Abstract
Background
Brugada phenocopy (BrP) is a condition that induces reversible Brugada-like electrocardiographic (ECG) changes in patients without true Brugada syndrome. We present two cases of fulminant eosinophilic myocarditis that showed Type 1 Brugada ECG changes in the early phase of the clinical course.
Case summary
Case 1 was a 76-year-old man who developed fulminant eosinophilic myocarditis with ventricular tachycardia while hospitalized for heart failure. Case 2 was a 60-year-old man who presented with cardiogenic shock and was diagnosed with fulminant eosinophilic myocarditis. Both patients showed a Type 1 Brugada ECG at onset, and their ventricular function was greatly reduced. Regarding mechanical circulatory support, Case 1 was treated with venous-arterial extracorporeal membrane oxygenation and intra-aortic balloon pumping. Case 2 had venous-arterial extracorporeal membrane oxygenation and Impella CP insertion. Steroid therapy was introduced in both cases. In Case 1, the Type 1 Brugada ECG took 7 days to improve. Left ventricular function improved with time but right heart function was poor and right heart enlargement remained. In Case 2, the Type 1 Brugada ECG improved on the second day, and left and right heart function improved over time.
Discussion
We report two cases of fulminant eosinophilic myocarditis with Brugada-like ECG and severe right heart dysfunction. BrP in acute myocarditis may be an indicator of right heart failure and an important ECG marker in determining the indication for mechanical circulatory support and improvement of right heart function.
Keywords: Brugada phenocopy, Fulminant eosinophilic myocarditis, Mechanical circulatory support, Right heart function, Case series
Learning points.
Brugada phenocopy (BrP) in acute myocarditis may be an indicator of right heart dysfunction, and early mechanical circulatory support (MCS) may be required.
Early induction of MCS and steroid therapy are important in fulminant eosinophilic myocarditis and may contribute to normalization of BrP.
Early normalization of BrP in acute myocarditis may be a useful indicator of improved right heart function.
Introduction
Brugada phenocopy (BrP) is characterized by reversible Brugada-like electrocardiographic (ECG) changes in individuals lacking the genetic basis for Brugada syndrome.1 Various environmental factors, such as pulmonary embolism, myocardial ischaemia, and electrolyte abnormalities, are potential triggers for BrP.2–4 Brugada-like ST-segment elevation abnormalities associated with myocarditis have also been reported.5,6
In the context of acute myocarditis, the use of mechanical circulatory support (MCS) devices, such as an intra-aortic balloon pump (IABP), the Impella, and venous-arterial extracorporeal membrane oxygenation (V-A ECMO), plays a pivotal role in managing left ventricular (LV) or right ventricular (RV) dysfunction. Acute fulminant myocarditis often causes biventricular failure, requiring LV and RV support. Therefore, early identification of the requirement for RV and LV support is critical in managing fulminant myocarditis. In this report, we present two cases of acute fulminant eosinophilic myocarditis with Type 1 Brugada ECG changes early in the clinical course. We also describe an interesting course of conflicting results regarding ECG and right heart function after acute treatment with MCS.
Summary figure
Case presentation
Patient 1
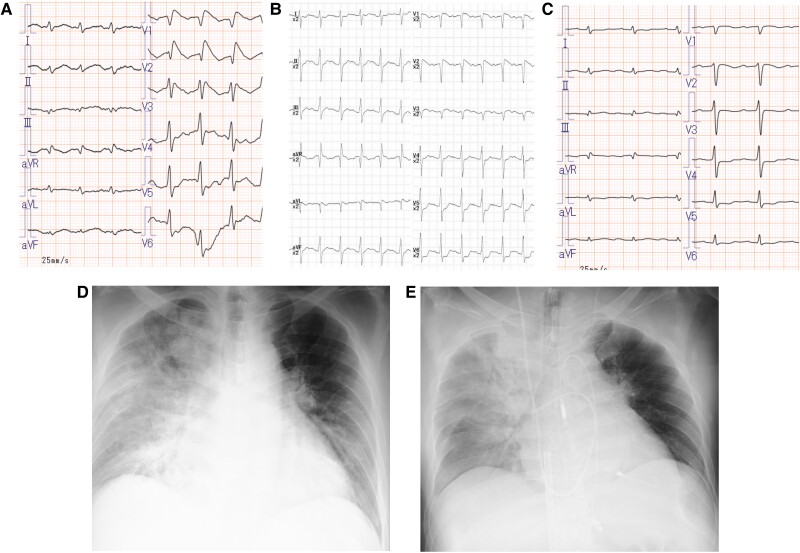
A 76-year-old man developed a fever of 37.8°C 8 days before admission and complained of general fatigue. Three days before admission, his fever had decreased, but dyspnoea on exertion was observed. The patient was diagnosed with congestive heart failure associated with atrial fibrillation at a primary care hospital and was referred to our hospital for admission. His medical history included treatment for hypertension and dyslipidaemia at a local hospital, and about 1 year ago, he was referred to our hospital for an abdominal aortic aneurysm and underwent endovascular aortic repair. Because coronary angiography prior to abdominal aortic aneurysm surgery showed a highly