Abstract
OBJECTIVE:
To assess the frequency of hepatitis C virus (HCV) testing among a population-based cohort of perinatally exposed children and identify factors associated with testing.
METHODS:
Using a population-based surveillance cohort of perinatally exposed children born from 2018 to 2020 from 4 US jurisdictions (Georgia; Massachusetts; Allegheny County, Pennsylvania; and Los Angeles County, California), we describe the frequency, timing, and type of HCV testing among children and identify characteristics associated with having an HCV test result by the age of 2 to 3 years. Data were obtained from electronic laboratory reporting, vital records, and medical records.
RESULTS:
Of 803 perinatally exposed children, 7 (1%) died before the age of 24 months. Of 796 children, health departments were unable to find medical records or laboratory reports for 181 (23%). Among those with medical record abstraction at 24 months or testing reported before the age of 3 years (n = 615), 50% had an HCV test. The majority (70% of those tested) were tested for HCV antibodies at the age of 18 months or later, although 9% had an HCV nucleic acid test at ages 2 to <6 months. No characteristics examined were found to be significantly associated with having testing reported.
CONCLUSIONS:
In this surveillance report, we identify the gaps in current testing among children perinatally exposed to hepatitis C. Provider education and resources for health departments for follow-up and linkage to care can improve the identification of children requiring treatment, a vital piece of HCV elimination.
Hepatitis C virus (HCV) is a bloodborne infection that can be transmitted from pregnant people to their infants, with transmission occurring in 5% to 10% of pregnancies in which HCV infection is present.1 HCV infection is often asymptomatic in early childhood, but over time, the infection can lead to cirrhosis, hepatocellular carcinoma, and continued transmission in the population.2,3 A successful cascade of care, beginning with the identification of HCV infection and leading to cure with direct-acting antivirals (available for children 3 years of age and older), will prevent long-term morbidity among impacted infants, avert the future spread of HCV, and support public health efforts toward HCV elimination.4
In the early 2010s, there was an epidemiological shift in increases in HCV infection among young adults, including pregnant people, mirroring the opioid overdose crisis.5,6 In response, during 2020 and 2021, the Centers for Disease Control and Prevention (CDC) and clinical organizations began recommending the universal screening of pregnant people for HCV infection during each pregnancy as an opportunity to link pregnant people to HCV treatment postpartum and to improve the identification of perinatally exposed infants.7,8
Before 2023, the standard of care for testing perinatally exposed children included HCV antibody testing at or after 18 months of age.2 However, past reports revealed significant gaps in the testing of exposed children and high loss to follow-up care. One report of Tennessee Medicaid enrollees revealed that only 25% of infants exposed to HCV from 2005 to 2014 had been tested by 2 years of age.9 The authors of another report describing infants exposed to HCV born at a single facility in Pennsylvania from 2006 to 2014 found that 30% had been tested for HCV.10 Reports from multiple states have revealed the underreporting of infants with perinatal HCV infection compared with expected case counts.11,12
In November 2023, the CDC published evidence-based recommendations for testing infants perinatally exposed to HCV at 2 to 6 months of age with a nucleic acid test (NAT).13 As a result, recent updates in screening and testing guidance among both pregnant people and infants can be expected to lead to increases in the identification of exposed infants, as well as children with perinatally acquired HCV infections. At the same time, baseline population data revealing the frequency and type of testing (ie, antibody versus NAT), as well as characteristics associated with testing, are lacking. Thus, using the CDC’s Surveillance for Emerging Threats to Pregnant People and Infants Network (SET-NET), we sought to describe testing patterns among a population-based cohort of children exposed to HCV perinatally and to determine the characteristics associated with being tested, or not being tested, from birth through 3 years of age. Understanding current testing patterns will inform the future evaluation of the impact of the 2023 perinatal HCV testing recommendations, as well as guide targeted strategies to increase appropriate perinatal HCV testing.
