX is modeled after the minimalist tag and contains a cyclobutane diazirine, a bioorthogonal handle, and a modular attachment site, allowing for the rapid development of probes with PALBOX.
Here, we report the development of a new PAL tag, PALBOX, which shares the compact, trifunctional features of the minimalist tag:18 an attachment handle, a diazirine, and an alkyne for bioorthogonal chemistry (Figure 1b). We show that diazirines embedded to a cyclobutane ring reduce labeling through the diazo intermediate and do not exhibit pH-dependent reactivity in vitro. The modular design of PALBOX is amenable to different coupling conditions and was readily incorporated into five PAL probes. These probes can label small molecule–protein interactions in cells for the enrichment and profiling of protein-binding partners. PALBOX probes additionally show lower inherent labeling of known diazirine off-targets. Therefore, the PALBOX tag will be especially useful in target identification experiments where the direct labeling specificity of the probe is essential or when detecting binding interactions independent of the pH environment is of importance.
RESULTS
Initial Explorations in Cyclic Diazirine Scaffolds.
We initially explored avenues to improve two different attributes of alkyl diazirines: first, we sought to restrict their isomerization to reactive diazo species, and second, to reduce the rate of nonproductive alkene formation from 1,2 H-migration (Figure 2a). We hypothesized that both of these nonproductive pathways could be mitigated by the use of strained rings to constrain the geometry of the diazo and carbene intermediates. First, the typical trigonal planar geometry of the diazo isomer would result in an unstable ring conformation, possibly reducing its stability or rate of formation. Second, upon conversion to the carbene, the strained ring would reduce the orbital overlap of the neighboring σC−H bonds with the adjacent carbene p-orbital, slowing the rate of 1,2 H-migration. To test these hypotheses, we evaluated a series of diazirine-substituted azacycles developed by Enamine.28 We first investigated the products following the photolysis of the azacycles as the HCl salt in D2O (Figure 2b, Supporting Information Figure S1). While the six- and five-membered azacycles formed alkene rearrangement products, we observed that the diazirine-substituted azetidine produced no alkenes or other rearrangement products upon photolysis to afford high yields of the hydrated adduct (95%). Unfortunately, these high yields were isolated to the HCl salt, as the conversion dropped upon deprotonation of the HCl salt or N-substitution with a Boc-protecting group, which led to the observation of rearrangement products upon photolysis (Supporting Information Figure S2).
Figure 2.
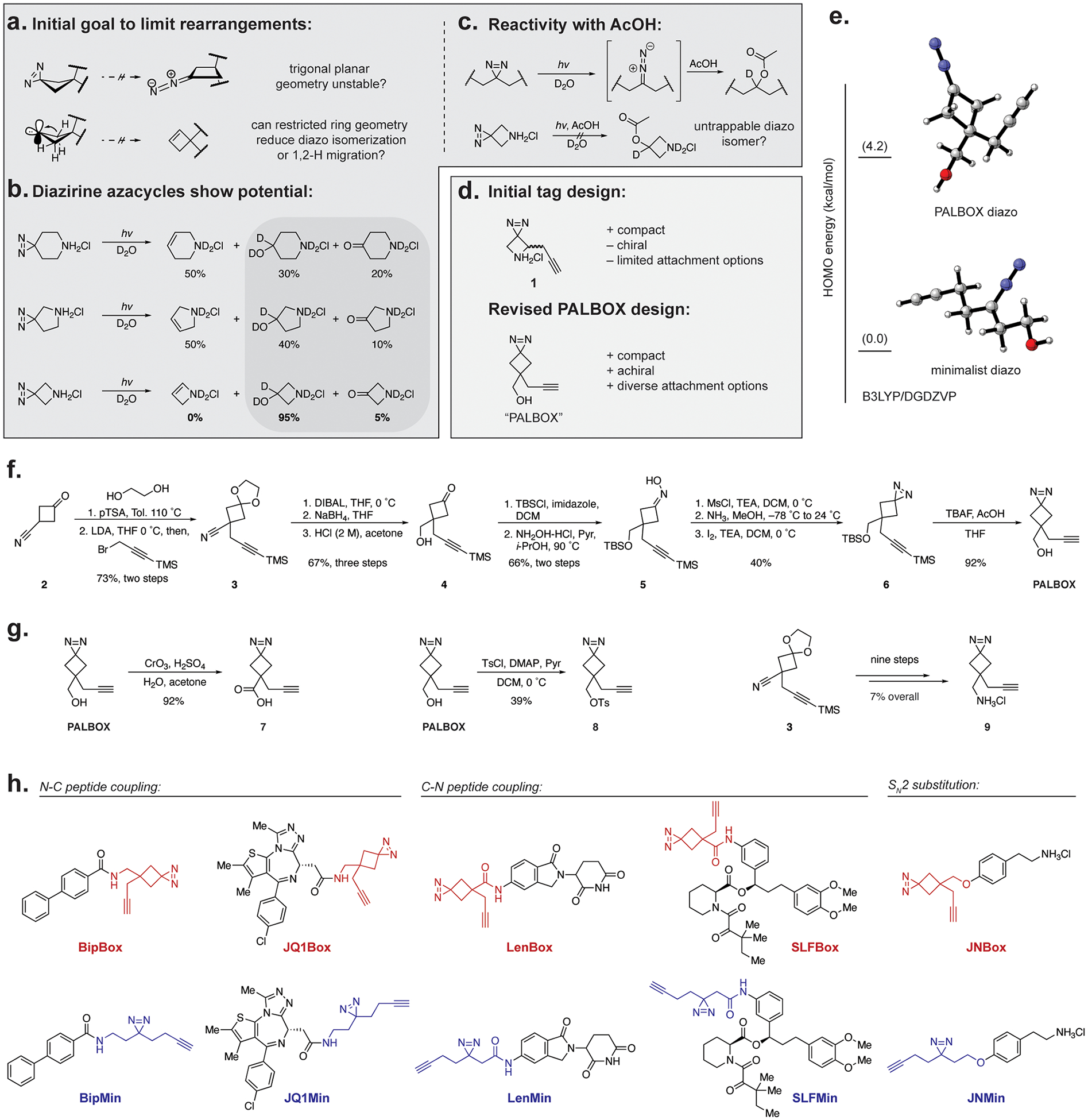
Reactivity profile of cyclic diazirine scaffolds and development of PALBOX. (a) Initial hypothesis that conformationally constrained cyclobutane-substituted diazirines would limit alternate rearrangement pathways during photolysis. (b) Product distribution following aqueous photolysis of diazirine-substituted azacycles. (c) Aqueous photoreaction of alkyl diazirines and azetidine diazirines. (d) Initial design of a PAL tag with the azetidine scaffold was redesigned with a cyclobutane core to avoid chirality and improve attachment options. (e) Optimized geometries of PALBOX and minimalist diazo intermediates with relative HOMO energies. Geometries were optimized with the B3LYP/DGDZVP basis set. (f) Synthesis of PALBOX over 11 steps in 11% overall yield. (g) Derivatization of PALBOX with several reactive functional groups. (h) Series of PALBOX and minimalist-tagged probes prepared for comparative studies.
