Abstract
Background:
Social determinants of health (SDoH) are environmental conditions that influence health outcomes. As olfactory dysfunction (OD) in older individuals is associated with increased morbidity and mortality, we sought to investigate the impact of specific SDoH on olfactory function.
Methods:
A cross-sectional analysis of the Health, Aging and Body Composition Study, a US population-based epidemiologic cohort study, was performed. Olfactory function was assessed utilizing both a self-report and a psychophysical olfactory test (CC-SIT test). Multivariable logistic regressions were performed to examine associations between specific SDoH with self-reported anosmia (sOD) and objective anosmia (oOD) as assessed by psychophysical testing. Differences in sensitivity and specificity were evaluated with sample tests for equality of proportions.
Results:
Of 2219 participants, 13% had oOD and 18% had objective hyposmia; only 10% had sOD. Individuals identifying as Black race had higher odds of oOD (odds ratio [OR]:1.41, 95% confidence interval [CI]:1.02–1.95), while females and those reporting family incomes ≥$50,000 had lower odds of oOD (OR: 0.46, CI:0.34–0.62; OR:0.52, CI:0.29–0.93), adjusting for covariates. No specific SDoH was significantly associated with sOD. The sensitivity and specificity of sOD for oOD was 23.1% and 92.0%, respectively. sOD had greater sensitivity in females than males (30.8% vs. 18.8%, p = 0.030), while specificity varied significantly depending on family income (range: 90.0%−94.8%, p = 0.033).
Conclusions:
Utilizing a large population-based study, we find disparities in the prevalence and self-recognition of OD among individuals of different gender, race, and income levels. Further effort is needed to evaluate factors propagating these disparities and to raise awareness of OD across all patient populations.
Keywords: anosmia, disparities, hyposmia, olfaction, social determinants of health
1 |. INTRODUCTION
Olfactory dysfunction (OD), and particularly complete olfactory loss, is an important public health concern that emerges disproportionately among aging individuals. Studies suggest that up to 27.5% of elderly adults in the United States have some form of OD, and up to one-third of this population reports dissatisfaction with their sense of smell.1–3 The health ramifications of OD are numerous, contributing to diminished appetite and malnutrition, injury due to failed perception of environmental hazards, reduced quality of life (QoL), and major adverse health outcomes.4–6 Indeed, recent studies provide mounting evidence that OD may be a harbinger of dementia and Parkinson’s disease and predict higher long-term morbidity and mortality.7–10
The US Department of Health and Human Services defines social determinants of health (SDoH) as “the conditions in the environments where people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and QoL outcomes and risks.”11 Racial and socioeconomic disparities have been found in outcomes of cancer, diabetes, and heart disease.12,13 In a recent study, we identified five themes which can be used to categorize specific SDoH that have been found to be associated with OD: socioeconomic status (SES), education status, occupational exposures, racial/ethnic disparities, and lifestyle/behavioral factors.14
The complexity and cost of performing broad population-based olfaction testing has led to a deficiency of knowledge about risk factors for age related, noninfectious OD. While several prior studies have looked at a limited set of available population-based datasets on OD, these investigations have shortcomings in not exploring both subjective olfactory dysfunction (sOD) and oOD in the same cohort or not fully accounting for facets of SDoH previously identified to be associated with OD.15–18 In this study, we expand upon a previous investigation by Dong et al., which utilized a pooled analysis of two community-based studies, and found that Black individuals had markedly higher odds of anosmia compared with White individuals in age- and sex-adjusted analyses.16 Here, we systematically investigate the association between the more comprehensive five independent categories of SDoH we previously identified, and both sOD and oOD, using a US population-based epidemiologic cohort study of older adults. We also investigate whether there are disparities in the prevalence of hyposmia or a reduced ability to detect odor that does not meet the criteria for anosmia. In addition, as previous studies have found a lack of correlation between subjective and objective measures of olfaction, we assess whether specific SDoH may account for differences in olfactory function perception and objective measurement.2,19
2 |. METHODS
2.1 |. Study population
Participants were drawn from the Health, Aging and Body Composition (HealthABC) study.20,21 Briefly, the HealthABC is an interdisciplinary study focused on risk factors for the decline of function in healthier older persons, particularly change in body composition with age.20 From 1997 to 1998, 3075 subjects were recruited from a random sample of Medicare beneficiaries and age-eligible participants in two cities. As part of the larger study, inperson interviews and examinations occurred at years 1, 2, and 3, with phone calls alternating every 6 months. A total of 2535 participants completed both the subjective and objective olfactory test, of whom 316 reported having a cold in the week prior to the exam and were excluded from further analysis. This secondary data analysis did not require institutional review board (IRB) review due to the utilization of a widely available, deidentified, national dataset.
2.2 |. Olfactory function testing
Olfactory function was tested as part of the year 3 clinical visit utilizing the 12-item cross-cultural smell identification test (CC-SIT), a psychophysical screening test commonly referred to as the B-SIT (Sensonics; Haddon Heights, NJ), which is widely used in clinical and epidemiological studies.22 One point was given for each correct answer with a total score ranging from 0 to 12. Psychophysical testing offers a reproducibly quantifiable description of olfactory performance and allows for evaluation of treatment response. As in prior studies, we treat it as a surrogate of objective assessment because we lack truly quantitative measurement capabilities.2,15,23 We defined objective olfactory dysfunction (oOD) as a CC-SIT score ≤ 6, a threshold previously utilized to define clinically confirmed anosmia among patients.16 We also analyzed the association between SDoH and hyposmia, using a more sensitive cutoff of CC-SIT score ≤8.24 sOD was also assessed as part of the year 3 clinical visit survey and was defined as an affirmative answer to the question: “Do you suffer from smell and/or taste problems?”16
2.3 |. Social determinates of health
Demographic and lifestyle information was obtained via interviews during the year 1 clinical visit, unless otherwise noted.16 {Dong, 2017 #14} Gender and race were dichotomously coded as male/female and White/Black, respectively. Education was categorized as “less than high school,” “high school,” and “above high school.” Measures of SES included: family income, which was categorized as “less than $10,000,” “$10,000 to $25,000,” “>$25,000 up to <$50,000,” “≥$50,000”; whether they possessed supplemental insurance to Medicare; and whether they had enough money for food. Alcohol drinking behaviors and smoking, assessed in year 3, were defined as current, former, or never.16
2.4 |. Potential confounding variables
Statistical analyses were adjusted for the following comorbidities which have been shown to be associated with OD. These data were obtained via in-clinic interviews and examinations during year 3. Participants were asked how often they “feel excessively (overly) sleepy during the day” with a response of “often (5−15 times/month)” or “almost always (16−30 times/month)” considered positive for daytime sleepiness.16 Prevalence of depressive symptoms was defined as ≥10 on the 15-item Center for Epidemiologic Studies depression (CES-D) scale.16 Prevalence of anxiety symptoms, previously found to be associated with OD,18 was defined as a positive response to “During the past week, have you felt nervous or shaky inside?” or “During the past week, have you felt tense or keyed up?” Head injury was defined as an affirmative answer to “Have you ever been hit in the head hard enough to make you faint?”16 Body mass index (BMI) was categorized as normal (<25 kg/m2), overweight (25−29.9 kg/m2), and obese (≥30 kg/m2). Self-reported general health status was defined as “excellent or very good,” “good,” and “fair or poor.” Cognition was assessed using the modified Mini-Mental State Examination (3MSE), a 100-point expansion of the 30-point Mini-Mental State Examination, with scores of <80 indicating dementia.20,25 Comorbid conditions such as Parkinson’s disease,26 pneumonia in the preceding 6 months,27 cardiovascular disease (myocardial infarction or congestive heart failure), hypertension, diastolic and systolic blood pressure,28 cerebrovascular disease,29 and diabetes mellitus30 were also reported based on self-report.