METHODS
All data came from SET-NET, which collects linked longitudinal surveillance data of pregnant people and their children who were perinatally exposed to HCV.14 Prenatal and child information through at least 2 years and up to 3 years of age was collected by state and local health jurisdictions from existing sources, including laboratory reports, vital statistics, birth hospitalization medical records, and well-child visit medical records. The urbanicity of the maternal county of residence at delivery was based on the 2013 National Center for Health Statistics Urban-Rural Classification Scheme for Counties.15
Data were included for dyads (pregnant people and their children) meeting either of the following criteria: (1) pregnant people with a positive NAT result for HCV RNA during pregnancy or in the year before pregnancy with no evidence of treatment or subsequent negative test results before to pregnancy or (2) children meeting the Council of State and Territorial Epidemiologists laboratory criteria for the diagnosis of perinatal HCV infection (positive NAT result for HCV RNA, HCV antigen, or detectable HCV genotype at ≥2 months and ≤36 months and not known to be exposed via another mechanism).16 Medical records were abstracted from well-child visits occurring at 12 months (visit occurring closest to 12 months of age between 6 and <18 months of age) and 24 months (visit occurring closest to 24 months of age occurring between 18 and <36 months of age). Because not all jurisdictions sent data after 36 months, data were censored at 36 months of age. Data collected included maternal, birth, or infant characteristics known to be associated with follow-up or HCV testing based on the previous literature (eg, maternal demographics and socioeconomic factors) or hypothesized to be predictors of improved medical record documentation of HCV exposure (eg, maternal substance use or medication for opioid use disorder, prematurity, NICU admission, neonatal abstinence syndrome [NAS] diagnosis).
This analysis included infants born to pregnant people with HCV infections between January 1, 2018 and December 31, 2020 in 4 jurisdictions (Georgia [2019 births]; Massachusetts [2018–2020 births]; Allegheny County, Pennsylvania [2018–2020 births]; and Los Angeles County, California [2018–2020 births]). We excluded child deaths that occurred during the follow-up period because they would not have had the full opportunity to be tested. Children who had no 24-month medical record abstraction (MRA) and no HCV testing data otherwise reported were considered lost to follow-up (LTFU). Although HCV-positive test results are reportable to the health department, negative results are not always reported. Therefore, for our main analysis, we limited the analysis to children with 24-month MRA data to better ascertain all HCV testing and reduce bias from the overestimation of the percent tested and percent positive. We performed a sub-analysis comparing selected characteristics of those who were excluded to the characteristics of those who were included in our main analysis.
For those with complete 24-month data, we reported the type of HCV testing conducted by age. To make comparisons of children known to the health department to have been tested versus not tested, we included children with 24-month MRA data, as well as those without 24-month MRA data but who had any testing reported to the health department. We also conducted sensitivity analyses to examine whether including children with any MRA data at 12 months but none at 24 months would change our findings.
Three jurisdictions abstracted data on all identified pregnant people with HCV infection and their perinatally exposed children. One jurisdiction conducted MRA on a random sample of patients at the end of pregnancy and provided data on all perinatally exposed children. Sampling weights were applied to account for selection probability, and the results presented include unweighted counts and weighted percentages.17 Rao–Scott χ2 tests were used to assess statistical significance. All analyses were conducted by using R 4.2.2. This activity was deemed public health surveillance and conducted according to applicable federal law and CDC policy. An institutional review board review was not required.
RESULTS
Four jurisdictions reported 803 children meeting the SET-NET inclusion criteria (Fig 1), including 802 children born to birth parents with a positive NAT result during pregnancy and 1 child identified only through positive child test results, with no birth parent testing available. Of these 803 children, 7 (0.9%) died at <24 months of age, with a median age at death of 19 days (interquartile range 0–263 days); these children were excluded from the remainder of the analysis. Of the remaining 796 children, 181 (23%) had neither 24-month MRA data nor testing data reported and were considered LTFU to the health department. MRA data at 24 months were reported for 582 (73%) of the children, and a further 33 (7%) had HCV testing data reported but no MRA data at 24 months. Compared with those without 24-month MRA data, children with 24-month MRA data reported were more likely to have been born to people who were white non-Hispanic, lived in a medium-large metropolitan area, and had a diagnosis code of NAS at birth (Table 1). In the sub-analysis (including children with 12-month MRA but not 24), there was also an association with not being admitted to a NICU (data not shown).
FIGURE 1.

Health department follow-up and testing for HCV among children perinatally exposed to HCV.
TABLE 1.