Promisingly, attempts to trap the diazo isomer of the diazirine-substituted azetidine with AcOH (10 mM) did not result in the formation of new products (Figure 2c, Supporting Information Figure S2). This observation shows that the potential diazo isomers produced by strained diazirine-substituted azetidines are nonreactive to dilute acids, in contrast to acyclic alkyl diazirines. This suggests that diazirine-substituted four-membered rings could exhibit less pH-dependent labeling bias than acyclic diazirines. Based on this observation, we incorporated this scaffold into a new PAL tag to evaluate the labeling specificity. Our initial design, 1, was based on the diazirine-substituted azetidine motif, which was elaborated with an alkyne for bioorthogonal labeling and enrichment (Figure 2d). However, our initial design had limited options for attachment to a compound of interest and was chiral, which could complicate PAL probe development and analyses. We subsequently developed an achiral PAL tag based on a cyclobutane scaffold with more functionalization options, which we dubbed PALBOX. To examine whether the diazo isomer of PALBOX would be more reactive than the diazo isomer of the minimalist tag, we calculated the optimized geometries of the diazo intermediates from PALBOX and the dialkyl minimalist at the B3LYP/DGDZVP level of theory (Figure 2e). When comparing the HOMO energies of each diazo intermediate, we found that the PALBOX diazo intermediate was approximately 4 kcal/mol higher than the analogous minimalist diazo intermediate. The predicted difference of 4 kcal/mol between the two diazo intermediates suggests a significant difference in reactivity, which may translate to a faster conversion of the PALBOX diazo intermediate to the carbene and effectively reduce its half-life in solution.
Synthesis of PALBOX and Derivatization for Ligand Functionalization.
We envisioned that PALBOX could be accessed via propargylation of cyclobutanone nitrile 2, followed by the reduction of nitrile 3 to a primary alcohol 4, and installation of the diazirine to afford the completed PAL tag (Figure 2f). The synthesis of PALBOX was initiated by the ketal protection of commercially available cyclobutanone nitrile 2, followed by alkylation with TMS-propargyl bromide (73% yield, two steps). Sequential reduction of nitrile 3 with DIBALH and NaBH4, followed by acidic deprotection of the ketal granted ketone 4 in three steps (67% yield). Protection of alcohol 4 with TBSCl, followed by hydroxyl amine condensation afforded the protected oxime 5 (66% yield). Oxime 5 was mesylated and then treated with ammonia overnight to produce a diaziridine intermediate that was oxidized to form the protected diazirine 6 (40% yield). Finally, treatment with tetrabutylammonium fluoride simultaneously deprotected the TBS and TMS groups to afford the completed PALBOX tag (92% yield).
Derivatization of PALBOX to access a variety of analogues for versatile functionalization to a desired ligand was straightforward (Figure 2g). The PALBOX alcohol could be converted to carboxylic acid 7 via the Jones oxidation reaction (92% yield) or activated with TsCl to afford an electrophilic reagent 8 for alkylation (39% yield).
Alternatively, the amine derivative 9 of the PALBOX tag was accessed by an analogous nine-step sequence initiated by the LAH reduction of nitrile 3, protecting group manipulation, installation of the diazirine, and final deprotection to afford the amine 9 as the HCl salt in 7% overall yield.
To evaluate the chemical versatility of PALBOX in the context of small molecule probes, we derivatized fragment-based compounds and those with known targets that span a range of sizes and conjugation chemistries.22,29,30 Small molecule fragments that exhibit high labeling propensity from earlier work22 were derivatized to afford BipBox and JNBox via peptide coupling with the amine tag 9 and via alkylation with 8, respectively (Figure 2h). The PALBOX versions of lenalidomide, LenBox, and SLF (simplified ligand to FKBP) probe SLFBox were accessed via peptide coupling with the acid tag 7, and the JQ1 probe JQ1Box was synthesized through coupling with amine 9. The analogous minimalist tag versions of these probes were also prepared for comparison.
Cyclobutane Diazirine Photolysis in Aqueous Solution.