2.5 |. Statistical analysis
Descriptive statistics were reported as median with interquartile range for continuous variables and as number and percent for categorical variables. Univariable logistic regressions were performed, separately for each SDoH, to identify factors associated with the presence of either sOD or oOD and were reported as an odds ratio (OR) with 95% confidence interval (CI) and p-value. All aforementioned SDoH independent variables, along with confounding variables that had a p < 0.2 on univariable regressions, were included in a stepwise forward multivariable logistic model. For ease of interpretation, diastolic and systolic blood pressure were converted to z-scores. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of self-awareness of olfactory loss in self-reported sOD in evaluating for oOD were calculated both in the entire cohort and in subgroups of race, gender, education, and income. Differences in CC-SIT scores, sensitivity, specificity, PPV, and NPV by race, gender, education, and incomes were calculated and tested for statistical significance using sample tests without continuity correction, Wilcoxon rank sum tests, and Dunn’s Kruskal–Wallis tests, as appropriate, and p-values were adjusted with the Benjamini–Hochberg method for multiple comparisons.31 Missing values (<1.1% of total datapoints) were imputed utilizing multivariate imputation by chained equations.32 Statistical analyses were performed using R, Version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria).
3 |. RESULTS
A total of 2219 individuals completed both the self-report olfactory awareness assessment and objective olfactory CC-SIT test and were included in our study. Fifty-two percent (n = 1164) of participants were female, 63% (n = 1,402) of participants self-described as White race, and the median age at the time of olfactory testing was 75 (interquartile range [IQR]: 73–78) (Table 1). Among all participants, the median CC-SIT score was 10 (IRQ: 8–11), 13% (n = 290) had anosmia or oOD with an additional 18% (n = 392) qualifying as having objective hyposmia, and 10% (n = 221) had sOD on questionnaire.
TABLE 1.
Patient characteristics and distribution of variables
| Characteristic | N = 2,219 |
|---|---|
| Site | |
| Memphis | 1,082 (49%) |
| Pittsburgh | 1,137 (51%) |
| Gender | |
| Male | 1,055 (48%) |
| Female | 1,164 (52%) |
| Race | |
| White | 1,402 (63%) |
| Black | 817 (37%) |
| Age at year 3 clinic visit | |
| 70–74 | 921 (42%) |
| 75–79 | 1,050 (47%) |
| 80–84 | 248 (11%) |
| Education | |
| Less than high school | 479 (22%) |
| High school graduate | 744 (34%) |
| Post-secondary schooling | 996 (45%) |
| Alcohol consumption | |
| Never | 629 (28%) |
| Current | 1,141 (51%) |
| Former | 449 (20%) |
| Smoking status | |
| Never | 1,019 (46%) |
| Former | 1,044 (47%) |
| Current | 156 (7.0%) |
| Family income | |
| <$10,000 | 262 (12%) |
| $10,000 to $25,000 | 822 (37%) |
| > $25,000 up to $50,000 | 750 (34%) |
| ≥ $50,000 | 385 (17%) |
| Supplemental insurance to Medicare | 1,850 (83%) |
| Enough money for food | 2,039 (92%) |
| Primary care received at | |
| Private doctor’s office | 1,891 (85%) |
| Public clinic | 96 (4.3%) |
| Health maintenance organization | 147 (6.6%) |
| Hospital outpatient clinic | 85 (3.8%) |
| Daytime sleepiness | 293 (13%) |
| Depressive symptoms | 249 (11%) |
| Anxiety symptoms | 735 (33%) |
| Hit head hard enough to faint | 211 (9.5%) |
| Parkinson’s disease | 12 (0.5%) |
| BMI | |
| <25 | 749 (34%) |
| 25–29.9 | 934 (42%) |
| >30 | 536 (24%) |
| Avg sitting systolic BP, mm Hg | 134.00 (120.00, 148.00) |
| Avg sitting diastolic BP, mm Hg | 70.00 (64.00, 78.00) |
| Self-reported health | |
| Excellent/very good | 1,003 (45%) |
| Good | 840 (38%) |
| Fair/poor | 376 (17%) |
| Cognitive impairment | 235 (11%) |
| Pneumonia, preceding 6 months | 15 (0.7%) |
| Ml/heart disease | 494 (22%) |
| Cerebrovascular disease | 195 (8.8%) |
| Hypertension | 1,251 (56%) |
| Diabetes mellitus | 368 (17%) |
| CC-SIT score | 10.00 (8.00, 11.00) |
| Objective olfactory dysfunction (anosmia) | 290 (13%) |
| Subjective olfactory dysfunction | 221 (10.0%) |
| Hyposmia or anosmia | 682 (30.7%) |
Note: n (%); Median (interquartile range [IQR[). Anosmia (objective olfactory dysfunction) defined as CC-SIT score ≤6. Hyposmia defined as CC-SIT score ≤8.
Abbreviations: BMI, body mass index; BP, blood pressure; CC-SIT, cross-cultural smell identification test; MI, myocardial infarction.
The factors associated with the prevalence of sOD differed from those associated with oOD. SDoH factors, including race, education, income, smoking or alcohol consumption, were not individually associated with sOD on unadjusted logistic analysis (Table 2). However, other participant factors, including anxiety symptoms (OR: 1.35, p = 0.038), a history of hitting one’s head hard enough to faint (OR: 1.95, p < 0.001), and cerebrovascular disease (OR: 1.59, p = 0.033), were all associated with higher odds of sOD. In our complete multivariable model, none of the SDoH we investigated were associated with sOD, although geographic location of clinical testing site, history of hitting one’s head hard enough to faint, and elevated systolic blood pressure were all significantly associated with sOD (Table 3).
TABLE 2.
