Abstract
Patients with LCHADD develop progressive chorioretinopathy with vision loss over time. To date, no data on the impact of vision loss on patient vision-specific activities of daily living or quality of life have been reported. We used validated ophthalmic patient-reported outcome measures (PROMs) to compare the impact of patient-perceived visual function to visual acuity and an ophthalmologist-graded stage of LCHADD chorioretinopathy. There was a strong correlation between the patient-reported visual function scores, visual acuity and the ophthalmologist's assigned stage. Adult patients reported lower driving and mental health scores compared to other visual subscales in the VFQ-25. Both children and their parents report a similar impact of their child's eye condition to their quality of life and worry about their vision. These validated PROMs captured functional vision in a group of 40 patients with LCHADD/TFPD that closely correlated with visual acuity and ophthalmologist-graded visual function.
Keywords: LCHADD, LCHAD deficiency, TFPD, TFP deficiency, Chorioretinopathy, Patient-reported outcomes
1. Introduction
Mitochondrial trifunctional protein (TFP) deficiency (TFPD, OMIM #609015) and long-chain 3-hydroxy acyl-CoA dehydrogenase deficiency (LCHADD; OMIM# 609016) are the result of different inherited autosomal recessive genetic defects that affect the enzymatic activity of the mitochondrial trifunctional protein, a protein complex within the inner mitochondrial membrane involved in catalyzing steps of the beta-oxidation pathway [1]. TFP is a multi-subunit enzyme with 2 alpha (TFPα) and 2 beta (TFPβ) subunits forming a holoenzyme; LCHADD is due to a the presence of a specific variant in TFPα (p.E510Q) that decreases LCHAD activity while TFPD is due to other mutations in either TFPα or TFPβ that result in a loss of all 3 enzymatic functions [[2], [3], [4], [5], [6]]. Unlike other inherited deficiencies of the beta-oxidation pathway, the development of chorioretinopathy is a complication common to LCHADD, but more rare in TFPD [1,[7], [8], [9]].
There has been an acknowledgment of the importance of using PROMs in ophthalmic research to measure how much patients are affected by changes in visual function which cannot be captured by clinical tests alone [10]. However, there is a current lack of data on the impact of progressive vision loss in patients with LCHADD or TFPD on their vision-related quality of life (QoL) and activities of daily living. The purpose of this analysis was to collect PRO scores on visual function and vision-related QoL from patients with LCHADD/TFPD and examine whether these agree with their visual acuity and ophthalmologist-graded scoring of visual function based on clinical testing.
2. Methods
2.1. Study design
Forty subjects with confirmed diagnosis of LCHADD or TFPD (53 % male, 78 % white, non-Hispanic), participating in our Natural History of LCHADD Retinopathy study (University of Pittsburgh IRB# PRO19040142), were asked to complete either the Pediatric Eye Questionnaire (PedEyeQ), ages 0–17 years, or the National Eye Institute Visual Functioning Questionnaire – 25 (NEI VFQ-25), ages 18+ years, during their initial study visit. Questionnaires were administered electronically using the REDCap survey tool with a requirement to answer each question in order to complete the questionnaire. For adults and older children, the questionnaires were self-administered or, if vision was so impaired that they could not read the text on the screen, with help from a member of the research team or study companion by reading the questions and response items to them. For younger children, depending on reading ability and comprehension, questionnaires were self-administered or completed with help from a member of the research team or the parent by reading the questions and response items to them.
2.2. Ethics approval and consent to participate
This study was approved by the University of Pittsburgh (IRB# PRO19040142) on August 22, 2019. The OHSU IRB deferred to the University of Pittsburgh for oversight, approved October 25, 2019. All study procedures were performed in compliance with institutional and national laws and guidelines for experiments involving humans and the ethical principles of the Declaration of Helsinki. Written informed consent and assent to participate in the study was obtained from all subjects and/or their legal guardians.