With PALBOX in hand, we next sought to evaluate its reactivity relative to the minimalist dialkyl diazirine tag. First, we investigated the general stability of PALBOX in methanol-d4, DMSO-d6, and chloroform-d in the dark or under ambient light and gratifyingly found negligible levels of decomposition after 56 days (Supporting Information Figure S3). Photolysis of the biphenyl PALBOX probe, BipBox, in water and analysis by UPLC−MS revealed the formation of an array of products matching the m/z of anticipated alcohol (~25%), ketone (~15%), and rearrangement (~50%) products (Supporting Information Figures S4 and S5). Notably, PALBOX produced a complex mixture of products, with multiple species matching the m/z of anticipated products. Four major peaks in the HPLC chromatogram match the m/z of alcohol products, while four major peaks match the m/z of alkene products. The alcohol products likely represent the insertion of water to the carbene or diazo intermediates to afford two major diastereomers and other uncharacterized products. The multiple alkene products may represent cyclobutene and ring-opened diene isomers. Overall, the yield of alcohol and ketone products from the photolysis of BipBox was similar to the minimalist analogue BipMin.
We next examined the photolysis rate of PALBOX relative to a dialkyl diazirine analogue by HPLC−MS (Supporting Information Figure S6). The UV−vis absorption spectrum of PALBOX has a maximum absorbance peak at 350 nm, in line with other alkyl diazirines. When photolyzed with a broadband UV light source (280−400 nm), BipBox and BipMin decomposed at a similar rate (t1/2 = ~1.5 s); however, when using a filtered UV-A light source (320−400 nm), BipBox exhibited faster conversion than the acyclic BipMin probe when adjusting for the photon dose (t1/2 BipBox = ~2 s vs t1/2 BipMin = ~4 s). Collectively, these data show that PALBOX probes require comparably shorter irradiation times under UV-A conditions relative to the minimalist probes.
Cyclobutane Diazirine Reactivity with Amino Acids.
We next interrogated the reactivity profile of PALBOX with individual amino acids under neat conditions or in solution, which are conditions that simulate a tight-binding event or a weak-binding event, respectively. We previously used these conditions to evaluate the reactivity profile of dialkyl and aryl trifluoromethyl diazirines.22 Incubation of BipBox under neat reaction conditions with N-acetyl, O-methyl-protected amino acids (4 equiv) and the analysis of the results with HPLC−MS showed that cysteine was the most reactive amino acid (Figure 3a). Notably, glutamic acid and aspartic acid were the second and third most reactive amino acids, suggesting that the cyclobutane diazirine diazo isomer may still be forming and selectively reacting with acidic amino acids. We observed measurable reaction yields (1−3%) with Tyr, Met, and Ser, and trace (<1%) product formation with the remaining amino acids, except for the two amino acids with amide side chains (Gln and Asn) where no reactivity was observed. The other products observed in these reactions matched the expected m/z of alkene isomers, the ketone, and a dimerized product, which are byproducts that can be formed in the absence of a solvent. The reactivity pattern observed with BipBox under neat conditions is comparable to the reactivity pattern observed with acyclic alkyl and aryl trifluoromethyl diazirines.22,31,32
Figure 3.

In vitro reactivity of cyclobutane diazirines. (a) Reactivity of cyclobutane diazirine with 20 N-acetyl, O-methyl-protected amino acids (4 equiv) in neat conditions. (b) Aqueous reaction yields of dialkyl and cyclobutane diazirines (0.5 mM) with N-acetyl, O-methyl-protected amino acids (5 mM) or increasing concentrations of AcOH. Reaction yields were calculated via UV peak integration from 250 to 280 nm by LC−MS. (c) Bovine serum albumin (BSA) was incubated with Min, Box, or ArCF2 in Tris buffer at pH 7.4. The diazirines were labeled to BSA by UV irradiation and visualized by click chemistry with Azide-Fluor 488 and in-gel fluorescence. Relative volume and lipophilicity parameters are displayed underneath each tag. (d) Relative BSA labeling in Tris buffer at varying pH. Protein labeling yields were calculated, with the fluorescence signal normalized to the Coomassie blue stain signal. Plotted values show the mean, with the error bars representing the standard deviation with n = 3. Statistical significance was determined with a t test between samples at pH 5.8 and 8.0 or 7.4 (* = p-value <0.05).
To evaluate the propensity of PALBOX to preferentially react with organic acids in the presence of excess water, which is a major characteristic of acyclic alkyl diazirines,22,23 we examined the photolysis of BipBox (500 μM) with acidic and weakly acidic N-acetyl, O-methyl-protected amino acids (5 mM) in 50% water in acetonitrile and observed the trace product formation with Cys and Asp (~0.5%, Figure 3b). By contrast, the alkyl diazirine BipMin generated product formation between 5% and 20% yield, in line with our previous observations.22 These results suggest that higher concentrations of amino acids are needed to outcompete water and react with the reactive intermediates produced by BipBox. To identify the minimal concentration needed to react with BipBox, we used AcOH to test the reactions with carboxylic acids at higher concentrations in water. With BipMin, we observed product formation with concentrations as low as 100 μM. Interestingly, the yield of the AcOH adduct with BipMin increased over two stages, with the first stage occurring from 100 μM to 10 mM AcOH up to a plateau of ~25% yield and the second stage occurring at higher concentrations around 5 M, where the yield again began to increase. These reaction kinetics suggest a change in reaction mechanism from the concentration of 100 μM−1 M, and 5 M−17 M, which may reflect the reactivity of the alkyl diazo in the first stage and reactivity through the alkyl carbene in the second stage with AcOH. Crucially, when we tested the cyclobutane diazirine probe in these same conditions, we saw no reactivity (<1% yield) with AcOH until reaching high concentrations. These data suggest that no preferential reactivity with AcOH occurs through the diazo intermediate of PALBOX at low concentrations and that AcOH adducts only form at concentrations high enough to react with a short-lived intermediate, presumably a carbene.