Univariable logistic regression analysis of variables associated with subjective olfactory dysfunction (left panel) and objective olfactory dysfunction (right panel)
| Subjective olfactory dysfunction (sOD), n = 221 | Objective olfactory dysfunction (oOD) n = 290 | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | p-value | OR | 95% CI | p-Value |
| Gender | ||||||
| Male | — | — | — | — | ||
| Female | 1.11 | 0.84, 1.47 | 0.472 | 0.46 | 0.35, 0.59 | <0.001 |
| Race | ||||||
| White | — | — | — | — | ||
| Black | 0.99 | 0.74, 1.32 | 0.957 | 1.81 | 1.41, 2.32 | <0.001 |
| Age at year 3 clinic visit | ||||||
| 70–74 | — | — | — | — | ||
| 75–79 | 1.14 | 0.85, 1.54 | 0.392 | 1.58 | 1.20, 2.09 | 0.001 |
| 80–84 | 1.2 | 0.75, 1.88 | 0.432 | 2.51 | 1.72, 3.65 | <0.001 |
| Education | ||||||
| Less than high school | — | — | — | — | ||
| High school graduate | 0.99 | 0.67, 1.49 | 0.978 | 0.49 | 0.36, 0.68 | <0.001 |
| Post-secondary schooling | 1.21 | 0.85, 1.77 | 0.3 | 0.52 | 0.39, 0.70 | <0.001 |
| Alcohol consumption | ||||||
| Never | — | — | — | — | ||
| Current | 1.2 | 0.86, 1.69 | 0.279 | 1.07 | 0.79, 1.45 | 0.681 |
| Former | 1.25 | 0.83, 1.88 | 0.285 | 1.58 | 1.12, 2.23 | 0.01 |
| Smoking status | ||||||
| Never | — | — | — | — | ||
| Former | 1.17 | 0.88, 1.57 | 0.286 | 1.35 | 1.04, 1.75 | 0.026 |
| Current | 1.12 | 0.62, 1.92 | 0.681 | 1.68 | 1.04, 2.62 | 0.027 |
| Family income | ||||||
| <$10,000 | — | — | — | — | ||
| $10,000 to $25,000 | 0.72 | 0.46, 1.13 | 0.141 | 0.62 | 0.43, 0.89 | 0.009 |
| > $25,000 up to $50,000 | 1 | 0.66, 1.57 | 0.988 | 0.52 | 0.36, 0.76 | <0.001 |
| ≥ $50,000 | 0.61 | 0.35, 1.04 | 0.066 | 0.44 | 0.28, 0.69 | <0.001 |
| Spirituality/religion | ||||||
| Not important | — | — | — | — | ||
| Important | 0.7 | 0.39,1.37 | 0.26 | 1.2 | 0.64, 2.48 | 0.601 |
| Site | ||||||
| Memphis | — | — | — | — | ||
| Pittsburgh | 0.68 | 0.51, 0.90 | 0.007 | 0.98 | 0.76, 1.25 | 0.841 |
| BMI | ||||||
| <25 | — | — | — | — | ||
| 25–29.9 | 0.8 | 0.58, 1.11 | 0.179 | 0.95 | 0.72, 1.25 | 0.706 |
| >30 | 0.88 | 0.61, 1.26 | 0.493 | 0.77 | 0.55, 1.08 | 0.136 |
| Avg sitting systolic BP, z-score | 0.85 | 0.73, 0.98 | 0.026 | 0.96 | 0.85, 1.09 | 0.529 |
| Avg sitting diastolic BP, z-score | 0.97 | 0.85, 1.12 | 0.698 | 1.12 | 0.99, 1.26 | 0.076 |
| Self-reported health | ||||||
| Excellent/very good | — | — | — | — | ||
| Good | 1.23 | 0.90, 1.68 | 0.187 | 1.15 | 0.87, 1.52 | 0.332 |
| Fair/poor | 1.38 | 0.93, 2.01 | 0.101 | 1.8 | 1.30, 2.49 | <0.001 |
| Daytime sleepiness | 1.22 | 0.82, 1.78 | 0.314 | 1.1 | 0.76, 1.55 | 0.615 |
| Depressive symptoms | 1.45 | 0.96, 2.12 | 0.067 | 1.18 | 0.80, 1.70 | 0.374 |
| Anxiety symptoms | 1.35 | 1.01, 1.80 | 0.038 | 0.91 | 0.70, 1.19 | 0.499 |
| Hit head hard enough to faint | 1.95 | 1.30, 2.85 | <0.001 | 1.07 | 0.69, 1.59 | 0.76 |
| Parkinson’s disease | 1.82 | 0.28, 6.94 | 0.443 | 3.36 | 0.89, 10.7 | 0.049 |
| Cognitive impairment | 0.83 | 0.50, 1.30 | 0.433 | 3.86 | 2.82, 5.24 | <0.001 |
| Pneumonia, preceding 6 months | 1.39 | 0.22, 5.09 | 0.663 | 0.47 | 0.03, 2.37 | 0.47 |
| MI/heart disease | 0.94 | 0.66, 1.30 | 0.708 | 0.88 | 0.64, 1.18 | 0.4 |
| Cerebrovascular disease | 1.59 | 1.02, 2.40 | 0.033 | 1.03 | 0.65, 1.55 | 0.909 |
| Hypertension | 0.93 | 0.70, 1.23 | 0.608 | 0.82 | 0.64, 1.05 | 0.113 |
| Diabetes mellitus | 1.24 | 0.86, 1.76 | 0.227 | 1.18 | 0.85, 1.61 | 0.318 |
Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval; MI, myocardial infarction OR, odds ratio.
TABLE 3.
Multivariable logistic regression models of variables associated with subjective olfactory dysfunction
| Subjective olfactory dysfunction (n = 221) - odds ratio (95% confidence interval), p-value | |||||
|---|---|---|---|---|---|
| Characteristic | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
| Gender | |||||
| Male | — | — | — | — | — |
| Female | 1.12 (0.84, 1.48), 0.443 | 1.14 (0.86, 1.51), 0.380 | 1.09 (0.82, 1.46), 0.554 | 1.19 (0.87, 1.61), 0.276 | 1.23 (0.89, 1.70), 0.207 |
| Race | |||||
| White | — | — | — | — | — |
| Black | 0.99 (0.74, 1.32), 0.939 | 1.04 (0.76, 1.42), 0.790 | 0.99 (0.71, 1.37), 0.959 | 1.00 (0.71, 1.39), 0.994 | 1.13 (0.79, 1.61), 0.499 |
| Age | |||||
| 70–74 | — | — | — | — | — |
| 75–79 | 1.14 (0.85, 1.55), 0.376 | 1.15 (0.85, 1.55), 0.375 | 1.14 (0.84, 1.54), 0.393 | 1.15 (0.85, 1.56), 0.366 | 1.16 (0.85, 1.58), 0.360 |
| 80–84 | 1.21 (0.75, 1.88), 0.424 | 1.20 (0.75, 1.88), 0.430 | 1.22 (0.76, 1.91), 0.401 | 1.24 (0.77, 1.94), 0.367 | 1.34 (0.82, 2.13), 0.229 |
| Education | |||||
| Less than high school | — | — | — | — | |
| High school graduate | 0.99 (0.66, 1.50), 0.956 | 1.00 (0.66, 1.53), 0.990 | 1.00 (0.66, 1.54), 0.988 | 1.09 (0.70, 1.72), 0.706 | |
| Some college | 1.23 (0.84, 1.84), 0.292 | 1.31 (0.87, 2.01), 0.204 | 1.30 (0.86, 2.01), 0.222 | 1.33 (0.85, 2.11), 0.214 | |
| Family income | |||||
| <$10,000 | — | — | — | ||
| $10,000 to $25,000 | 0.70 (0.44, 1.13), 0.136 | 0.70 (0.44, 1.13), 0.135 | 0.72 (0.45, 1.18), 0.184 | ||
| $25,000 to $50,000 | 0.93 (0.57, 1.55), 0.774 | 0.93 (0.56, 1.54), 0.762 | 1.06 (0.64, 1.79), 0.834 | ||
| ≥ $50,000 | 0.53 (0.28, 0.98), 0.042 | 0.52 (0.28, 0.97), 0.041 | 0.58 (0.31, 1.10), 0.096 | ||
| Alcohol consumption | |||||
| Never | — | — | |||
| Current | 1.21 (0.84, 1.75), 0.318 | 1.29 (0.89, 1.88), 0.190 | |||
| Former | 1.25 (0.81, 1.92), 0.316 | 1.29 (0.83, 2.01), 0.258 | |||
| Smoking status | |||||
| Never | — | — | |||
| Former | 1.16 (0.85, 1.60), 0.346 | 1.14 (0.83, 1.59), 0.416 | |||
| Current | 1.09 (0.60, 1.90), 0.761 | 0.99 (0.53, 1.74), 0.971 | |||
| Site | |||||
| Memphis | — | ||||
| Pittsburgh | 0.66 (0.49, 0.89), 0.006 | ||||
| BMI | |||||
| <25 | — | ||||
| 25–29.9 | 0.82 (0.59, 1.15), 0.251 | ||||
| >30 | 0.88 (0.59, 1.30), 0.526 | ||||
| Systolic BP | 0.83 (0.70, 0.99), 0.042 | ||||
| Diastolic BP | 1.08 (0.92, 1.29), 0.344 | ||||
| Self-reported health | |||||
| Excellent/very good | |||||
| Good | 1.21 (0.88, 1.68), 0.240 | ||||
| Fair/poor | 1.32 (0.85, 2.03), 0.214 | ||||
| Depressive symptoms | 1.28 (0.81, 1.98), 0.275 | ||||
| Anxiety symptoms | 1.18 (0.85, 1.61), 0.316 | ||||
| Hit head hard | 1.97 (1.29, 2.94), 0.001 | ||||
| Parkinson’s disease | 1.40 (0.21, 5.67), 0.674 | ||||
| Cognitive impairment | 0.73 (0.41, 1.25), 0.271 | ||||
| Hypertension | 0.92 (0.68, 1.25), 0.602 | ||||
| Cerebrovascular disease | 1.54 (0.97, 2.36), 0.057 | ||||
Note: Model 1: Gender + race + age; Model 2: Model 1 + education; Model 3: Model 2 + socioeconomic status; Model 4: Model 3 + lifestyle/behavioral factors; Model 5: Model 4 + comorbidities.