2.3. Questionnaires
2.3.1. PedEyeQ
The PedEyeQ is a validated, Rasch-calibrated survey used to assess the impact of eye conditions in children 0–17 years on the their eye-related quality of life (QoL) and visual function [11]. The PedEyeQ consists of a child questionnaire to be completed by children ages 5–17 years, and a proxy and parent questionnaire to be completed by the parent or legal guardian. The child questionnaire has two formats depending on the age of the child (5–11 years or 12–17 years) and includes four separately scored domains: functional vision, bothered by eyes/vision, social and frustration/worry. The proxy questionnaire has the parent/guardian answer questions about their child's QoL and visual function. There are 3 formats depending on the age of the child (0–4 years, 5–11 years, or 12–17 years). All age formats include similar separately scored domains of functional vision, bothered by eyes/vision, and social, with additional scored domains for ages 5–11 and 12–17 years in frustration/worry and eyecare. The parent questionnaire asks the parent/guardian questions about their own experience with their child's eye condition. The same format is administered to all ages 0–17 years and is scored into 4 separate domains including: impact on parent and family, worry about child's eye condition, worry about self-perception and interactions, and worry about functional vision. Each question on the PedEyeQ utilizes a 3-point frequency scale for responses of “Never”, “Sometimes” or “All the time”. For the PedEyeQ, Rasch calibrated scores for each domain were obtained using the PedEyeQ look-up table (http://www.pedig.net/) and reported on a scale from 0 to 100, with 0 being the worst measure of QoL and visual function and 100 being the best.
2.3.2. NEI VFQ-25
The NEI VFQ-25 is a validated survey used to assess the impact of eye-related symptoms and disability of persons with chronic eye conditions on visual function, emotional well-being, social function and health [12]. Responses from the survey are assigned a numerical value which are then re-coded on a scale from 0 to 100 with 0 representing the worst functioning and 100 the best. Items within each of the 12 sub-scales (general health, general vision, ocular pain, near activities, distance activities, social functioning, mental health, role difficulties, dependency, driving, color vision and peripheral vision) are then averaged to obtain a sub-scale score. A composite score is calculated by taking the average of the vision-related sub-scale scores, excluding the general health score.
Clinical eye testing was performed at the same initial study visit during which participants and their parents completed the vision-related PROs. LCHADD chorioretinopathy staging for each eye was evaluated as described by Wongchaisuwat et al [13]. and was used in our correlation analysis. Chorioretinopathy stage between each eye was equivalent across all subjects. Visual acuity testing was performed as described by Gillingham et al. [8] and expressed as the logarithmic minimal angle of resolution (LogMAR). The eye with lower LogMAR (i.e. better visual acuity) was used in our correlation analysis.
2.4. Statistical analysis
Statistical analysis was performed with Prism Software (Version 10.2.2, GraphPad, La Jolla, CA). Correlation between ophthalmologist-graded chorioretinopathy stage or visual acuity (LogMAR) and VFQ-25 composite score was analyzed using Pearson correlation coefficient. Correlation between ophthalmologist-graded chorioretinopathy stage or visual acuity (LogMAR) and PedEyeQ score in the functional vision domain (ages 5–17 years) was analyzed using Spearman rank correlation for non-normal data. Differences in the domains of functional vision, bothered vision, social and frustration/worry between child and proxy questionnaires was analyzed by a non-parametric test for non-normal data, using the Wilcoxon matched-pairs signed rank test. Results are reported as mean ± standard deviation. For all analyses, a p < .05 was considered statistically significant.
3. Results/discussion
Fourteen subjects ages 18–36 years were given the VFQ-25 and 26 subjects, ages 2–17 years, and their parent/guardian were given the PedEyeQ. The completion rate of each questionnaire is shown in Table 1.
Table 1.