To further interrogate the reactivity of the diazo intermediate formed during the photolysis of PALBOX, we used a strained cyclo-octyne reagent (BCN) to trap the diazo intermediate via a cycloaddition reaction. In line with previous studies with dialkyl diazirines,24 BipMin (1 mM) reacted with BCN (10 mM) in 50% water−acetonitrile to form a cycloadduct (~5% yield, Supporting Information Figure S7). In contrast, no cycloaddition product was observed from the photolysis of BipBox (1 mM) with BCN in either 50% water−acetonitrile (10 mM BCN) or MeOH (50 mM BCN).
Cyclobutane Diazirines Label Single Proteins Independent of pH.
To further confirm the limited reactivity of PALBOX through the diazo intermediate, we next examined pH-dependent protein labeling, which is another characteristic of the reactive diazo intermediate. We incubated PALBOX (Box), the minimalist tag (Min), or an aryl difluorodiazirine (ArCF2), with BSA in Tris buffer at varying pH. The samples were photoirradiated, reacted with Azide-Fluor 488 using copper-catalyzed azide−alkyne click chemistry (CuAAC), and visualized via (sodium dodecyl sulfate−polyacrylamide gel electrophoresis) SDS−PAGE in-gel fluorescence. We found that Box labeled ~20% as much as the minimalist tag and labeled at similar levels as ArCF2, despite possessing physicochemical attributes more similar to Min (Figure 3c). The aryl diazirine ArCF2 has an electronically stabilized diazo isomer and is expected to label the protein solely through a carbene. The comparable labeling amounts from PALBOX and the aryl diazirine may suggest that the reactive species generated by each have similar half-lives. We next sought to evaluate whether the cyclobutane diazirine could label single proteins independent of pH. While labeling from Min decreased nearly 50% from pH 5 to pH 8, labeling from Box was not dependent on pH (Figure 3d, Supporting Information Figure S8). Overall, these data suggest that the labeling profile of the cyclobutane diazirine resembles labeling through a carbene intermediate and not a diazo species.
Cyclobutane PAL Probes Show Reduced Background Labeling in Cells.
We next examined whether the in vitro reactivity differences between alkyl and PALBOX diazirines correspond to differences in protein labeling in cells. We incubated an assortment of minimalist and PALBOX probes in HEK293T cells at 10 μM for 1 h (Figure 4a). Following incubation, the cells were photoirradiated, harvested, lysed, and reacted with Azide-Fluor 488 for visualization by SDS−PAGE (Figure 4b, Supporting Information Figure S9). In comparison to the minimalist PAL probes, the global labeling from PALBOX probes was generally lower and comparable to the rate of labeling observed with BSA (Figure 3c). We interpret this reduced global labeling as a reduction in the background labeling of PALBOX probes.
Figure 4.

Whole proteome cellular labeling and target enrichment with PAL probes. (a) Workflow schematic showing the generation of photocross-linked samples and their analysis via in-gel fluorescence or quantitative proteomics. (b) Labeling of HEK293T cells with the indicated minimalist or PALBOX probes, visualized by fluorescence imaging. (c) Quantitative proteomic comparison of proteins labeled by probes functionalized by minimalist or PALBOX tags. Reported alkyl diazirine ist or PALBOX tags. Reported alkyl diaziriack and labeled with their gene name. Top minimalist-enriched proteins shared in both datasets are colored in blue, and shared top PALBOX-enriched proteins are colored in red. (d) Western blots of VDAC1 and vimentin following dose-dependent labeling by fragment probes JNMin or JNBox. (e) Western blots of BRD4(BD2)-GFP fusion protein, VDAC1, or vimentin following labeling and enrichment with JQ1 probes in HEK-BD2 cells. (f) Western blots of BRD4(BD2)−GFP fusion protein following treatment with JQ1 probes with or without a competitor in HEK-BD2 cells. (g) Western blots of CRBN, VDAC1, or vimentin following treatment with lenalidomide probes with or without a competitor from a Flag-CRBN overexpressing HEK293T cell line.
We then compared the relative labeling profiles generated by the biphenyl BipBox and BipMin probes and the phenyl amine JNBox and JNMin probes via quantitative proteomics. We incubated each probe (10 μM) in HEK293T cells in triplicate for 1 h. Following incubation, the cells were photoirradiated, harvested, lysed, and reacted with biotin azide. The biotinylated proteins were enriched with streptavidin, digested with trypsin, labeled with an appropriate TMT channel, and analyzed via quantitative proteomics. As expected, we observed several proteins that are commonly labeled by alkyl diazirines (Supporting Information Table S1).22,25 Comparison of the relative labeling of each protein by the minimalist or PALBOX probes showed that nearly every known “backgroundome” protein was labeled by the minimalist PAL probes to a greater degree than the PALBOX analogues, which indicates that the PALBOX probes proportionally label known alkyl diazirine off-targets less than other proteins (Figure 4c, black circles). To examine the overlap of proteins that are preferentially labeled by PALBOX, we compared the top 50 most PALBOX-enriched proteins from both BipBox and JNBox datasets and found four proteins in common (Figure 4c, red dots). In contrast, 18 of the top 50 most minimalist-enriched proteins from the BipMin and JNMin datasets were commonly enriched (Figure 4c, blue dots). Several alkyl diazirine backgroundome proteins were still enriched by PALBOX probes relative to DMSO, but they constituted a smaller fraction of the most enriched proteins relative to the minimalist probes (Supporting Information Figure S10). We validated the labeling and enrichment of two alkyl diazirine backgroundome proteins, VDAC1 and vimentin, with JNMin and JNBox, observing a dose-dependent enrichment of these off-targets with JNMin, but not JNBox (Figure 4d, Supporting Information Figure S11).