Abbreviations: BMI, body mass index; BP, blood pressure.
When analyzing factors associated with oOD, female gender, increased educational attainment, and higher family income were all associated with lower odds of oOD on unadjusted analysis. Black race, status as a former drinker as opposed to never drinking alcohol, and being a current or former smoker as opposed to never smoking were all associated with higher odds of oOD on unadjusted analysis (Table 2). On stepwise multivariable logistic regression, we found that individuals identifying as Black race had higher odds of oOD (OR:1.41, 95% CI: 1.02–1.95), while females and those reporting family incomes ≥$50,000 had lower odds of oOD (OR:0.46, CI:0.34–0.62; OR:0.52, CI:0.29–0.93) even after considering all confounding variables (Table 4). Education, smoking status, and alcohol status did not maintain significance after accounting for all covariates. We also found that higher diastolic blood pressure (OR:1.19; CI:1.03–1.39) and cognitive impairment (OR:2.73; CI:1.86–3.98) were both associated with oOD. Liberalizing the definition of oOD to include both anosmia and hyposmia (CC-SIT score ≤8) expanded the prevalence of OD to 30.7% within this population. When comparing those with hyposmia with individuals with intact olfactory function, we found that female gender and higher income were associated with lower odds of hyposmia, while education and race did not maintain significance after accounting for all covariates (Table S1 and Table S2).
TABLE 4.
Multivariable logistic regression models of variables associated with objective olfactory dysfunction
| Objective olfactory dysfunction (n = 290) - odds ratio (95% confidence interval), p-value | |||||
|---|---|---|---|---|---|
| Characteristic | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
| Gender | |||||
| Male | — | — | — | — | — |
| Female | 0.42 (0.32, 0.55), <0.001 | 0.43 (0.33, 0.56), <0.001 | 0.39 (0.30, 0.52), <0.001 | 0.41 (0.31, 0.55), <0.001 | 0.46 (0.34, 0.62), <0.001 |
| Race | |||||
| White | — | — | — | — | — |
| Black | 2.13 (1.65, 2.76), <0.001 | 1.85 (1.40, 2.44), <0.001 | 1.64 (1.22, 2.20), <0.001 | 1.61 (1.20, 2.17), <0.001 | 1.41 (1.02, 1.95), <0.001 |
| Age | |||||
| 70–74 | — | — | — | — | — |
| 75–79 | 1.59 (1.20, 2.11), 0.001 | 1.60 (1.20, 2.13), 0.001 | 1.56 (1.18, 2.09), 0.002 | 1.58 (1.19, 2.11), 0.002 | 1.54 (1.15, 2.07), 0.004 |
| 80–84 | 2.70 (1.83, 3.96), <0.001 | 2.75 (1.86, 4.03), <0.001 | 2.72 (1.84, 4.00), <0.001 | 2.77 (1.87, 4.09), <0.001 | 2.57 (1.71, 3.84), <0.001 |
| Education | |||||
| Less than high school | — | — | — | — | |
| High school graduate | 0.62 (0.44, 0.87), 0.006 | 0.68 (0.48, 0.97), 0.031 | 0.68 (0.48, 0.97), 0.032 | 0.90 (0.62, 1.33), 0.604 | |
| Some college | 0.63 (0.46, 0.88), 0.006 | 0.75 (0.52, 1.07), 0.108 | 0.76 (0.53, 1.09), 0.132 | 1.03 (0.69, 1.54), 0.891 | |
| Family income | |||||
| <$10,000 | — | — | — | ||
| $10,000 to $25,000 | 0.64 (0.43, 0.95), 0.025 | 0.65 (0.44, 0.97), 0.032 | 0.70 (0.47, 1.06), 0.086 | ||
| $25,000 to $50,000 | 0.58 (0.37, 0.91), 0.018 | 0.59 (0.38, 0.93), 0.023 | 0.64 (0.40, 1.03), 0.062 | ||
| ≥ $50,000 | 0.47 (0.27, 0.81), 0.007 | 0.48 (0.28, 0.84), 0.011 | 0.52 (0.29, 0.93), 0.027 | ||
| Alcohol consumption | |||||
| Never | — | — | |||
| Current | 1.03 (0.74, 1.46), 0.852 | 1.05 (0.74, 1.50), 0.769 | |||
| Former | 1.15 (0.79, 1.68), 0.456 | 1.20 (0.81, 1.77), 0.362 | |||
| Smoking status | |||||
| Never | — | — | |||
| Former | 1.08 (0.80, 1.44), 0.624 | 1.12 (0.83, 1.52), 0.445 | |||
| Current | 1.31 (0.79, 2.11), 0.279 | 1.13 (0.67, 1.87), 0.633 | |||
| Site | |||||
| Memphis | — | ||||
| Pittsburgh | 1.10 (0.84, 1.45), 0.480 | ||||
| BMI | |||||
| <25 | — | ||||
| 25–29.9 | 0.94 (0.70, 1.28), 0.701 | ||||
| >30 | 0.79 (0.54, 1.14), 0.205 | ||||
| Systolic BP | 0.87 (0.74, 1.02), 0.101 | ||||
| Diastolic BP | 1.19 (1.03, 1.39), 0.023 | ||||
| Self-reported health | |||||
| Excellent/very good | |||||
| Good | 1.13 (0.84, 1.53), 0.421 | ||||
| Fair/poor | 1.42 (0.97, 2.07), 0.073 | ||||
| Depressive symptoms | 0.87 (0.56, 1.33), 0.533 | ||||
| Anxiety symptoms | 0.94 (0.69, 1.27), 0.690 | ||||
| Hit head hard | 1.01 (0.64, 1.55), 0.963 | ||||
| Parkinson’s disease | 3.38 (0.87, 11.4), 0.057 | ||||
| Cognitive impairment | 2.73 (1.86, 3.98), <0.001 | ||||
| Hypertension | 0.77 (0.58, 1.01), 0.063 | ||||
| Cerebrovascular disease | 0.92 (0.57, 1.45), 0.735 | ||||
Note: Model 1: Gender + race + age; Model 2: Model 1 + education; Model 3: Model 2 + socioeconomic status; Model 4: Model 3 + lifestyle/behavioral factors; Model 5: Model 4 + comorbidities.