Characteristics of Children Born From 2018 to 2020 Perinatally Exposed to HCV, With and Without Reported HCV Testing by 3 Years of Age, SET-NET
| Total, N = 615 | Any HCV Testing Reported, N = 314 | No HCV Testing Reported, N = 301 | P a | ||||
|---|---|---|---|---|---|---|---|
| n | Weighted Column %b (95% CI) | n | Weighted Row % (95% CI) | n | Weighted Row % (95% CI) | ||
| Year of birth | 615 | 314 | 301 | .97 | |||
| 2018 | 166 | 29.3 (24.9 – 33.8) | 81 | 49.5 (40.2 – 58.7) | 85 | 50.5 (41.3 – 59.8) | |
| 2019 | 300 | 43.7 (39.1 – 48.3) | 168 | 50.6 (43.7 – 57.6) | 132 | 49.4 (42.4 – 56.3) | |
| 2020 | 149 | 27 (22.6 – 31.4) | 65 | 51.0 (41.3 – 60.8) | 84 | 49.0 (39.2 – 58.7) | |
| Maternal residence at delivery | 615 | 314 | 301 | .12 | |||
| Medium-large metropolitan (≥250 000) | 542 | 88.9 (86.1 – 91.8) | 262 | 49.0 (43.9 – 54.2) | 280 | 51.0 (45.8 – 56.1) | |
| Rural–small metropolitan (≤249 999) | 73 | 11.1 (8.2 – 13.9) | 52 | 61.3 (46.8 – 75.9) | 21 | 38.7 (24.1 – 53.2) | |
| Maternal race and ethnicity | 615 | 314 | 301 | .96 | |||
| White, non-Hispanic | 490 | 83.1 (79.9 – 86.4) | 256 | 50.3 (44.9 – 55.8) | 234 | 49.7 (44.2 – 55.1) | |
| Other | 125 | 16.9 (13.6 – 20.1) | 58 | 50.6 (40.0 – 61.3) | 67 | 49.4 (38.7 – 60.0) | |
| Maternal education | 525 | 286 | 239 | .14 | |||
| High school graduate and below | 222 | 48.5 (43.4 – 53.7) | 135 | 56.7 (48.8 – 64.6) | 87 | 43.3 (35.4 – 51.2) | |
| Some college and higher | 303 | 51.5 (46.3 – 56.6) | 151 | 48.8 (41.9 – 55.7) | 152 | 51.2 (44.3 – 58.1) | |
| Maternal health insurance | 605 | 313 | 292 | .15 | |||
| Public | 528 | 86.2 (82.7 – 89.6) | 278 | 52.3 (47.0 – 57.5) | 250 | 47.7 (42.5 – 53.0) | |
| Private/other/none | 77 | 13.8 (10.4 – 17.3) | 35 | 41.7 (28.3 – 55.2) | 42 | 58.3 (44.8 – 71.7) | |
| Maternal illicit substance usec | 581 | 303 | 278 | .77 | |||
| Yes | 276 | 48.6 (36.6 – 60.5) | 153 | 52.0 (44.8 – 59.3) | 123 | 48.0 (40.7 – 55.2) | |
| No | 305 | 51.4 (39.5 – 63.4) | 150 | 50.6 (43.7 – 57.4) | 155 | 49.4 (42.6 – 56.3) | |
| Maternal medication for opioid use disorder | 608 | 314 | 294 | .73 | |||
| Yes | 301 | 52.2 (47.4 – 57.1) | 154 | 51.7 (44.8 – 58.6) | 147 | 48.3 (41.4 – 55.2) | |
| No | 307 | 47.8 (42.9 – 52.6) | 160 | 50.0 (43.1 – 56.9) | 147 | 50.0 (43.1 – 56.9) | |
| Gestational age | 613 | 314 | 299 | .44 | |||
| Term (≥37 wk) | 505 | 83.4 (79.9 – 86.9) | 248 | 49.8 (44.5 – 55.1) | 257 | 50.2 (44.9 – 55.5) | |
| Preterm (<37 wk) | 108 | 16.6 (13.1 – 20.1) | 66 | 54.9 (43.1 – 66.6) | 42 | 45.1 (33.4 – 56.9) | |
| NICU admission | 615 | 314 | 301 | .49 | |||
| Yes | 221 | 26 (22.4 – 29.7) | 109 | 47.9 (40.3 – 55.5) | 112 | 52.1 (44.5 – 59.7) | |
| No | 394 | 74 (70.3 – 77.6) | 205 | 51.3 (45.3 – 57.3) | 189 | 48.7 (42.7 – 54.7) | |
| NAS (ICD-10 code of P96.1) | 601 | 308 | 293 | .45 | |||
| Yes | 234 | 41.8 (37 – 46.6) | 110 | 48.9 (41.1 – 56.8) | 124 | 51.1 (43.2 – 58.9) | |
| No | 367 | 58.2 (53.4 – 63) | 198 | 52.8 (46.6 – 59.1) | 169 | 47.2 (40.9 – 53.4) | |
CI, confidence interval; ICD-10, International Classification of Diseases, Tenth Revision.