We next tested the ability of these PAL probes to label and enrich known binding partners in cells. Using a HEK293T cell line stably expressing BRD4(BD2)-GFP (HEK-BD2),33 we observed a dose-dependent enrichment of BRD4(BD2) with both JQ1Min and JQ1Box probes from 10 to 50 μM (Figure 4e, Supporting Information Figure S11). Labeling of BRD4(BD2) from the JQ1Min probe appears to plateau at lower concentrations, but both probes exhibit comparable labeling beginning at 15−22 μM. Importantly, we observed a dose-dependent enrichment of off-targets VDAC1 and vimentin only with the acyclic alkyl diazirine JQ1Min probe, and not JQ1Box. We next confirmed that the labeling of BRD4(BD2) could be competed by preincubation with excess JQ1 (Figure 4f). Interestingly, while we observed only a partial competition of BRD4(BD2) labeling, we found that the labeling of VDAC1 and vimentin was competed strongly using these conditions (Figure 4f, Supporting Information Figure S12).
We extended this analysis to the labeling and enrichment of CRBN by LenMin and LenBOX in a Flag-CRBN overexpressing HEK293T cell line.29,34 We observed competitive labeling and enrichment in the target protein, CRBN (Figure 4g, Supporting Information Figure S12). Notably, the amount of labeling from LenMin was approximately fivefold higher than that from LenBox. Interestingly, neither probe enriched the off-targets, VDAC1 and vimentin, which could reflect the relatively low overall protein labeling of the lenalidomide probes, as shown in Figure 4b.
Cyclobutane Diazirine Probes can Map Small Molecule Binding Sites.
A major application of PAL is binding site mapping.35 This technique can be performed in cells or in vitro with purified proteins to identify peptides in the binding site of a small molecule ligand. MS-based binding site mapping is a highly informative approach for studying the small molecule mechanism of action and is particularly useful for structural studies that are challenging to profile with crystallography. We therefore sought to ensure that PALBOX could be used to profile binding sites of small molecule–protein interactions.
We previously showed that LenMin selectively labeled His353 of CRBN, located within the tri-tryptophan binding pocket.29 To investigate if LenBox gives a similar labeling profile, we identified the binding site of LenBox by using the thalidomide-binding domain of CRBN (CULT, aa318–442).36 To compare the labeling efficiency of LenMin and LenBox against CULT, we incubated CULT with each probe at different concentrations, and the interactions were captured by photoirradiation. The conjugated proteins were labeled with Azide-Fluor 488 via CuAAC, and the labeling was visualized by in-gel fluorescence (Figure 5a). Notably, the relative labeling of LenMin was approximately fivefold higher than that of LenBox at similar concentrations, which corresponds to the labeling of CRBN in cells (Figure 4g).
Figure 5.

Binding site mapping of lenalidomide PALBOX probe with CULT. (a) CULT domain of CRBN was incubated with LenMin or LenBox, cross-linked with UV irradiation, and visualized by click chemistry with Azide-Fluor 488 and in-gel fluorescence. (b) Annotated mass spectra for the labeled site on CULT. (c) Amino acid sequence of CULT, with the numbers corresponding to the position in full-length CRBN. The segment underlined in blue represents the LenBox-conjugated peptide detected by LC−MS/MS. The bold portion corresponds to the highest confidence peptide spectral match. (d) Model of CRBN docked with lenalidomide (green) and LenBox (red), adapted from PDB 4CI2. The labeled peptide is shown in blue.
Next, CULT was incubated with LenBox (50 μM) for 30 min before photoirradiation. The samples were conjugated with an acid-cleavable isotopically coded biotin-azide probe,37 which embeds an isotopic signature to enhance confidence in the labeled peptide assignment. The samples were digested with chymotrypsin, treated with formic acid to cleave the tag, and analyzed via tandem MS. The resulting mass spectra were searched with Sequest HT against the database of semi-chymotryptic peptides of CULT, allowing for one modification of the corresponding probe on any amino acid. Tandem mass spectrometry spectra (MS2) assigned to conjugated peptides were manually validated for the isotopic code in the precursor spectra.
Gratifyingly, we identified one directly labeled region by LenBox based on three unique peptides across 10 peptide spectral matches (Supporting Information Table S2). The region spans amino acids 347−363, with the modification primarily localized to His353 (Figure 5b,c, Supporting Information Figure S13). Notably, this is the same region and amino acid we observed as modified by LenMin in our prior studies.29 The LenBox-labeled peptides showed similar fragmentation patterns that we previously observed from the LenMin-labeled peptide, without notable internal fragmentation of the PALBOX moiety. LenBox-labeled peptides gave comparatively low S/N ratio in the MS2 spectra, which may be due to the low overall abundance of the labeled peptides. We finally performed a docking analysis of LenBox at the lenalidomide binding site in the crystal structure of CRBN (4CI2),38 which positioned the PALBOX diazirine in close proximity to the labeled peptide (shown in blue, Figure 5d). These data illustrate that PALBOX probes, like minimalist probes, can be used for binding site mapping to produce models of the binding interaction.
DISCUSSION
The development of trifunctional PAL tags has accelerated the construction of PAL probes and enabled many target identification and interactome mapping studies. Here, we report the development of the PALBOX tag, which harnesses ring strain to alter the reactivity of diazirines during PAL experiments. The synthesis of PALBOX is straightforward with an alkyne handle for enrichment and a modular attachment site for ready late-stage derivatization to a PAL probe. In conjunction with our synthetic efforts, we showed that cyclobutane-substituted diazirines are not readily trapped by organic acids or diazo-trapping cyclo-octynes in water, in contrast to their linear alkyl diazirine counterparts. In turn, the protein labeling from these cyclobutane diazirines does not exhibit a dependence on pH that was observed with linear alkyl diazirines.22
Mechanistically, the differences in reactivity between the cyclobutane diazirine presumably arise from the destabilization of the cyclobutane diazo isomer, which increases the relative HOMO energy and therefore accelerates the transition to a carbene. As a result, the half-life of the diazo intermediate is shorter and thereby reduces pH-dependent reactivity. Additionally, we note that cyclobutane diazirines react faster than alkyl diazirines with photoirradiation from 320 to 400 nm, and were unaffected by the inclusion of 300 nm light, which is known to convert alkyl diazos into carbenes.39 This observation may imply that the cyclobutane diazo isomers react more rapidly than alkyl diazos during photolysis without 300 nm light. Alternatively, the diazo intermediate may be circumvented altogether and not formed as a distinct intermediate upon photolysis or have a different reactivity pattern with biological acids than expected. Future investigation by methods like heavy isotope labeling24 or ultrafast spectroscopy40 would improve our understanding of the formation of these reactive intermediates and the product distribution, as well as confirm the half-lives of the carbene and diazo intermediates.