stenotic lesion in the proximal part of the right coronary artery, a percutaneous coronary intervention was performed 9 months ago. During that hospitalization, paroxysmal atrial fibrillation was observed. He had no history of syncope or family history of sudden cardiac death. On admission, his consciousness was clear, blood pressure was 157/92 mmHg, heart rate was irregular at 125 beats/minute, body temperature was 36.6°C, and oxygen saturation was 99% in room air. A physical examination showed mild oedema of the face and lower legs. An ECG showed atrial fibrillation (Figure 1A), and a chest X-ray showed cardiac enlargement with enhanced vascular shadows in the lung fields (Figure 1B). Laboratory results on arrival are shown in Table 1. Transthoracic echocardiography (TTE) showed preserved LV systolic function (see Supplementary material online, Video S1). The patient’s heart failure improved with intravenous furosemide, but on day 5, he suddenly developed haemodynamically stable ventricular tachycardia (Figure 2A) and underwent direct electrical cardioversion. Post-direct electrical cardioversion ECG showed atrial fibrillation and coved-type ST-segment elevation in leads V1–3 (Figure 2B). Considering the possibility of acute coronary syndrome, an emergency coronary angiography was performed. Emergency coronary angiography showed no considerable coronary artery stenosis, an IABP was inserted as MCS, and dobutamine and norepinephrine infusion was started because of low blood pressure. On day 6, because of the findings of prolonged hypotension and impaired peripheral circulation, the patient was intubated and V-A ECMO was started (Figure 2D). Laboratory data showed an elevated peripheral blood eosinophil count (white blood cell count: 9.3 × 103/µL [normal range: 3.3–8.6 × 103/µL], eosinophils: 16.6% [0.0%–10.0%]) and elevated myocardial enzymes (creatine kinase concentration: 271 U/L [59–248 U/L], creatine kinase-MB concentration: 64 IU/L [≤12 IU/L], high-sensitivity troponin T concentration: 7.23 ng/mL [≤0.014 ng/mL]), and a myocardial biopsy showed eosinophilic infiltration between myocardial fibres (Figure 3). These findings led to a diagnosis of fulminant eosinophilic myocarditis. Serum antinuclear antibody tests, parasite screening, and blood cultures were negative. TTE after V-A ECMO insertion showed oedematous changes in the LV wall and decreased RV contraction along with the LV (see Supplementary material online, Video S2). The pulmonary artery pulsatility index (PAPi) ([systolic pulmonary artery pressure−diastolic pulmonary artery pressure]/central venous pressure) was 0.69 (reference >1) after V-A ECMO insertion, which indicated RV failure. High-dose methylprednisolone was started at 1 g/day on days 6–8, and this was switched to prednisolone 40 mg/day on day 9 and then tapered to 30 mg/day on day 12. High-sensitivity troponin T concentrations were highest (29.36 ng/mL [≤0.014 ng/mL]) on day 6 and began to decrease from day 7. Creatine kinase concentration peaked at 799 U/L (59–248 U/L) on day 7. Intravenous immunoglobulin was administered at 2.0 g/kg on days 11–12 because of persistent dysfunction in both ventricles. The Brugada ECG showed improvement on day 12 (Figure 2C), and the PAPi improved to within the reference range on day 13. Prednisolone was tapered to 20 mg/day on day 15. Finally, the patient was weaned from V-A ECMO on day 17 and from IABP on day 24 and extubated. An angiotensin-converting enzyme inhibitor and beta blocker were initiated on day 25. Following rehabilitation, he was discharged on day 98. Prednisolone was tapered during hospitalization and the dose at discharge was 7.5 mg/day, which was tapered to a maintenance dose of 5 mg at the outpatient clinic. Follow-up TTE in the outpatient setting showed recovery of LV systolic function. However, right heart diameter enlargement and prolonged RV dysfunction (tricuspid annular plane systolic excursion: 17 mm [normal range: 23 ± 7 mm], RV fractional area change: 26% [49% ± 7%], and tissue Doppler peak velocity at the annulus: 7.3 cm/s [15 ± 5 cm/s]; Supplementary material online, Video S3) was observed.