Questionnaire completion rate.
| VFQ-25 (n = 14), No. (%) | PedEyeQ Ages 0–4 y (n = 4), No. (%) |
PedEyeQ Ages 5–11 y (n = 10), No. (%) |
PedEyeQ Ages 12–17 y (n = 12), No. (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| Proxy | Parent | Child | Proxy | Parent | Child | Proxy | Parent | |
| 14 (100) | 3 (75) | 4 (100) | 10 (100) | 9 (90) | 10 (100) | 12 (100) | 12 (100) | 12 (100) |
y = years.
Vision-related PRO scores for each subject were correlated with visual acuity (LogMAR) and LCHADD chorioretinopathy stage using the VFQ-25 composite score for subjects 18+ years and the PedEyeQ Child functional vision score for subjects aged 5–17 years. Individual PRO scores, LogMAR and chorioretinopathy staging are listed in Table 2.
Table 2.
Individual PROM scores, visual acuity and chorioretinopathy stage by subject age.
| Age (y) | Sex | Genotype |
VFQ-25 Composite Score | PedEyeQ Child - Functional Vision Score | Visual Acuity (LogMAR) | Chorioretinopathy Stage | |
|---|---|---|---|---|---|---|---|
| Allele 1 | Allele 2 | ||||||
| 2 | M | c.1528G > C | c.1654G > C | NA | NA | NA | 1 |
| 3 | M | c.1528G > C | c.274_278del | NA | NA | NA | 3A |
| 3 | M | c.1528G > C | c.919-2A > G | NA | NA | NA | 2A |
| 4 | F | c.1528G > C | c.274_278delTCATC | NA | NA | NA | 2A |
| 7 | F | c.1528G > C | c.1916-1919dup | NA | 94.99 | 0.00 | 2A |
| 7 | M | c.1025 T > C | c.1493A > G | NA | 100.00 | −0.10 | 2A |
| 7 | M | c.1528G > C | c.180 + 3A > G | NA | 100.00 | −0.10 | 1 |
| 7 | M | c.1528G > C | c.2225_2228dup | NA | 100.00 | 0.20 | 3A |
| 9 | M | c.1528G > C | c.1036C > T | NA | 64.98 | 0.40 | 3A |
| 9 | M | c.1528G > C | c.180 + 3A > G | NA | 94.99 | −0.10 | 1 |
| 10 | F | c.1528G > C | c.1654G > C | NA | 64.98 | 0.00 | 1 |
| 11 | M | c.1528G > C | c.180 + 3A > G | NA | 94.99 | −0.22 | 1 |
| 11 | F | c.1528G > C | c.1085 + 5G > C | NA | 94.99 | 0.00 | 2A |
| 11 | F | c.1528G > C | c.180 + 3A > G | NA | 100.00 | −0.22 | 1 |
| 12 | F | c.1528G > C | EX11del | NA | 79.98 | 0.00 | 2B |
| 12 | M | c.1528G > C | c.1528G > C | NA | 49.98 | 0.10 | 2B |
| 13 | F | c.1528G > C | c.315-1G > A | NA | 100.00 | −0.10 | 2A |