During probe development, there will be instances where the probe design will benefit from the use of the minimalist tag or from PALBOX. Our findings with PAL probes constructed from PALBOX show enrichment of the known cellular targets with the reduced enrichment of common alkyl diazirine “backgroundome” proteins.22,25 Therefore, in experiments that aim to maximize labeling specificity, such as small molecule target identification or binding site mapping, PALBOX may be a preferred choice of PAL tag as PALBOX probes reduce the labeling of weaker binding interactions with acidic surfaces that are favored by the other alkyl diazirines. Conversely, for experiments that aim to maximize the labeling signal, the minimalist tag may be a better option given that the overall labeling is several fold higher than the PALBOX probes. These experiments include cellular fully functionalized fragment experiments5,21 or single protein ligand screens.41 As these experiments rely on ligands with low binding affinity, the larger signal from the minimalist tag could be more desirable than the increased specificity reported by PALBOX. Other approaches, such as using enantiomeric probes,4 can be used to increase the confidence in the specificity of these low-affinity interactions. Investigation into the reactivity profiles of exciting developments in other diazirine scaffolds,21 photoactivatable functional groups,13 and other activation chemistries42 will expand the scope of labeling experiments and assist in probe design in the future. Finally, probe design should take into consideration additional physicochemical properties, such as the lipophilicity and branching of the probe compound, the latter of which has been posited to increase the labeling specificity.21,43
CONCLUSIONS
PALBOX is a multifunctional diazirine-based PAL tag with pH-independent reactivity profiles that enhance the development of PAL probes for small molecule target identification and binding site mapping experiments in vitro and in cells.
Supplementary Material
ACKNOWLEDGMENTS
We thank H. Flaxman, N. Vallavoju, M. Leon-Duque, W. Xu, and A. D’Souza for their advice and helpful discussions. Mass spectrometry data were collected at the Harvard University Mass Spectrometry and Proteomics Resource Laboratory (R. Robinson), and NMR data were collected at the Laukin-Purcell Instrumentation Center (D. Cui). Support from NIH NIDA (DP1DA046586), Camille and Henry Dreyfus Foundation, and Harvard University are gratefully acknowledged.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c08257.
Full description of the experimental methods used in this study; synthetic protocols; representative spectra for analytical reactions; and characterization of novel compounds (PDF)
Quantitative proteomics for BipMin versus BipBox and JNMin versus JNBox (XLSX)
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.2c08257
The authors declare no competing financial interest.
All proteomics data are available within this paper, in the Supporting Information, or in the Supporting Information Tables. The full mass spectrometry proteomics data have been deposited to the ProteomeXchange44 Consortium via the PRIDE45 partner repository with the data set identifier PXD035954.
Contributor Information
Alexander V. West, Department of Chemistry and Chemical Biology, Harvard University, Cambridge, Massachusetts 02138, United States
Yuka Amako, Department of Chemistry and Chemical Biology, Harvard University, Cambridge, Massachusetts 02138, United States.
Christina M. Woo, Department of Chemistry and Chemical Biology, Harvard University, Cambridge, Massachusetts 02138, United States
REFERENCES
- (1).Singh A; Thornton ER; Westheimer FH The Photolysis of Diazoacetylchymotrypsin. J. Biol. Chem 1962, 237, PC3006–PC3008. [PubMed] [Google Scholar]
- (2).Burton NR; Kim P; Backus KM Photoaffinity labelling strategies for mapping the small molecule–protein interactome. Org. Biomol. Chem 2021, 19, 7792–7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Smith E; Collins I Photoaffinity labeling in target-and binding-site identification. Future Med. Chem 2015, 7, 159–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wang Y; Dix MM; Bianco G; Remsberg JR; Lee H-Y; Kalocsay M; Gygi SP; Forli S; Vite G; Lawrence RM; et al. Expedited mapping of the ligandable proteome using fully functionalized enantiomeric probe pairs. Nat. Chem 2019, 11, 1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Parker CG; Galmozzi A; Wang Y; Correia BE; Sasaki K; Joslyn CM; Kim AS; Cavallaro CL; Lawrence RM; Johnson SR; et al. Ligand and Target Discovery by Fragment-Based Screening in Human Cells. Cell 2017, 168, 527–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Reisman BJ; Guo H; Ramsey HE; Wright MT; Reinfeld BI; Ferrell PB; Sulikowski GA; Rathmell WK; Savona MR; Plate L; et al. Apoptolidin family glycomacrolides