Figure 1.
(A) Twelve-lead electrocardiogram on admission. (B) Chest X-ray on admission.
Table 1.
Laboratory data on admission
| A: Case 1 | B: Case 2 | Normal range | |
|---|---|---|---|
| White blood cell (×103/μL) | 9.9 | 9.4 | 3.3–8.6 |
| Basophil (%) | 0.1 | 0.4 | 0.0–3.0 |
| Eosinophil (%) | 7.1 | 0.0 | 0.0–10.0 |
| Neutrophil (%) | 77.6 | 80.1 | 50.0–70.0 |
| Lymphocytes (%) | 13.2 | 9.3 | 30.0–45.0 |
| Monocytes (%) | 2.0 | 10.2 | 2.0–8.0 |
| CRP (mg/dL) | 1.92 | 5.56 | 0.00–0.14 |
| AST (U/L) | 20 | 1124 | 13–30 |
| ALT (U/L) | 22 | 618 | 10–42 |
| BUN (mg/dL) | 22.7 | 70.4 | 8.0–20.0 |
| Creatinine (mg/dL) | 0.62 | 1.43 | 0.65–1.07 |
| CK (U/L) | 125 | 1228 | 59–248 |
| CK-MB (IU/L) | 11 | N/A | ≤12 |
| Qualitative-TnT | (+) | N/A | (−) |
| hs-TnT (ng/mL) | 0.745 | 4.611 | A ≤ 0.014/B < 0.100 |
| BNP (pg/mL) | 1305.5 | N/A | ≤18.4 |
| Lactate (mmol/L) | 1.3 | 7.5 | 0.44–1.78 |
CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; CK, creatine kinase; CK-MB, creatine kinase-MB; TnT, troponin T; hs-TnT, high-sensitive troponin T; BNP, brain natriuretic peptide.
Figure 2.
(A) Twelve-lead electrocardiogram (ECG) showing ventricular tachycardia (VT) on day 5 of hospitalization. (B) Twelve-lead ECG after electrical cardioversion for VT. (C) Twelve-lead ECG on day 12 of hospitalization with improved Type 1 Brugada ECG. (D) Chest X-ray after mechanical circulatory support with venous-arterial extracorporeal membrane oxygenation and intra-aortic balloon pumping.
Figure 3.
Right ventricular endomyocardial biopsy shows eosinophilic infiltration and degranulation (arrow).
Patient 2
A 60-year-old man developed a fever of 38.5°C 3 days before admission and complained of general fatigue. On the day of admission, he suddenly developed severe dyspnoea and visited our Emergency Department. He had a history of hypertension and Type 2 diabetes mellitus and was receiving medications at his local physician. The patient had no history of syncope or family history of sudden cardiac death. On admission, his consciousness was clear, blood pressure was 121/85 mmHg, heart rate was regular at 128 beats/minute, body temperature was 36.0°C, and oxygen saturation was 87% in room air. A physical examination showed sweating, weak pulse palpation, and respiratory distress. He was thought to have pre-shock vitals and received non-invasive positive pressure ventilation. An ECG showed sinus tachycardia with coved-type ST-segment elevation in leads V1–3 (Figure 4A). A chest X-ray showed cardiac enlargement with severe pulmonary congestion (Figure 4D). Laboratory results on arrival are shown in Table 1. Emergency coronary angiography showed no major stenosis in the coronary arteries, and an endocardial biopsy, with rapid testing, showed eosinophilic infiltration of the myocardium (Figure 5). These findings led to a diagnosis of eosinophilic myocarditis. Serum antinuclear antibody testing, parasite screening, and blood cultures were negative. There was also no recent drug exposure. An Impella CP (Abiomed Inc., Danvers, Massachusetts, USA) was inserted with a diagnosis of cardiogenic shock, and V-A ECMO was also started along with intubation owing to prolonged hypotension and signs of an impaired peripheral circulation and a low PAPi of 0.5 (reference >1) (Figure 4E). TTE after insertion of MCS showed considerably reduced LV and RV systolic function (see Supplementary material online, Video S4). Type 1 Brugada ECG changes were present at the start of V-A ECMO (Figure 4B) but normalized about 1 h after starting MCS (Figure 4C). After admission, an angiotensin receptor blocker and beta blocker were initiated, and high-dose methylprednisolone was started at 1 g/day for 3 days. The methylprednisolone was switched to prednisolone 40 mg/day on day 4, and then tapered to 30 mg/day on day 10 and further tapered by 10 mg weekly up to 10 mg/day. Creatine kinase and high-sensitivity troponin T concentrations were highest on admission and began to decrease on day 2, and the PAPi also improved to within the reference range on day 2. These findings resulted in successful weaning from V-A ECMO on day 9 and from the Impella CP on day 12. The patient was transferred to a rehabilitation hospital on day 61. The maintenance dose of prednisolone was 5 mg/day. Outpatient TTE follow-up confirmed improvement in LV and RV size and function (tricuspid annular plane systolic excursion: 22 mm [23 ± 7 mm], RV fractional area change: 34% [49% ± 7%], and peak velocity: 12 cm/s [15 ± 5 cm/s]; Supplementary material online, Video S5).