P values were calculated from Rao–Scott χ2 tests.
Weighted percentages for total are column percentages. Weighted percentages for any and no testing are row percentages.
Illicit substance use included illicit opioids and other illicit substances (eg, cocaine, methamphetamines).
Of 615 children with complete 24-month MRA data or HCV testing data reported, 50% (n = 314) had an HCV laboratory result reported (Fig 2A). However, 7% (n = 22) of these children were inappropriate for their age, including 4 NAT <2 months of age and 18 antibody tests <18 months of age. Of those with appropriate testing (n = 292), the majority had a first test result at ≥18 months of age (220, 75%), and of those, the majority (194, 88%) were antibody tests (Fig 2B). Twenty-nine children (10%) had a NAT for HCV RNA reported at 2 to <6 months of age. Although SET-NET systematically obtained laboratory results only up to 36 months of age, 40 children were reported with a first test result reported at ≥36 months of age (of 56 children with any data reported after 36 months; data not shown). In total, 25 children had an appropriate positive HCV test result reported for a weighted percent positivity of 5.8% (95% confidence interval 3.3% to 8.3%).
FIGURE 2.
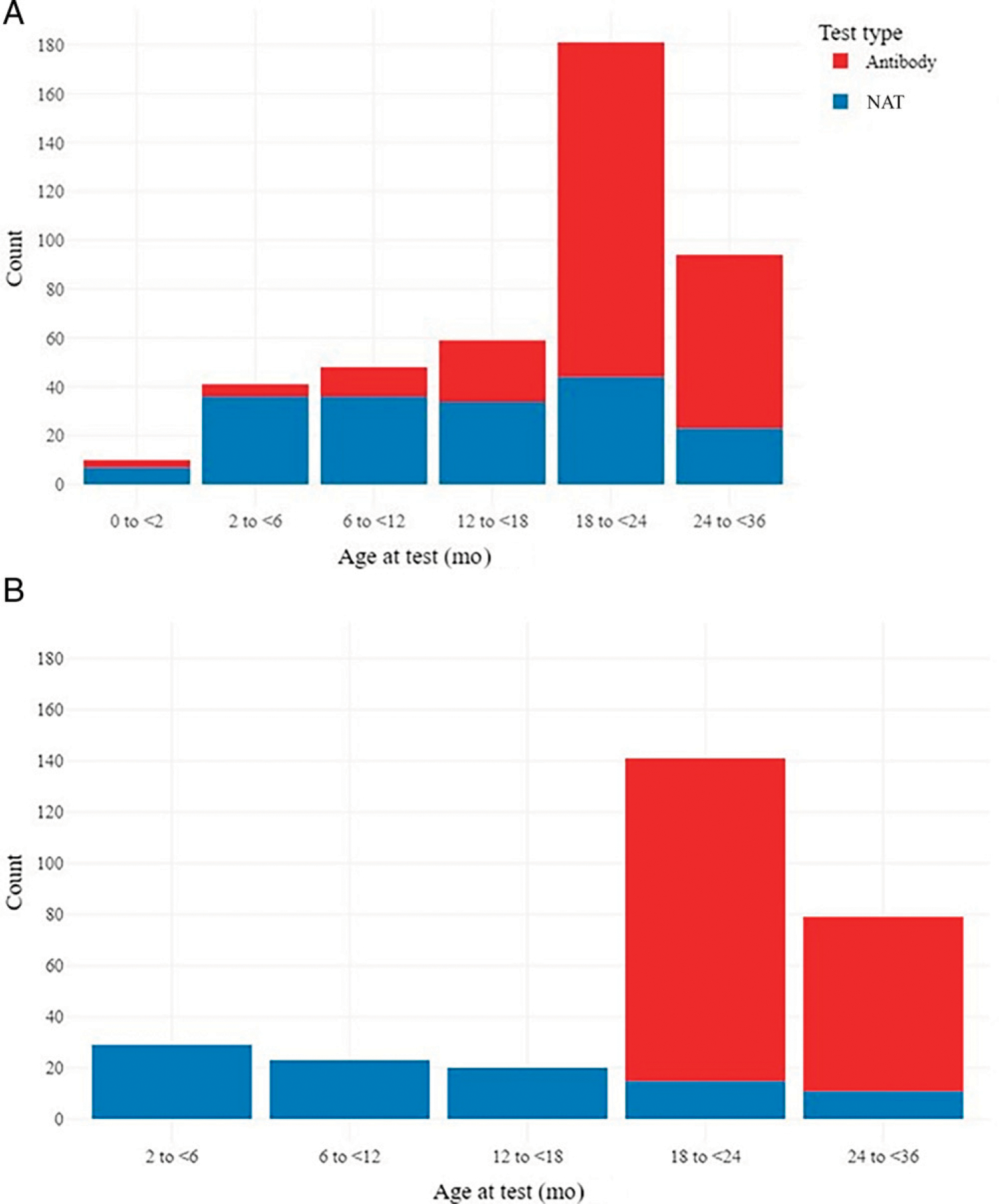
Type and timing of HCV testing among children perinatally exposed to HCV by (A) type of test and age at first test overall (n = 314 children and 433 unique tests) and (B) timing of the first test among appropriate test results (ie, excluding NATs <2 months or antibody testing <18 months) reported from 292 children with appropriate testing. Of note, for 20 children, their first test included both a NAT and antibody test collected on the same day.
Again, among the 615 children with complete 24-month MRA data or HCV testing data reported, the majority were born to people who resided in medium-large metropolitan areas (89%), were white non-Hispanic (83%), and had public insurance (86%), and 49% had a history of illicit substance use in pregnancy (Table 1). No statistically significant differences were seen in the likelihood of laboratory results reported by maternal demographics, including race and ethnicity (white non-Hispanic compared with other racial and ethnic groups), urbanicity, education, and insurance. Prematurity, history of NICU admission, and diagnosis code for NAS were also not associated with an increased likelihood of HCV testing. In the sub-analysis, including children with MRA performed at 12 months but not at 24 months and no HCV testing reported (n = 78; Supplemental Table 2), none of the examined characteristics were statistically associated with HCV testing.