Abbreviations: BMI, body mass index; BP, blood pressure.
When comparing sOD with oOD within the entire cohort, we found a self-reported perception of sOD had a low sensitivity (23.1%) and PPV (30.3%) for positive oOD, while having a better specificity (92.0%) and NPV (88.8%) for negative oOD (Table 5). We explored whether specific SDoH may mediate the relationship between sOD and oOD, and if the association between the two would be strengthened within certain subgroups. We found that self-perception of olfactory function had greater sensitivity in females than males (30.8% vs. 18.8%, p = 0.030), while the specificity significantly varied depending on family income (p = 0.033). We also found that the NPV of sOD for oOD varied between subgroups of gender, race, education, and income, ranging from as low as 81.8% to as high as 93.1% depending on which population we were testing in (Table 5). Evaluating the entire cohort, we found that our objective measure of OD was associated with sOD, as participants who endorsed sOD performed worse on the CC-SIT test than those who reported normal olfaction (CC-SIT score = 8 vs. 10, p < 0.001). We also found that CC-SIT scores were associated with sOD when divided into subgroups based on gender, race, and education; however, it was not related to sOD in those with family incomes below $10k (Figure 1A–D). Lastly, when looking at only patients without sOD, we found that males, Black race, those with less than high school educational attainment, and those with family income <$10,000 all had significantly lower CC-SIT scores than other subgroups within gender, race, education, and family income (Figure 2A–D).
TABLE 5.
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value of self-reported olfactory dysfunction (subjective OD) for objective OD
| Sensitivity | p | Specificity | p | PPV | p | NPV | p | |
|---|---|---|---|---|---|---|---|---|
| Entire cohort | 23.1% | – | 92.0% | – | 30.3% | – | 88.8% | – |
| Gender | 0.030 | 0.513 | 0.219 | <0.001 | ||||
| Male | 18.8% | 92.5% | 35.0% | 84.2% | ||||
| Female | 30.8% | 91.6% | 26.4% | 93.1% | ||||
| Race | 0.479 | 0.684 | 0.133 | <0.001 | ||||
| White | 25.2% | 91.8% | 26.4% | 91.3% | ||||
| Black | 21.0% | 92.4% | 37.0% | 84.6% | ||||
| Education | 0.115 | 0.635 | 0.539 | <0.001 | ||||
| Less than high school | 16.8% | 92.7% | 36.4% | 81.8% | ||||
| High school graduate | 22.2% | 92.5% | 26.5% | 90.7% | ||||
| Post-secondary schooling | 28.9% | 91.4% | 30.3% | 90.9% | ||||
| Family income | 0.429 | 0.033 | 0.723 | <0.001 | ||||
| <$10,000 | 18.9% | 90.0% | 32.3% | 81.4% | ||||
| $10,000 to $25,000 | 19.8% | 93.0% | 30.6% | 88.1% | ||||
| $25,000 to $50,000 | 27.6% | 90.2% | 27.0% | 90.5% | ||||
| ≥ $50,000 | 28.2% | 94.8% | 37.9% | 92.1% |
Note: p-value from sample test of equality of proportions.
FIGURE 1.
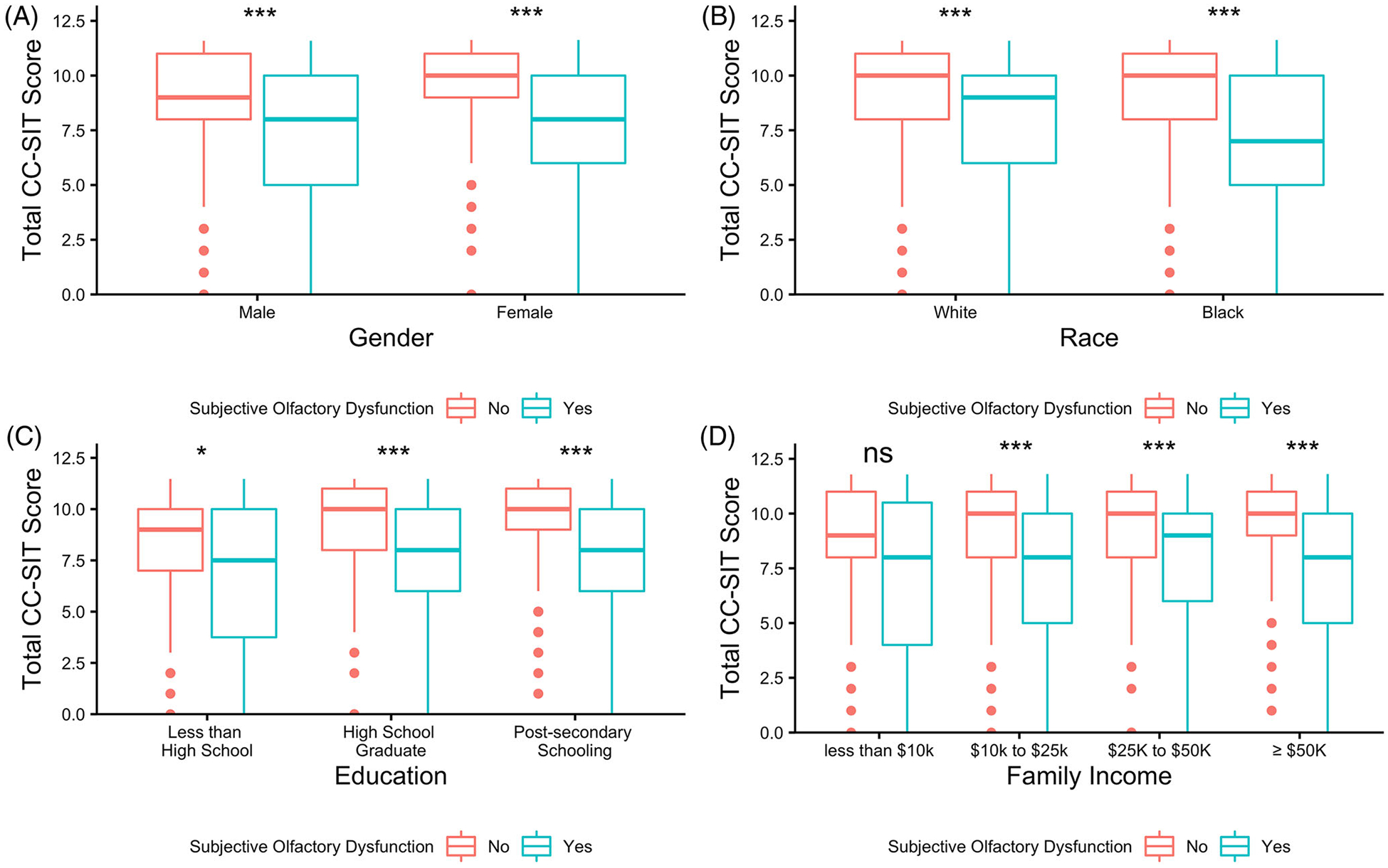
Comparison of cross-cultural smell identification test (CC-SIT) scores between those with and without subjective olfactory dysfunction, within subgroups of gender (A), race (B), education (C), and family income (D). p-value from Wilcoxon rank sum test. ns = not significant (p ≥ 0.05). *< 0.05, **< 0.01, ***< 0.001
FIGURE 2.