| 13 | M | c.1528G > C | c.274_278del | NA | 95.00 | −0.10 | 2A |
| 15 | F | c.1528G > C | c.703C > T | NA | 100.00 | −0.10 | 1 |
| 15 | M | c.1528G > C | c.1152dup | NA | 84.99 | 0.00 | 2A |
| 16 | F | c.1528G > C | c.467G > A | NA | 9.99 | 0.50 | 4 |
| 16 | M | c.1528G > C | c.2000 + 1G > T | NA | 95.00 | −0.22 | 2A |
| 17 | M | c.1528G > C | c.703C > T | NA | 100.00 | −0.10 | 1 |
| 17 | M | c.1528G > C | c.1528G > C | NA | 100.00 | 0.00 | 2B |
| 17 | M | c.1528G > C | 1 bp deletion A2059 | NA | 34.99 | 1.00 | 3B |
| 17 | F | c.1528G > C | c.1620 + 2_1620 + 6del | NA | 15.03 | 0.80 | 4 |
| 18 | F | c.1528G > C | c.1678C > T | 74.89 | NA | 0.20 | 3A |
| 18 | M | c.1528G > C | c.1528G > C | 93.83 | NA | 0.10 | 2B |
| 18 | F | c.1528G > C | c.1528G > C | 84.55 | NA | 0.20 | 3B |
| 18 | M | c.1528G > C | c.1528G > C | 98.67 | NA | −0.10 | 3A |
| 21 | M | c.1528G > C | c.1528G > C | 45.04 | NA | 0.70 | 4 |
| 24 | F | c.1528G > C | c.1528G > C | 56.50 | NA | 0.00 | 3B |
| 24 | F | c.1528G > C | c.1528G > C | 30.25 | NA | 0.20 | 4 |
| 26 | F | c.901G > A* | c.1390-23A > G* | 89.77 | NA | −0.10 | 1 |
| 27 | M | c.1528G > C | c.1528G > C | 45.67 | NA | 0.30 | 4 |
| 28 | F | c.1528G > C | c.1132C > T | 58.98 | NA | 0.10 | 3B |
| 29 | F | c.1528G > C | c.479_482delinsAATA | 63.98 | NA | 0.20 | 4 |
| 30 | M | c.1528G > C | EX2_4del | 33.45 | NA | 1.60 | 4 |
| 31 | F | c.901G > A* | c.1390-23A > G* | 93.37 | NA | −0.10 | 1 |
| 36 | F | c.1528G > C | c.1528G > C | 68.14 | NA | 0.30 | 4 |
M = Male; F = Female; y = years; NA = Not applicable. All genetic variants were identified in theTFPα gene HADHA transcript NM_000182.5 except those marked with an asterisk (*) that were identified in theTFPβ gene HADHB transcript NM_000183.2.
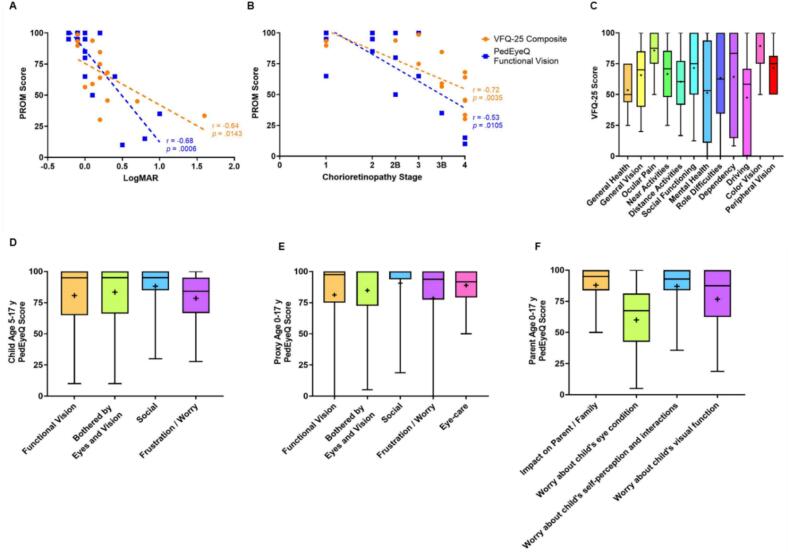
Higher LogMAR, or worse visual acuity, and more advanced chorioretinopathy stage strongly correlated with lower vision-related PRO scores (Fig. 1A and B), suggesting strong agreement between the patients' perceptions of declining visual function with the advancement of their eye disease and the measured functional vision as scored by the ophthalmologist.
Fig. 1.