DISCUSSION
Our analysis of this population-based multijurisdiction cohort revealed that only half of perinatally exposed children with follow-up data available were tested for HCV infection by 2 to 3 years of age. Additionally, some had testing performed that was inappropriate for their age (NAT at <2 months or positive antibody test result at <18 months of age), leaving their actual perinatal HCV status unknown. Of the birth parent demographic and socioeconomic and neonatal factors assessed, none were associated with the likelihood of having an HCV test reported. Additionally, we found that nearly a quarter of exposed children were LTFU to the health department before 24 months of age.
Gaps in Perinatal HCV Testing
For goals of HCV elimination to be met, efforts surrounding identification and linkage to care for HCV infection must include infections both in pregnant people and their children.18 Our report highlights that before the publication of recommendations in November 2023 specifying earlier testing, nearly half of perinatally exposed children were found not to have been tested for HCV infection. The frequency of testing was on the high end of past estimates in the published literature (8.6%–53.1%13), but there is a great deal of room for improvement.
Exploring risk factors for failure to test could inform ongoing public health and clinical efforts to improve the identification of children with perinatal HCV infection; however, barriers to testing are likely to be manifold. The exposure status of the newborn is identified by obstetric care providers and must be documented and communicated to the newborn’s hospital providers and again, potentially, to multiple outpatient pediatric care providers over the first 18 months of the child’s life. Gaps and challenges in this communication and documentation of perinatal exposures to follow-up providers are not unique to hepatitis C.19 Although we postulated that neonatal factors that may be associated with a more robust handoff or frequent medical care (eg, children who were born premature and admitted to a NICU) or those with documentation of factors known to be associated with perinatal HCV infection (eg, birth parent history of substance use or International Classification of Diseases, 10th Revision code for NAS) may be associated with appropriate testing, we did not find this to be the case.