Figure 4.
(A) Twelve-lead electrocardiogram (ECG) on arrival at the hospital. (B) Twelve-lead ECG immediately after starting mechanical circulatory support (MCS) with venous-arterial extracorporeal membrane oxygenation (V-A ECMO) and the Impella CP (2 h after the visit). (C) One hour after starting MCS with V-A ECMO and the Impella CP, the 12-lead ECG showed improvement in Type 1 Brugada ECG changes. (D) Chest X-ray on arrival at the hospital. (E) Chest X-ray after MCS with V-A ECMO and the Impella CP.
Figure 5.
Right ventricular endomyocardial biopsy shows eosinophilic infiltration and degranulation (arrow).
Discussion
We experienced two cases of Type 1 Brugada ECG changes in the acute phase of fulminant eosinophilic myocarditis. Case 1 required 7 days for improvement of the Brugada ECG after MCS support with V-A ECMO and an IABP, and steroid therapy. However, Case 2 showed improvement of the Brugada ECG after a few hours of MCS support with V-A ECMO and the Impella CP, and steroid therapy. Case 1 had residual right heart dysfunction, whereas Case 2 had relatively preserved right heart function. To the best of our knowledge, this is the first report to document the course of Brugada ECG changes and their relationship with right heart function in acute myocarditis.
BrP is classified into six categories of metabolic conditions, mechanical compression, ischaemia, myocardial and pericardial disease, ECG modulation, and miscellaneous.7 Genetic screening and provocative testing are recommended to differentiate BrP from true Brugada syndrome.8 Both of our patients had no history of syncope or a family history of sudden cardiac death. However, neither genetic screening nor provocative testing was performed because of a low pre-test probability and lack of patient consent. Therefore, both patients were classified as Type 1 (BrP with typical Type 1 ‘coved’ Brugada ECG morphological characteristics), class B (highly suspected BrP, but not all mandatory criteria were complete) BrP according to the BrP morphological classification system.7
The appearance of Type 1 Brugada ECG changes in myocarditis may be induced by several factors, such as inflammation of the RV involving the RV outflow tract, local myocardial injury, and ischaemia of the RV wall.5 Pieroni et al. showed the pathological finding of inflammation in the RV outflow tract in patients with Brugada syndrome, with or without mutations,9 providing insight into the mechanism of Brugada-type ECG formation in myocarditis. Moreover, Brugada-type ECG changes induced by transient conus branch ischaemia have been reported.10 This finding suggests that RV ischaemia including the RV outflow tract region induced by hypotension, hypoxemia, or RV overload during the acute phase of myocarditis may cause Brugada-type ECG abnormalities. In particular, in Case 2, the Brugada-type ECG normalized immediately after the improvement of haemodynamics and oxygenation by aggressive RV unloading with V-A ECMO support. This finding suggests that not only RV inflammation but also RV ischaemia play an important role in BrP formation. Therefore, aggressive RV unloading with early introduction of V-A ECMO may have facilitated early normalization of the Brugada-type ECG. Interestingly, improvement in Brugada-type ECG changes preceded improvement in the PAPi, which is a measure of right heart function, in both cases. If Brugada-type ECG reflects an injury (e.g. inflammation or ischaemia of the RV), rapid improvement of Brugada-type ECG changes in myocarditis may be a more favourable prognostic ECG marker for RV function.
Two hypotheses were considered regarding the differential improvement in RV function during the chronic phase in both cases, other than the time course to the early introduction of V-A ECMO as described above. First, in Case 2, steroid therapy was initiated immediately, which may have rapidly alleviated the inflammation and avoided irreversible RV changes. The time from onset of cardiogenic shock to steroid pulse therapy was ∼29 h in Case 1 vs. 2.5 h in Case 2. The relatively rapid suppression of inflammation may have preserved RV function in Case 2. Second, a potent inhibitory effect of the Impella on inflammatory cell infiltration during LV unloading in myocarditis has been reported.11 Therefore, this ‘unloading’-dependent immunosuppressive mechanism, not present with the IABP, may have contributed to early haemodynamic stability and avoidance of irreversible inflammatory changes.