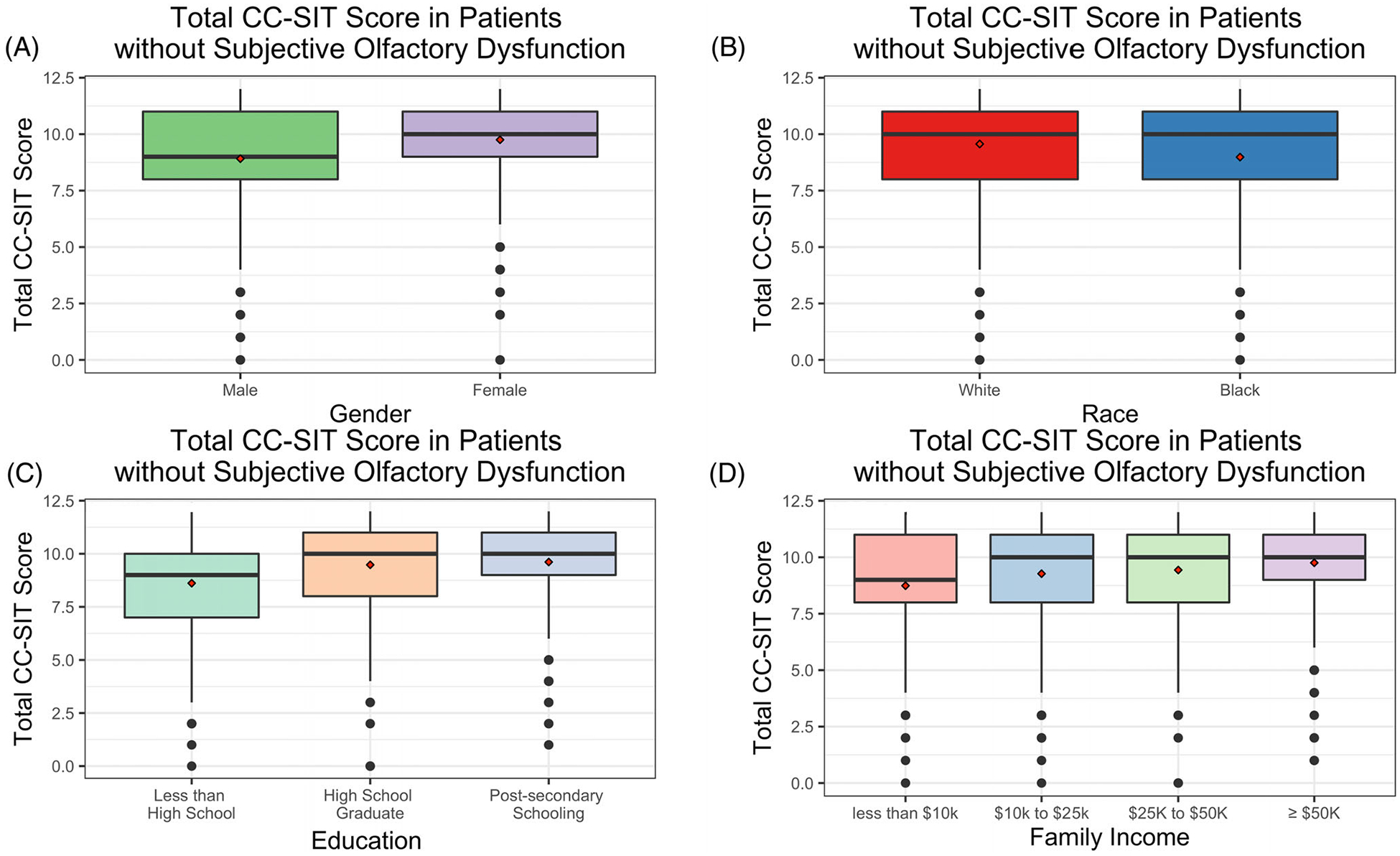
Among those without subjective olfactory dysfunction, comparison of cross-cultural smell identification test (CC-SIT) scores within subgroups of gender (A), race (B), education (C), and family income (D). Red diamond represents the mean. Panels A and B: Wilcoxon p < 0.001 for both. Panels C and D: Kruskal–Wallis p < 0.001 for both
4 |. DISCUSSION
Utilizing data from 2219 participants in the HealthABC study, we found that 13% of individuals had objective absence of smell or anosmia (oOD) and 18% had objectively reduced sense of smell (hyposmia), compared with only 10%, who self-reported subjective olfactory impairment (sOD). When assessing risk factors for oOD, gender, race, and income remained associated with oOD even after accounting for comorbidities, while education, smoking status, and alcohol status were associated with oOD on univariable analysis but not after accounting for comorbidities. When looking at risk factors for sOD, we did not find that gender, race, education, income, smoking status, or alcohol status were associated with sOD on univariate analysis or on multivariate analysis after considering comorbidities. While participants who self-reported sOD performed worse on the CC-SIT than those who reported normal olfaction, self-reports showed a very poor sensitivity (23.1%) for identifying individuals with oOD. The NPV of sOD for oOD varied significantly between subgroups of gender, race, education, and family income. In addition, when looking at individuals who did not self-report OD, males, individuals of Black race, individuals who did not complete high school, and individuals with lower family income all had lower CC-SIT scores than females, White race, high school graduates, and higher income families, respectively. These results necessitate further efforts to evaluate factors propagating these disparities and compel physicians to consider differences in self-reporting when interpreting a negative response to a subjective olfactory screen.