Correlation analysis and PROM scores in adults and children with LCHAD/TFP deficiency. A) Pearson correlation of VFQ-25 composite score (N = 14) and Spearman correlation of PedEyeQ Child Functional Vision score (ages 5–17 years, N = 22) with visual acuity (LogMAR) B) Pearson correlation of VFQ-25 composite score (N = 14) and Spearman correlation of PedEyeQ Child Functional Vision score (ages 5–17 years, N = 22) with ophthalmologist-graded chorioretinopathy stage C) VFQ-25 subscale scores D) PedEyeQ Child questionnaire scores for ages 5–17 years E) PedEyeQ Proxy questionnaire scores for ages 0–17 years F) PedEyeQ Parent questionnaire scores for ages 0–17 years. Boxes represent 1st quartile, median, and 3rd quartile range; whiskers represent the minimum and maximum range; + denotes mean score.
LCHADD chorioretinopathy is a progressive disease that affects patients in early adulthood and is without current treatment. Similar to results of qualitative interviews and vision-related PRO scores of patients with other inherited retinal diseases, such as retinitis pigmentosa [14] or Stargardt disease [15,16], LCHADD/TFPD patients indicate suffering not only functional declines in their vision but also psychological difficulties as a result of their vision loss. The lowest mean score related to vision outcomes from adults was in vision-related driving difficulties (47.51 ± 36.44, Fig. 1C). In other interviews of adults suffering from vision loss, the inability to drive has been connected to feelings of isolation, less independence, and an inability to participate in work or social activities [17,18], which has negative impacts on vision-specific health-related QoL [19]. For adults, this is closely followed by low scores in mental health (51.34 ± 39.69, Fig. 1C) and from children in frustration/worry (78.48 ± 20.36, Fig. 1D).
Parents of children with LCHADD/TFPD share a similar awareness of their child's vision-related QoL and visual function to that of their child, with no significant differences measured between child and proxy scores in functional vision, bothered by eyes and vision, social or frustration/worry (Fig. 1D & E). These parents also experience their own difficulties with their child's condition reporting the lowest scores in worry about their child's eye condition (59.99 ± 25.56) and worry about their child's visual function (76.68 ± 24.91) (Fig. 1F).
4. Conclusions
Ophthalmic PRO data has not previously been captured in patients with LCHADD chorioretinopathy. As such, the extent to which gradual changes in their progressive vision loss impacts their vision-related QoL and functional vision hasn't been reported. Although selection bias in our cohort of patients may have been present in that participants needed to be willing and have the time to travel to the study site for multiple days of testing, the strength of this study includes having a large cohort of LCHADD/TFPD patients across a broad age range. In this study, patient-reported visual function from the VFQ-25 in adults and PedEyeQ in children shows strong agreement with their visual acuity and ophthalmologist-graded chorioretinopathy stage. This indicates that the effect of vision loss experienced in each chorioretinopathy stage results in a noticeable loss of functional vision in these patients and that any preservation of vision that may be achieved in future treatment trials could have a measurable positive effect on the lives of these patients.
Funding
This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health & Human Development (R01HD095968) and with support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1TR002369 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Author statements
All authors have read and approved the final draft of this manuscript.
CRediT authorship contribution statement
Ashley N. Gregor: Writing – review & editing, Writing – original draft, Visualization, Project administration, Investigation, Formal analysis. Danielle Black: Writing – review & editing, Project administration, Investigation, Formal analysis. Nida Wongchaisuwat: Writing – review & editing, Methodology, Formal analysis. Mark E. Pennesi: Writing – review & editing, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Melanie B. Gillingham: Writing – review & editing, Writing – original draft, Visualization, Supervision, Methodology, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
Ashley Gregor, Danielle Black and Nida Wongchaisuwat have no competing interests. Melanie B Gillingham has received speaker honorium from Ultragenyx Pharmaceutical Inc., Vitaflow, and Nutricia, and received research grant/funds from Nestle Bioscience and Reneo Pharmaceutical. Mark E Pennesi has received consulting fees from 4D Molecular Therapeutics, Adverum, Arrowhead Pharmaceuticals, AGTC, Aldebraran, Ascidian, Atsena, Astellas, BlueRock-Opsis, Coave, ClarisBio, Dompe, Editas, Edigene, Endogena, FFB, Ingel Therapeutics J-Cyte, Janssen, KalaTherapeutics, Kiora, Nacuity Pharmaceuticals, Ocugen, Ora, ProQR, Prime Editing, PTC Therapeutics, PYC Therapeutics, Ray Therapeutics, Rejuvitas, RestoreVision, RegenexBio, Sparing Vision, SpliceBio, Spotlight Therapeutics, Thea and Theranexus. He has received clinical trial support from AGTC, Biogen, Editas, FFB, ProQR, Reneuron. He has received fees as part of the Data Safety Montitoring Board (DSMB) for Akous, Gensight. He has equity in the following companies: Aldebaran, Atsena, Endogena, EnterX, Ingel Therapeutics, Kiora, Nacuity Pharmaceuticals, Ocugen, and ZipBio.