A previous report on HCV testing among a cohort of exposed children born from 2006 to 2014 in Pittsburgh, Pennsylvania, revealed that maternal opioid use was a predictor of HCV testing in the child, as were prematurity and NICU admission. These findings may reflect practices and communication (eg, handoff of exposure status) within a single health care system.10 In many cases, especially when pregnant people and children move between clinicians and facilities, information regarding HCV status and laboratory test results may not be transferred. Collaboration with local obstetric and newborn nursery colleagues and systems-level changes in, for example, templates for birth hospital discharge information could increase the transfer of HCV infection status. Improved transfer of information is critical to increase the identification of exposed children and subsequently improve testing for the diagnosis and treatment of children with perinatal HCV infection.
In our analysis, none of the examined factors were associated with testing. Previous reports have revealed socioeconomic factors associated with the reduced likelihood of HCV testing among exposed children, including birth parents of African American race, lower birth parent education level, and living in a rural area.9,20,21 These factors may be markers of complex societal issues leading to reduced care among disenfranchised populations (eg, experiences of racism and stigmatization in health care, low health literacy, and lack of access to quality health care). Our analysis may differ from these reports because we used a more stringent inclusion to examine HCV testing patterns focusing only on those with 24-month MRA or testing reported. Unfortunately, low numbers of individuals within select racial and ethnic categories led to unstable estimates in the current analysis, and statistical testing was only performed to compare white non-Hispanic individuals to all other racial and ethnic groups, which might mask inequities. Similarly, although we did not see testing differences by urbanicity, the homogeneity of this cohort (>90% from medium-large metropolitan areas) makes assessing this challenging, and other reports have revealed a decreased likelihood of HCV testing among exposed children whose birth parents resided in rural areas.9
Systemic changes are needed to improve testing rates among children, regardless of socioeconomic background or geographic region. The new CDC guideline recommending earlier testing for all perinatally exposed children, rather than risk-based consideration of early NAT testing, should reduce loss to follow-up, relative to both health care providers and the health department for care coordination and surveillance efforts.
Provider Education on Appropriate Testing
Another critical step for the increased identification of children with perinatal HCV infection is provider education.18 Our analysis revealed, in addition to overall low testing, frequent inappropriate testing (antibody testing before 18 months, NAT testing before 2 months) and delayed testing (after 3 years of age). Several jurisdictions participating in this analysis have since implemented extensive provider education outreach, including collaborating with local American Academy of Pediatrics chapters, partnering with Perinatal Quality Collaboratives, and providing direct outreach to primary care providers caring for exposed children to recommend testing and provide education around testing recommendations. Indeed, the jurisdiction with the highest frequency of children with testing results reported performed proactive outreach starting in 2020 to primary care providers who were caring for exposed children when they were <18 months of age to ensure awareness of the perinatal HCV exposure and recommend testing at ≥18 months of age (the recommendation at the time) and again reached out in 2022 to ensure that testing had occurred.
Public Health Surveillance
Public health surveillance of hepatitis C, at both the national and jurisdiction levels, fulfills a critical goal of establishing burden and identifying epidemiological trends and is used to inform resource allocation and quality improvement. Jurisdictions also play a key role in the identification of exposed children through laboratory test results received and vital record linkages; they can also help coordinate and communicate with pediatric care providers across disparate health care systems. In the absence of resources to provide case ascertainment through linkages and longitudinal follow-up for all exposed children, an alternative, automated process could improve the identification of pregnant people and children with HCV infections. First, the improved reporting of pregnancy status in electronic laboratory reports or via electronic case reporting could help with the more timely identification of affected pregnancies that may require referral to treatment and longitudinal follow-up.22 Additionally, some jurisdictions have implemented reporting requirements for negative HCV test results among children <3 years of age, allowing them to identify and focus resources and outreach on exposed children who were not known to have been tested. Better collaboration between clinical and public health partners can improve surveillance to drive innovation and resource allocation and directly impact the identification of exposed pregnancies and children, as well as the navigation of clinical care for both.