Evaluating our results using the previously reported thematic construct for SDoH, such as racial/ethnic disparities, SES, education status, occupational exposures, and lifestyle/behavioral factors, we found that race, similar to results from other studies, was an important predictor of OD. Individuals reporting a Black racial identity had greater odds of oOD than those reporting White race, even when accounting for comorbidities (OR: 1.41, p = 0.037).16–18 The complexity of race as a construct challenges this interpretation, where rather than a true biologic or genetic relation to OD, race is more likely a proxy for different occupational exposures, life experience, and conditions that we are not capturing in our limited dataset. For example, prior studies have found that Black race, people at lower educational levels, and people at lower income levels were significantly more likely to live within a mile of a polluting facility—an exposure that can damage olfactory capabilities.33 Unlike in prior studies, we did not find that race was associated with elevated rates of self-reported poor olfaction.15,17
We find mixed results when exploring the role of SES in OD. In multivariate analysis, those with family incomes >$50,000 were approximately half as likely to have oOD than those with families with family income <$10,000 (OR = 0.52, p = 0.027). Similarly, in an analysis of the Beaver Dam Offspring Study, the authors found that an income of >$50,000/year was associated with less OD (OR = 0.48).34 We did not, however, find an association between SES and sOD. Patients with lower SES may be more at risk for true OD due to a myriad of factors, such as living in areas with greater air pollution or having reduced access to healthcare that may otherwise delay the deterioration of their condition.33,35
Similarly, we found the relationship between educational attainment and OD to be inconsistent. There was no apparent association between education and sOD in this population. After adjusting for covariates, those with less than a high school degree were not more likely to have oOD than those with a high school degree. Importantly, the HealthABC utilizes the CC-SIT, a psychophysical olfactory assessment using only odorant identification to stratify olfactory performance. This contrasts with other tests which assess olfactory threshold, the minimum concentration at which an odor can be detected by participants, and discrimination, the ability of a participant to discern between two odorants. Previous studies have found that odor identification test are more susceptible to cognitive and neurologic impairments, with patients with Alzheimer’s disease and Parkinson’s disease found to do worse on odor identification than odor threshold tests.36,37 When interpreting the effects of race, education, and SES on oOD in our study, responses and perceptions of odorants used in the CC-SIT may be attributable to differences in cultural norms or life experience, thus leading to lower odor identification rather than worse underlying disease.38 This highlights the importance of continuing to develop objective measures of disease that perform consistently across all populations.
Our findings that a large proportion of individuals with objective olfactory deficits did not self-report a diminished sense of smell is in-line with a recent review which found that OD prevalence was significantly greater when measured by objective olfactory assessments compared with subjective measures (28.8% vs. 9.5%, p < 0.001).2 Other studies have found discordance between self-assessed measures of chemosensation and objective findings. In an analysis of the National Social Life, Health, and Aging Project, the authors found that 74.2% of older adults with measured OD did not recognize it.39 In a recent analysis of the National Health and Nutrition Examination Survey, the authors found that increased age was associated with underreporting of olfactory impairment, though the analysis did not reveal how other demographic characteristics affect the sensitivity of self-report.40 In another recent analysis of the Sister Study, a study of middle-aged and older women, the authors found that self-reports showed a low sensitivity (22.6%) for poor olfaction.15 Here, we found that the sensitivity of sOD for oOD is 23.1%, with a sensitivity as low as 16.8% in those with less than a high school degree. Moreover, we found that the NPV of sOD for oOD—whether a patient reports no OD and truly has no olfactory issues—varied significantly based on gender, race, education, and income. In total, these results suggest that physicians should raise awareness of OD across all patient populations while strongly considering the use of objective olfactory testing to evaluate patients where there exists suspicion for OD.
In contrast to prior studies, in the HealthABC population we did not find that gender, race, education, SES, or behaviors such as smoking or alcohol consumption were associated with differences in sOD, though we did find differences in self-awareness and reporting accuracy depending on gender, race, education, and SES.15,17 Direct comparison across studies is difficult due to differences in study population and survey formulation; for example, the HealthABC asks participants whether they have smell or taste problems, and not if they have a decrease in smell as other surveys do.15 Overall, we postulate that the accuracy of self-report of OD for objective disease is poor, as people interpret the question differently and disparities may exist in awareness and education of sensory deficits across various populations.
Our analysis has several limitations. Due to the study design of the dataset using an affirmative answer to the question: “Do you suffer from smell and/or taste problems?” to identify sOD, there remains ambiguity as to whether the participant’s response is driven by olfactory or gustatory dysfunction. Our use of this question was based on prior analyses of this dataset,16 and even when using this question encompassing both smell and taste, self-report of chemosensory dysfunction is lower than that uncovered by psychophysical testing. Moreover, prior studies have found that individuals often self-report codysfunction of these two senses, and individuals who do not report taste dysfunction rarely report OD.41 Secondly, the variables provided within the HealthABC dataset, while comprehensive, may not fully account for all confounders. For example, the database lacks questions about vocation, thereby limiting our analysis into the association between OD and occupational exposure risks, a previously identified category of SDoH.14 Additionally, the dataset does not include diseases, such as chronic rhinosinusitis (CRS), and environmental exposures, such as lead, that have previously been found to be associated with OD and might exist as underlying confounding factors; this is especially true considering previously published studies that have found disparities in care for CRS and that lower SES was associated with a higher CRS rate.42–44 Lastly, the HealthABC cohort comprised only elderly adults above the age of 70(median age: 75, IQR:73–78) limiting its generalizability to younger patients. Nonetheless, the known association between OD and morbidity and mortality in elderly adults necessitates further investigation into a population with a disproportionate burden of noninfectious OD. A recent meta-analysis found that in studies with a mean age >55 years, the prevalence of OD was 34.5%, compared with an OD prevalence rate of 7.5% in studies with a mean age <55 years.2 The association between specific SDoH and OD remains underexplored across all ages due to the hurdles to widespread olfactory testing in population studies, and we believe this study provides valuable insight into the disparities in olfactory function within older adults.
5 |. CONCLUSION
While our study reports no association between SDoH and awareness of sOD, certain SDoH factors may impact olfactory function when measured using the CC-SIT psychophysical olfactory test (oOD). We also found that the accuracy of self-reports of sOD for oOD varies based on gender, race, education, and income. As OD is associated with morbidity and mortality, it is imperative that we make an effort to further evaluate factors propagating these disparities while providing more equitable care for our patients.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by National Institute on Aging (NIA) contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant R01-AG028050; and NINR grant R01-NR012459. This research was funded in part by the Intramural Research Program of the National Institutes of Health (NIH), NIA. Additional support (JBO) provided by grant K23DC019678 from the National Institute on Deafness and Other Communication Disorders and the NIH, with support provided through grant UL1TR001873 (Clinical and Translational Science Award Program at Columbia University Irving Medical Center) from the National Center for Advancing Translational Sciences, NIH.
The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders did not play any role in study design, data collection/analysis, decision to publish, or manuscript preparation.
Footnotes
CONFLICT OF INTEREST
None to disclose.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
Accepted for oral presentation at the 2022 American Rhinologic Society Annual Meeting, Philadelphia, PA, September 9–10, 2022.