target leukemia through inhibition of ATP synthase. Nat. Chem. Biol 2022, 18, 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ma T; Tian X; Zhang B; Li M; Wang Y; Yang C; Wu J; Wei X; Qu Q; Yu Y; et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 2022, 603, 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Niphakis MJ; Lum KM; Cognetta AB; Correia BE;Ichu T-A; Olucha J; Brown SJ; Kundu S; Piscitelli F; Rosen H; et al. A Global Map of Lipid-Binding Proteins and Their Ligandability in Cells. Cell 2015, 161, 1668–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Nielsen PE; Buchardt O Aryl Azides as Photoaffinity Labels. A Photochemical Study of some 4-substituted Aryl Azides. Photochem. Photobiol 1982, 35, 317–323. [Google Scholar]
- (10).Galardy RE; Craig LC; Jamieson JD; Printz MP Photoaffinity Labeling of Peptide Hormone Binding Sites. J. Biol. Chem 1974, 249, 3510–3518. [PubMed] [Google Scholar]
- (11).Smith RAG; Knowles JR Aryldiazirines. Potential Reagents for Photolabeling of Biological Receptor Sites. J. Am. Chem. Soc 1973, 95, 5072–5073. [DOI] [PubMed] [Google Scholar]
- (12).Goldman DW; Pober JS; White J; Bayley H Selective labelling of the hydrophobic segments of intrinsic membrane proteins with a lipophilic photogenerated carbene. Nature 1979, 280, 841–843. [DOI] [PubMed] [Google Scholar]
- (13).Bach K; Beerkens BLH; Zanon PRA; Hacker SM Light-Activatable, 2,5-Disubstituted Tetrazoles for the Proteome-wide Profiling of Aspartates and Glutamates in Living Bacteria. ACS Cent. Sci 2020, 6, 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Li Z; Qian L; Li L; Bernhammer JC; Huynh HV; Lee J-S; Yao SQ Tetrazole Photoclick Chemistry: Reinvestigating Its Suitability as a Bioorthogonal Reaction and Potential Applications. Angew. Chem., Int. Ed 2016, 55, 2002–2006. [DOI] [PubMed] [Google Scholar]
- (15).Ota E; Usui K; Oonuma K; Koshino H; Nishiyama S; Hirai G; Sodeoka M Thienyl-Substituted alpha-Ketoamide: A Less Hydrophobic Reactive Group for Photo-Affinity Labeling. ACS Chem. Biol 2018, 13, 876–880. [DOI] [PubMed] [Google Scholar]
- (16).Crump CJ; Murrey HE; Ballard TE; am Ende CW; Wu X; Gertsik N; Johnson DS; Li Y-M Development of Sulfonamide Photoaffinity Inhibitors for Probing Cellular γ-Secretase. ACS Chem. Neurosci 2016, 7, 1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Xu H; Hett EC; Gopalsamy A; Parikh MD; Geoghegan KF; Kyne RE; Menard CA; Narayanan A; Robinson RP; Johnson DS; et al. A library approach to rapidly discover photoaffinity probes of the mRNA decapping scavenger enzyme DcpS. Mol. BioSyst 2015, 11, 2709–2712. [DOI] [PubMed] [Google Scholar]
- (18).Li Z; Hao P; Li L; Tan CY; Cheng X; Chen GY; Sze SK; Shen HM; Yao SQ Design and synthesis of minimalist terminal alkyne-containing diazirine photo-crosslinkers and their incorporation into kinase inhibitors for cell- and tissue-based proteome profiling. Angew. Chem., Int. Ed. Engl 2013, 52, 8551–8556. [DOI] [PubMed] [Google Scholar]
- (19).Kumar NS; Young RN Design and synthesis of an all-in-one 3-(1,1-difluoroprop-2-ynyl)-3H-diazirin-3-yl functional group for photo-affinity labeling. Bioorg. Med. Chem 2009, 17, 5388–5395. [DOI] [PubMed] [Google Scholar]
- (20).Chang C-F; Mfuh A; Gao J; Wu H-Y; Woo CM Synthesis of an electronically-tuned minimally interfering alkynyl photo-affinity label to measure small molecule–protein interactions. Tetrahedron 2018, 74, 3273–3277. [Google Scholar]
- (21).Conway LP; Jadhav AM; Homan RA; Li W; Rubiano JS; Hawkins R; Lawrence RM; Parker CG Evaluation of fully-functionalized diazirine tags for chemical proteomic applications. Chem. Sci 2021, 12, 7839–7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).West AV; Muncipinto G; Wu H-Y; Huang AC; Labenski MT; Jones LH; Woo CM Labeling Preferences of Diazirines with Protein Biomolecules. J. Am. Chem. Soc 2021, 143, 6691–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Iacobucci C; Götze M; Piotrowski C; Arlt C; Rehkamp A; Ihling C; Hage C; Sinz A Carboxyl-Photo-Reactive MS-Cleavable Cross-Linkers: Unveiling a Hidden Aspect of Diazirine-Based Reagents. Anal. Chem 2018, 90, 2805–2809. [DOI] [PubMed] [Google Scholar]
- (24).O’Brien JGK; Jemas A; Asare-Okai PN; Am Ende CW; Fox JM Probing the Mechanism of Photoaffinity Labeling by Dialkyldiazirines through Bioorthogonal Capture of Diazoalkanes. Org. Lett 2020, 22, 9415–9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kleiner P; Heydenreuter W; Stahl M; Korotkov VS; Sieber SA A Whole Proteome Inventory of Background Photocrosslinker Binding. Angew. Chem., Int. Ed 2017, 56, 1396–1401. [DOI] [PubMed] [Google Scholar]
- (26).Agard NJ; Prescher JA; Bertozzi CR A Strain-Promoted [3 + 2] Azide−Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc 2004, 126, 15046–15047. [DOI] [PubMed] [Google Scholar]