Strengths and Limitations
This surveillance cohort allows us to examine population-based data from a large number of dyads and link birth parent demographics and characteristics with child outcomes. However, this analysis is subject to several limitations. First, we report findings from 4 jurisdictions, which are not nationally representative. Additionally, the catchment area is predominantly urban, in which access to testing might be different than in rural areas. Second, we had low numbers for some characteristics examined, which made estimates unstable for more granular categories to examine health inequities (eg, race and ethnicity, private insurance), and medical records may not appropriately ascertain indicators of social vulnerability. Third, LTFU was substantial, and we required children to be included if they had MRA performed at 24 months of age. In sensitivity analyses, when examining those children with MRA performed at 12 months of age but not at 24 months of age, none of the examined characteristics remained statistically associated with HCV testing. Fourth, for those who were LTFU, we were unable to ascertain the reasons why (eg, moved out of state, unable to locate primary care provider, not receiving routine medical care). Finally, it is possible that the coronavirus disease 2019 pandemic may have impacted clinicians’ ability to perform HCV testing or reduce follow-up in unmeasured ways; however, we did not see differences in testing by year of birth.
CONCLUSIONS
This population-based estimate of HCV testing among perinatally exposed children highlights persistent challenges in the identification and follow-up of exposed children. Changes to HCV testing recommendations advising early NAT for HCV RNA may improve the diagnosis of children with perinatal HCV infection. Provider education, innovative communication strategies, and partnerships between clinicians and public health agencies can ensure that we increase testing and improve the entire continuum of care for children with perinatal exposure to HCV infection.
Supplementary Material
WHAT’S KNOWN ON THIS SUBJECT:
Single-site reports demonstrate insufficient testing among children exposed to HCV perinatally. Previous guidance recommended testing at age ≥18 months; in 2023, the Centers for Disease Control and Prevention recommended earlier testing of exposed infants at ages 2 to 6 months.
WHAT THIS STUDY ADDS:
High loss to follow-up and low testing within this large, population-based cohort indicate that earlier HCV testing recommendations might result in more children tested and retained in care. Improved identification and follow-up are needed for successful HCV elimination.
ACKNOWLEDGMENTS
We would like to thank the following individuals for their contributions to SET-NET and review of the paper: Raiza Amiling, Michael Andrews, Katherine Barter, Jerusha Barton, Amaris Beatty, Catherine M. Brown, Bonnie Dao, Micheleange Etienne, Kerry A. Fenton, Ami Gandhi, Prabhu Grounder, Tracy Kavanaugh, Irene Lerman, Allison Longenberger, Lisa McHugh, Tonia Ruddock, Nehali Shah, Susan Soliva, Kirsten Waller, Michelle Whelan, Jamil Williams, Andre’a Wilson, Mahsa M. Yazdy, and Carol Zafiratos.
FUNDING:
This study was performed as regular work of the Centers for Disease Control and Prevention (CDC) and is supported through the Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases Cooperative Agreement (ELC CK19–1904) and through contractual mechanisms, including the Local Health Department Initiative to Chickasaw Health Consulting (200-2021-F-12655). Staffing support for this work was funded by the CDC through a contract with G2S, LLC (47QRAA19D00C5). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
ABBREVIATIONS
- CDC
Centers for Disease Control and Prevention
- HCV
hepatitis C virus
- LTFU
lost to follow-up
- MRA
medical record abstraction
- NAS
neonatal abstinence syndrome
- NAT
nucleic acid test
- SET-NET
Surveillance for Emerging Threats to Pregnant People and Infants Network
Footnotes
Dr Woodworth conceptualized the study, interpreted data, and drafted the initial manuscript; Ms Distler conducted the data analysis and participated in drafting the initial manuscript; Mr Chang and Ms Luong conducted the data analysis and replication; Ms Newton calculated sampling weights; Drs Orkis, Halai, and Lyu, Ms Reynolds, Ms Carpentieri, Ms Willabus, Mr Osinski, and Ms Shephard coordinated data collection, data quality checks, and data reporting; Ms Tong, Ms Sizemore, and Dr Sandul interpreted data; and all authors reviewed and revised the manuscript, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no potential conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis 2014;59(6):765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Infectious Diseases. Hepatitis C. In: Kimberlin DW, Banerjee R, Barnett ED, eds. Red Book: 2021–2024 Report of the Committee on Infectious Diseases. American Academy of Pediatrics; 2021 [Google Scholar]