REFERENCES
- 1.Cain WS, Stevens JC. Uniformity of olfactory loss in aging. Ann N Y Acad Sci. 1989;561:29–38. [DOI] [PubMed] [Google Scholar]
- 2.Desiato VM, Levy DA, Byun YJ, Nguyen SA, Soler ZM, Schlosser RJ. The prevalence of olfactory dysfunction in the general population: a systematic review and meta-analysis. Am J Rhinol Allergy. 2021;35(2):195–205. [DOI] [PubMed] [Google Scholar]
- 3.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA, J Am Med Assoc. 2002;288(18):2307–2312. [DOI] [PubMed] [Google Scholar]
- 4.Santos DV, Reiter ER, DiNardo LJ, Costanzo RM. Hazardous events associated with impaired olfactory function. Arch Otolaryngol Head Neck Surg. 2004;130(3):317–319. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman SS, Graham BG. Taste and smell perception affect appetite and immunity in the elderly. Eur J Clin Nutr. 2000;54:S54–63. [DOI] [PubMed] [Google Scholar]
- 6.Schubert CR, Fischer ME, Pinto AA, et al. Sensory impairments and risk of mortality in older adults. J Gerontol A Biol Sci Med Sci. 2017;72(5):710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu B, Luo Z, Pinto JM, et al. Relationship between poor olfaction and mortality among community-dwelling older adults: a cohort study. Ann Intern Med. 2019;170(10):673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R, Cruickshanks KJ. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc. 2008;56(8):1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64(7):802–808. [DOI] [PubMed] [Google Scholar]
- 10.Yuan Y, Luo Z, Li C, Simonsick E, Shiroma E, Chen H, Olfaction and physical function in older adults: findings from health ABC. 530–531. [Google Scholar]
- 11.Healthy People 2030. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Accessed https://health.gov/healthypeople/objectives-and-data/social-determinants-health
- 12.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2021;44(1):258–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav. 2010;51:S28–40. [DOI] [PubMed] [Google Scholar]
- 14.James J, Tsvik AM, Chung SY, Usseglio J, Gudis DA, Overdevest JB. Association between social determinants of health and olfactory function: a scoping review. Int Forum Allergy Rhinol. 2021;11(10):1472–1493. [DOI] [PubMed] [Google Scholar]
- 15.Cao Z, Yang A, D’Aloisio AA, et al. Assessment of self-reported sense of smell, objective testing, and associated factors in middle-aged and older women. JAMA Otolaryngol Head Neck Surg. 2022;148(5):408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong J, Pinto JM, Guo X, et al. The prevalence of anosmia and associated factors among U.S. black and white older adults. J Gerontol A Biol Sci Med Sci. 2017;72(8):1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noel J, Habib AR, Thamboo A, Patel ZM. Variables associated with olfactory disorders in adults: a U.S. population-based analysis. World J Otorhinolaryngol Head Neck Surg. 2017;3(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinto JM, Schumm LP, Wroblewski KE, Kern DW, McClintock MK. Racial disparities in olfactory loss among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2014;69(3):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philpott CM, Wolstenholme CR, Goodenough PC, Clark A, Murty GE. Comparison of subjective perception with objective measurement of olfaction. Otolaryngol Head Neck Surg. 2006;134(3):488–490. [DOI] [PubMed] [Google Scholar]
- 20.Helzner EP, Cauley JA, Pratt SR, et al. Race and sex differences in age-related hearing loss: the health, aging and body composition study. J Am Geriatr Soc. 2005;53(12):2119–2127. [DOI] [PubMed] [Google Scholar]
- 21.Rooks RN, Simonsick EM, Miles T, et al. The association of race and socioeconomic status with cardiovascular disease indicators among older adults in the health, aging, and body composition study. J Gerontol: Series B. 2002;57(4):S247–S256. [DOI] [PubMed] [Google Scholar]
- 22.Doty RL, Marcus A, Lee WW. Development of the 12-item cross-cultural smell identification test (CC-SIT). Laryngoscope. 1996;106(3):353–356. Pt 1. [DOI] [PubMed] [Google Scholar]
- 23.Jumaily JS, Fayad C, Mardirossian V, Singh A, Stram J, Spiegel J. Preoperative incidence of olfactory dysfunction in nasal surgery patients. Otolaryngol Head Neck Surg. 2012;147(1):157–160. [DOI] [PubMed] [Google Scholar]
- 24.Felix C, Chahine LM, Hengenius J, et al. Diffusion tensor imaging of the olfactory system in older adults with and without hyposmia. Front Aging Neurosci. 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 26.Doty RL. Olfaction in Parkinson’s disease and related disorders. Neurobiol Dis. 2012;46(3):527–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan Y, Luo Z, Li C, et al. Poor olfaction and pneumonia hospitalisation among community-dwelling older adults: a cohort study. Lancet Healthy Longev. 2021;2(5):e275–e282. [DOI] [PubMed] [Google Scholar]
- 28.Siegel JK, Wroblewski KE, McClintock MK, Pinto JM. Olfactory dysfunction persists after smoking cessation and signals increased cardiovascular risk. Int Forum Allergy Rhinol. 2019;9(9):977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wehling E, Naess H, Wollschlaeger D, et al. Olfactory dysfunction in chronic stroke patients. BMC Neurology. 2015;15(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SJ, Windon MJ, Lin SY. The association between diabetes and olfactory impairment in adults: a systematic review and meta-analysis. Laryngoscope Investig Otolaryngol. 2019;4(5):465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6(3):241–252. [Google Scholar]
- 32.van Buuren S, Groothuis-Oudshoorn K. Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 33.Mohai P, Lantz PM, Morenoff J, House JS, Mero RP. Racial and socioeconomic disparities in residential proximity to polluting industrial facilities: evidence from the Americans’ Changing Lives Study. Am J Public Health. 2009;99:S649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Olfactory impairment in an adult population: the beaver dam offspring study. Chem Senses. 2012;37(4):325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kullgren JT, Galbraith AA, Hinrichsen VL, et al. Health care use and decision making among lower-income families in high-deductible health plans. Arch Intern Med. 2010;170(21):1918–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koss E, Weiffenbach JM, Haxby JV, Friedland RP. Olfactory detection and identification performance are dissociated in early Alzheimer’s disease. Neurology. 1988;38(8):1228–1232. [DOI] [PubMed] [Google Scholar]
- 37.Lötsch J, Reichmann H, Hummel T. Different odor tests contribute differently to the evaluation of olfactory loss. Chem Senses. 2007;33(1):17–21. [DOI] [PubMed] [Google Scholar]
- 38.Seo HS, Jeon KJ, Hummel T, Min BC. Influences of olfactory impairment on depression, cognitive performance, and quality of life in Korean elderly. Eur Arch Otorhinolaryngol. 2009;266(11):1739–1745. [DOI] [PubMed] [Google Scholar]
- 39.Adams DR, Wroblewski KE, Kern DW, et al. Factors associated with inaccurate self-reporting of olfactory dysfunction in older US adults. Chem Senses. 2016;42(3):223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jang SS, Choi JS, Kim JH, Kim N, Ference EH. Discordance between subjective and objective measures of smell and taste in US adults. Otolaryngol Head Neck Surg. 2022;166(3):572–579. [DOI] [PubMed] [Google Scholar]
- 41.Bhattacharyya N, Kepnes LJ. Contemporary assessment of the prevalence of smell and taste problems in adults. Laryngoscope. 2015;125(5):1102–1106. [DOI] [PubMed] [Google Scholar]
- 42.Banglawala SM, Oyer SL, Lohia S, Psaltis AJ, Soler ZM, Schlosser RJ. Olfactory outcomes in chronic rhinosinusitis with nasal polyposis after medical treatments: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2014;4(12): 986–994. [DOI] [PubMed] [Google Scholar]
- 43.Grashow R, Sparrow D, Hu H, Weisskopf MG. Cumulative lead exposure is associated with reduced olfactory recognition performance in elderly men: the normative aging study. Neurotoxicology. 2015;49:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuelson MB, Chandra RK, Turner JH, Russell PT, Francis DO. The relationship between social determinants of health and utilization of tertiary rhinology care. Am J Rhinol Allergy. 2017;31(6):376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