- (27).Baskin JM; Prescher JA; Laughlin ST; Agard NJ; Chang PV; Miller IA; Lo A; Codelli JA; Bertozzi CR Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. U.S.A 2007, 104, 16793–16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Martyloga OV; Myronenko A; Tkachenko AM; Matvienko VO; Kuchkovska YO; Grygorenko OO Multigram Synthesis of Functionalized Spirocyclic Diazirines. Eur. J. Org. Chem 2019, 2019, 3744–3750. [Google Scholar]
- (29).Lin Z; Amako Y; Kabir F; Flaxman HA; Budnik B; Woo CM Development of Photolenalidomide for Cellular Target Identification. J. Am. Chem. Soc 2022, 144, 606–614. [DOI] [PubMed] [Google Scholar]
- (30).Flaxman HA; Chang CF; Wu HY; Nakamoto CH; Woo CM A Binding Site Hotspot Map of the FKBP12-Rapamycin-FRB Ternary Complex by Photoaffinity Labeling and Mass Spectrometry-Based Proteomics. J. Am. Chem. Soc 2019, 141, 11759–11764. [DOI] [PubMed] [Google Scholar]
- (31).Ziemianowicz DS; Bomgarden R; Etienne C; Schriemer DC Amino Acid Insertion Frequencies Arising from Photoproducts Generated Using Aliphatic Diazirines. J. Am. Soc. Mass Spectrom 2017, 28, 2011–2021. [DOI] [PubMed] [Google Scholar]
- (32).Sigrist H; Mühlemann M; Dolder M Philicity of amino acid side-chains for photogenerated carbenes. J. Photochem. Photobiol., B 1990, 7, 277–287. [Google Scholar]
- (33).Nowak RP; DeAngelo SL; Buckley D; He Z; Donovan KA; An J; Safaee N; Jedrychowski MP; Ponthier CM; Ishoey M; et al. Plasticity in binding confers selectivity in ligand-induced protein degradation. Nat. Chem. Biol 2018, 14, 706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Van Nguyen T; Lee JE; Sweredoski MJ; Yang S-J; Jeon S-J; Harrison JS; Yim J-H; Lee SG; Handa H; Kuhlman B; et al. Glutamine Triggers Acetylation-Dependent Degradation of Glutamine Synthetase via the Thalidomide Receptor Cereblon. Mol. Cell 2016, 61, 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Gao J; Mfuh A; Amako Y; Woo CM Small Molecule Interactome Mapping by Photoaffinity Labeling Reveals Binding Site Hotspots for the NSAIDs. J. Am. Chem. Soc 2018, 140, 4259–4268. [DOI] [PubMed] [Google Scholar]
- (36).Lupas AN; Zhu H; Korycinski M The ThalidomideBinding Domain of Cereblon Defines the CULT Domain Family and Is a New Member of the β-Tent Fold. PLoS Comput. Biol 2015, 11, No. e1004023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Miyamoto DK; Flaxman HA; Wu H-Y; Gao J; Woo CM Discovery of a Celecoxib Binding Site on Prostaglandin E Synthase (PTGES) with a Cleavable Chelation-Assisted Biotin Probe. ACS Chem. Biol 2019, 14, 2527–2532. [DOI] [PubMed] [Google Scholar]
- (38).Fischer ES; Böhm K; Lydeard JR; Yang H; Stadler MB; Cavadini S; Nagel J; Serluca F; Acker V; Lingaraju GM; et al. Structure of the DDB1−CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 2014, 512, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Pezacki JP; Pole DL; Warkentin J; Chen T; Ford F;Toscano JP; Fell J; Platz MS Laser Flash and Dual Wavelength Photolysis of 3,4-Diaza-2,2-dimethoxy-1-oxa[4.5]spirooct-3-ene. Migration of Hydrogen and Carbon in Cyclobutylidene and in the Excited State of Its Precursor. J. Am. Chem. Soc 1997, 119, 3191–3192. [Google Scholar]
- (40).Zhang Y; Burdzinski G; Kubicki J; Vyas S; Hadad CM; Sliwa M; Poizat O; Buntinx G; Platz MS Study of the S1 Excited State of para-Methoxy-3-phenyl-3-methyl Diazirine by Ultrafast Time Resolved UV−Vis and IR Spectroscopies and Theory. J. Am. Chem. Soc 2009, 131, 13784–13790. [DOI] [PubMed] [Google Scholar]
- (41).Grant EK; Fallon DJ; Hann MM; Fantom KGM; Quinn C; Zappacosta F; Annan RS; Chung C.-w.; Bamborough P; Dixon DP; et al. A Photoaffinity-Based Fragment-Screening Platform for Efficient Identification of Protein Ligands. Angew. Chem., Int. Ed 2020, 59, 21096–21105. [DOI] [PubMed] [Google Scholar]
- (42).Kawamata Y; Ryu KA; Hermann GN; Sandahl A; Vantourout JC; Olow AK; Adams L-TA; Rivera-Chao E; Roberts LR; Oslund RC, et al. Electroaffinity Labeling: A New Platform for Chemoproteomic-based Target Identification, 2022. ChemRxiv: 10.26434/chemrxiv-2022-s2m8f (accessed Oct 25, 2022). [DOI] [PubMed] [Google Scholar]
- (43).Park H; Koo JY; Srikanth YVV; Oh S; Lee J; Park J; Park SB Nonspecific protein labeling of photoaffinity linkers correlates with their molecular shapes in living cells In. Chem. Commun 2016, 52, 5828. [DOI] [PubMed] [Google Scholar]
- (44).Deutsch EW; Csordas A; Sun Z; Jarnuczak A; Perez-Riverol Y; Ternent T; Campbell DS; Bernal-Llinares M; Okuda S; Kawano S; Moritz RL; Carver JJ; Wang M; Ishihama Y; Bandeira N; Hermjakob H; Vizcaíno JA The ProteomeXchange consortium in 2017: supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 2016, 45, D1100–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Perez-Riverol Y; Bai J; Bandla C; García-Seisdedos D; Hewapathirana S; Kamatchinathan S; Kundu DJ; Prakash A;Frericks-Zipper A; Eisenacher M; Walzer M; Wang S; Brazma A; Vizcaíno JA The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