Acknowledgements
The authors thank the participants and their families for their time and effort completing the clinical evaluations and PROMs.
Data availability
The datasets generated and analyzed during the current study are not publicly available as this study is ongoing, but are available from the corresponding author upon reasonable request.
References
- 1.Fletcher A.L., Pennesi M.E., Harding C.O., Weleber R.G., Gillingham M.B. Observations regarding retinopathy in mitochondrial trifunctional protein deficiencies. Mol. Genet. Metab. 2012;106:18–24. doi: 10.1016/j.ymgme.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IJlst L., Ruiter J.P., Hoovers J.M., Jakobs M.E., Wanders R.J. Common missense mutation G1528C in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Characterization and expression of the mutant protein, mutation analysis on genomic DNA and chromosomal localization of the mitochondrial trifunctional protein alpha subunit gene. J. Clin. Invest. 1996;98:1028–1033. doi: 10.1172/JCI118863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IJlst L., Wanders R.J., Ushikubo S., Kamijo T., Hashimoto T. Molecular basis of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: identification of the major disease-causing mutation in the alpha-subunit of the mitochondrial trifunctional protein. Biochim. Biophys. Acta. 1994;1215:347–350. doi: 10.1016/0005-2760(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 4.Orii K.E., Aoyama T., Souri M., Jiang L.L., Orii K.O., Hayashi S., Yamaguchi S., Kondo N., Orii T., Hashimoto T. Formation of the enzyme complex in mitochondria is required for function of trifunctional beta-oxidation protein. Biochem. Biophys. Res. Commun. 1996;219:773–777. doi: 10.1006/bbrc.1996.0309. [DOI] [PubMed] [Google Scholar]
- 5.Spiekerkoetter U., Khuchua Z., Yue Z., Bennett M.J., Strauss A.W. General mitochondrial trifunctional protein (TFP) deficiency as a result of either alpha- or beta-subunit mutations exhibits similar phenotypes because mutations in either subunit alter TFP complex expression and subunit turnover. Pediatr. Res. 2004;55:190–196. doi: 10.1203/01.PDR.0000103931.80055.06. [DOI] [PubMed] [Google Scholar]
- 6.Ushikubo S., Aoyama T., Kamijo T., Wanders R.J., Rinaldo P., Vockley J., Hashimoto T. Molecular characterization of mitochondrial trifunctional protein deficiency: formation of the enzyme complex is important for stabilization of both alpha- and beta-subunits. Am. J. Hum. Genet. 1996;58:979–988. [PMC free article] [PubMed] [Google Scholar]
- 7.Rinaldo P., Matern D., Bennett M.J. Fatty acid oxidation disorders. Annu. Rev. Physiol. 2002;64:477–502. doi: 10.1146/annurev.physiol.64.082201.154705. [DOI] [PubMed] [Google Scholar]
- 8.Gillingham M.B., Choi D., Gregor A., Wongchaisuwat N., Black D., Scanga H.L., Nischal K.K., Sahel J.A., Arnold G., Vockley J., Harding C.O., Pennesi M.E. Early diagnosis and treatment by newborn screening (NBS) or family history is associated with improved visual outcomes for long-chain 3-hydroxyacylCoA dehydrogenase deficiency (LCHADD) chorioretinopathy. J. Inherit. Metab. Dis. 2024;47(4):746–756. doi: 10.1002/jimd.