Conclusion
In our case series, we report two cases of BrP in fulminant eosinophilic myocarditis. Their clinical course suggests that, in fulminant eosinophilic myocarditis with BrP, early steroid administration and aggressive and rapid mechanical unloading by V-A ECMO and the Impella are important to restore not only left heart but also right heart function.
Supplementary Material
Acknowledgements
We thank Ellen Knapp, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Consent: The authors confirm that written consent for submission and publication of this case report, including images and associated text, has been obtained from the patient in line with COPE guidance.
Funding: None declared.
Contributor Information
Dai Kawauchi, Department of Cardiology, Tsuyama Chuo Hospital, 1756 Kawasaki, Tsuyama, Okayama 708-0841, Japan.
Kei Yunoki, Department of Cardiology, Tsuyama Chuo Hospital, 1756 Kawasaki, Tsuyama, Okayama 708-0841, Japan.
Tomohiro Yoshino, Department of Cardiology, Tsuyama Chuo Hospital, 1756 Kawasaki, Tsuyama, Okayama 708-0841, Japan.
Takefumi Oka, Department of Cardiology, Tsuyama Chuo Hospital, 1756 Kawasaki, Tsuyama, Okayama 708-0841, Japan.
Lead author biography

Dai Kawauchi graduated from the Faculty of Medicine, Jichi medical University in 2018. He is currently working as general cardiologist and interested in ischemic heart disease and percutaneous coronary intervention. He sometimes plays jazz-saxophone in front of his patients.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
References
- 1. de Oliveira Neto NR, de Oliveira WS, Mastrocola F, Sacilotto L. Brugada phenocopy: mechanisms, diagnosis, and implications. J Electrocardiol 2019;55:45–50. [DOI] [PubMed] [Google Scholar]
- 2. Elikowski W, Łazowski S, Fertała N, Zawodna-Marszałek M, Szczęśniewski P, Bolewski A, et al. Brugada phenocopy in pulmonary embolism—clinicopathological case study and literature review. Pol Merkur Lekarski 2022;50:378–383. [PubMed] [Google Scholar]
- 3. Xu G, Gottschalk BH, Pérez-Riera A, Barbosa-Barros R, Dendramis G, Carrizo AG, et al. Link between Brugada phenocopy and myocardial ischemia: results from the international registry on Brugada phenocopy. Pacing Clin Electrophysiol 2019;42:658–662. [DOI] [PubMed] [Google Scholar]
- 4. Genaro NR, Anselm DD, Cervino N, Estevez AO, Perona C, Villamil AM, et al. Brugada phenocopy clinical reproducibility demonstrated by recurrent hypokalemia. Ann Noninvasive Electrocardiol 2014;19:387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim YH, Lim HE, Kim SH, Pak HN, Ahn JC, Song WH, et al. Brugada-like ST-segment abnormalities associated with myocardial involvement of hematologic diseases. Pacing Clin Electrophysiol 2008;31:761–764. [DOI] [PubMed] [Google Scholar]
- 6. Bergamo D, Nelson C. Brugada pattern in adolescent with acute myocarditis due to SARS-CoV-2. J Am Coll Emerg Physicians Open 2022;3:e12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anselm DD, Gottschalk BH, Baranchuk A. Brugada phenocopies: consideration of morphologic criteria and early findings from an international registry. Can J Cardiol 2014;30:1511–1515. [DOI] [PubMed] [Google Scholar]
- 8. Gottschalk B, Anselm DD, Baranchuk A. Brugada phenocopy: morphological classification and importance of provocative testing. Ann Noninvasive Electrocardiol 2014;19:604–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pieroni M, Notarstefano P, Oliva A, Campuzano O, Santangeli P, Coll M, et al. Electroanatomic and pathologic right ventricular outflow tract abnormalities in patients with brugada syndrome. J Am Coll Cardiol 2018;72:2747–2757. [DOI] [PubMed] [Google Scholar]
- 10. Yamaki M, Sato N, Myojo T, Nishiura T, Nishimura M, Nakamura H, et al. Possible contribution of ischemia of the conus branch to induction or augmentation of Brugada type electrocardiographic changes in patients with coronary artery disease. Int Heart J 2010;51:68–71. [DOI] [PubMed] [Google Scholar]
- 11. Tschöpe C, Van Linthout S, Klein O, Mairinger T, Krackhardt F, Potapov EV, et al. Mechanical unloading by fulminant myocarditis: LV-IMPELLA, ECMELLA, BI-PELLA, and PROPELLA concepts. J Cardiovasc Transl Res 2019;12:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.