- 3.Centers for Disease Control and Prevention. Hepatitis C. Available at: https://www.cdc.gov/hepatitis/hcv/index.htm. Accessed November 15, 2023 [Google Scholar]
- 4.Centers for Disease Control and Prevention. Division of Viral Hepatitis 2025 Strategic Plan. US Department of Health and Human Services; 2020 [Google Scholar]
- 5.Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P-H, Johnson L, Limketkai BN, et al. Trends in the prevalence of hepatitis C infection during pregnancy and maternal-infant outcomes in the US, 1998 to 2018. JAMA Netw Open. 2023;6(7):e2324770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schillie S, Wester C, Osborne M, et al. CDC recommendations for hepatitis C screening among adults—United States, 2020. MMWR Recomm Rep 2020;69(2):1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists. Routine hepatitis C virus screening in pregnant individuals: practice advisory. Available at: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/05/routine-hepatitis-c-virus-screening-in-pregnant-individuals. Accessed November 22, 2023 [Google Scholar]
- 9.Lopata SM, McNeer E, Dudley JA, et al. Hepatitis C testing among perinatally exposed infants. Pediatrics. 2020;145(3): e20192482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chappell CA, Hillier SL, Crowe D, et al. Hepatitis C virus screening among children exposed during pregnancy. Pediatrics. 2018; 141(6):e20173273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuncio DE, Newbern EC, Johnson CC, Viner KM. Failure to test and identify perinatally infected children born to hepatitis C virus-infected women. Clin Infect Dis 2016;62(8):980–985 [DOI] [PubMed] [Google Scholar]
- 12.Newton SM, Woodworth KR, Chang D, et al. Frequency of children diagnosed with perinatal hepatitis C, United States, 2018–2020. Emerg Infect Dis 2024;30(1):202–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panagiotakopoulos L, Sandul AL, Conners EE, et al. ; Collaborators. CDC recommendations for hepatitis C testing among perinatally exposed infants and children - United States, 2023. MMWR Recomm Rep 2023;72(4):1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodworth KR, Reynolds MR, Burkel V, et al. A preparedness model for mother-baby linked longitudinal surveillance for emerging threats. Matern Child Health J 2021;25(2):198–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingram DD, Franco SJ. 2013. NCHS urban-rural classification scheme for counties. Vital Health Stat 2. 2014;(166):1–73 [PubMed] [Google Scholar]
- 16.Council for State and Territorial Epidemiologists. Public health reporting and national notification of perinatal hepatitis C virus infection 2017. Available at: https://cdn.ymaws.com/www.cste.org/resource/resmgr/2017PS/2017PSFinal/17-ID-08.pdf. Accessed November 21, 2023 [Google Scholar]
- 17.Surveillance for Emerging Threats to Pregnant People and Infants Network; Division of Birth Defects and Infant Disorders; National Center of Birth Defects and Developmental Disabilities; Centers of Disease Controal and Prevention. Sampling and weighting methodology for end of pregnancy and infant follow-up medical record abstraction. Available at: https://www.cdc.gov/set-net/media/pdfs/Pregnancy_Sampling_Methods_2023_clearance_CLEARED-P.pdf. Accessed November 21, 2023 [Google Scholar]
- 18.Saleh E, Jhaveri R. Earlier testing of infants with perinatal hepatitis C exposure: a key step toward elimination. Pediatrics. 2024; 153(1):e2023064242. [DOI] [PubMed] [Google Scholar]
- 19.Leeb RT, Cree RA, Aird L, et al. A framework for coordination between obstetric and pediatric providers in public health emergencies: lessons learned from the Zika outbreak in the United States, 2015 to 2017. Am J Perinatol 2020;37(10):982–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foley MK, Djerboua M, Kushner T, et al. Maternal neighbourhood-level social determinants of health and their association with paediatric hepatitis C screening among children exposed to hepatitis C in pregnancy. Paediatr Perinat Epidemiol 2024;38(2):152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuncio DE, Waterman EJ, Robison SZG, Roberts A. Factors associated with perinatal hepatitis C screening among exposed children: 2016–2020. Pediatrics. 2024;154(1):e2023064745. [DOI] [PubMed] [Google Scholar]
- 22.Council of State and Territorial Epidemologists; CSTE COVID-19 Pregnancy Status Reporting Workgroup. Guidance to improve pregnancy status reporting accuracy and completeness 2023. Available at: https://cdn.ymaws.com/www.cste.org/resource/resmgr/weblayers/guidance_document_final_0824.pdf. Accessed November 21, 2023 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