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boese E.A., Jain N., Jia Y., Schlechter C.L., Harding C.O., Gao S.S., Patel R.C., Huang D., Weleber R.G., Gillingham M.B., Pennesi M.E. Characterization of chorioretinopathy associated with mitochondrial trifunctional protein disorders: long-term follow-up of 21 cases. Ophthalmology. 2016;123:2183–2195. doi: 10.1016/j.ophtha.2016.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denniston A.K., Kyte D., Calvert M., Burr J.M. An introduction to patient-reported outcome measures in ophthalmic research. Eye (Lond.) 2014;28:637–645. doi: 10.1038/eye.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatt S.R., Leske D.A., Castaneda Y.S., Wernimont S.M., Liebermann L., Cheng-Patel C.S., Birch E.E., Holmes J.M. Development of pediatric eye questionnaires for children with eye conditions. Am. J. Ophthalmol. 2019;200:201–217. doi: 10.1016/j.ajo.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangione C.M., Lee P.P., Gutierrez P.R., Spritzer K., Berry S., Hays R.D., I. National eye Institute visual function questionnaire field test, development of the 25-item National eye Institute visual function questionnaire. Arch. Ophthalmol. 2001;119:1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 13.Wongchaisuwat N., Gillingham M.B., Yang P., Everett L., Gregor A., Harding C.O., Sahel J.A., Nischal K.K., Scanga H.L., Black D., Vockley J., Arnold G., Pennesi M.E. A proposal for an updated staging system for LCHADD retinopathy. Ophthalmic Genet. 2024;45:140–146. doi: 10.1080/13816810.2024.2303682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prem Senthil M., Khadka J., Pesudovs K. Assessment of patient-reported outcomes in retinal diseases: a systematic review. Surv. Ophthalmol. 2017;62:546–582. doi: 10.1016/j.survophthal.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Roborel de Climens A., Tugaut B., Dias Barbosa C., Buggage R., Brun-Strang C. Living with Stargardt disease: insights from patients and their parents. Ophthalmic Genet. 2021;42:150–160. doi: 10.1080/13816810.2020.1855663. [DOI] [PubMed] [Google Scholar]
- 16.Miedziak A.I., Perski T., Andrews P.P., Donoso L.A. Stargardt's macular dystrophy--a patient's perspective. Optometry. 2000;71:165–176. [PubMed] [Google Scholar]
- 17.Lange R., Kumagai A., Weiss S., Zaffke K.B., Day S., Wicker D., Howson A., Jayasundera K.T., Smolinski L., Hedlich C., Lee P.P., Massof R.W., Stelmack J.A., Carlozzi N.E., Ehrlich J.R. Vision-related quality of life in adults with severe peripheral vision loss: a qualitative interview study. J. Patient Rep. Outcomes. 2021;5:7. doi: 10.1186/s41687-020-00281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coyne K.S., Margolis M.K., Kennedy-Martin T., Baker T.M., Klein R., Paul M.D., Revicki D.A. The impact of diabetic retinopathy: perspectives from patient focus groups. Fam. Pract. 2004;21:447–453. doi: 10.1093/fampra/cmh417. [DOI] [PubMed] [Google Scholar]
- 19.DeCarlo D.K., Scilley K., Wells J., Owsley C. Driving habits and health-related quality of life in patients with age-related maculopathy. Optom. Vis. Sci. 2003;80:207–213. doi: 10.1097/00006324-200303000-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available as this study is ongoing, but are available from the corresponding author upon reasonable request.