Abstract
Acute myeloid leukemia (AML) with RUNX1::CBFA2T2 fusion is rare with largely unknown clinicopathological features and genomic characterization. We present one such case of AML transformed from JAK2 V617F mutated primary myelofibrosis and review the literature on this topic. The immunophenotype and the landscape of cooperative gene alterations in AML with RUNX1::CBFA2T2 resemble those of AML with RUNX1::RUNX1T1, including expression of CD19, cooperative gene alterations in signaling pathway (JAK2), epigenetic/chromatin and cell cycle regulation (TET2, SMC3, and CDKN2A/B), and additional chromosomal abnormalities (trisomies 8 and 15). This case study provides insights into the pathogenesis of this rare subtype of AML.
Keywords: acute myeloid leukemia, JAK2, primary myelofibrosis, RUNX1::CBFA2T2, RUNX1::RUNX1T1
1. INTRODUCTION
Acute myeloid leukemia (AML) with RUNX1::RUNX1T1 fusion, resulting from chromosomal translocation t(8;21)(q22;q22.1), is one of the core‐binding factor (CBF) leukemias. This subtype constitutes 1%–5% of AML, characterized by blasts expressing CD19/CD34, presence of additional cytogenetic abnormalities, and a favorable clinical outcome [1, 2, 3]. The RUNX1::RUNX1T1 fusion forms a co‐repressor complex, blocking the RUNX1‐mediated genes involved in regulation of myeloid differentiation [4]. Cooperative gene mutations, such as KIT, NRAS, FLT3‐ITD, ASXL1, and SMC3, are thought to be required in the RUNX1::RUNX1T1 fusion‐induced leukemogenesis [5, 6].
RUNX1T1 is one of the myeloid translocation gene (MTG) family members, which consists of RUNX1T1 (also known as MTG8, CBFA2T1, or ETO), CBFA2T2 (MTGR1), and CBFA2T3 (MTG16 or RUNX1T3) [7]. The high homology between CBFA2T2, RUNX1T1, and CBFA2T3 suggests that when fused with RUNX1, all three chimeric genes may have similar effects on leukemogenesis [8]. Several reports have shown similarity of AML with t(16;21)(q24;q22)/RUNX1::CBFA2T3 to AML with RUNX1::RUNX1T1 with regard to morphology, immunophenotype, gene expression profiling, and response to therapy [9, 10].
RUNX1::CBFA2T2 is a rare gene rearrangement in AML, with only four patients having been previously reported [8, 11–13]. The clinicopathological and molecular features of AML harboring RUNX1::CBFA2T2 are largely unknown. This case report is the first study to characterize the morphology, immunophenotype, cooperative gene alterations, and clonal evolution of this rare subtype of AML.
2. METHOD
Clinical data were retrieved from the electronic medical records. Flow cytometric immunophenotyping, morphologic evaluation, genetic karyotyping, and comprehensive pan‐cancer next‐generation sequencing (NGS) were performed, as described previously [14].
3. CASE DESCRIPTIONS
A 62‐year‐old female with a history of breast cancer (treated with lumpectomy, anastrozole, radiation, and adjuvant chemotherapy) was diagnosed with primary myelofibrosis (PMF, MF1‐2, prefibrotic, 1% blasts) 5 years later, with mutations in JAK2 (p.V617F, variant allele frequency [VAF%] 51%), IDH2 (p.R140, 48%), and SRSF2 (p.P95R, 47%), and normal cytogenetics (Figure 1). Four years after the PMF diagnosis, she presented with petechiae and generalized weakness. Laboratory studies showed leukocytosis (WBC 15.7 × 109/L), anemia (Hb 10.4 g/dL), thrombocytopenia (platelet 38 × 109/L), and 44% circulating myeloblasts in peripheral blood (PB). A bone marrow (BM) evaluation was limited by a dry tap. Flow cytometric analysis on a PB sample revealed myeloblasts with the following immunophenotype: CD34bright+/CD19partial+/CD38+/CD13+/CD15+/CD33+/CD117+/HLA‐DR+/MPO+/CD79−/CD20−/CD22−/CD3− (Figure 2A, Table 1). Cytogenetic analysis revealed an abnormal karyotype with two related clones harboring t(20;21):46,XX,t(20;21)(q13.1;q22)[17]/47,idem,+8[3] (Figure 2B). An AML FISH panel analysis showed extra copies of RUNX1 and RUNX1T1 in 93% and 7% of examined cells, respectively. An NGS study demonstrated persistent mutations in JAK2, IDH2, and SRSF2 (at similar VAF to the previously diagnosed PMF except for an increased JAK2 VAF to 97.5%), with additional gene alterations in SMC3 (p.R381Q), CDKN2A/2B (deletion), and RUNX1::CBFA2T2 (resulting from t(20;21)(q13.1;q22)) (Figure 1). These results demonstrate clonal evolution and transformation of PMF to AML.
FIGURE 1.
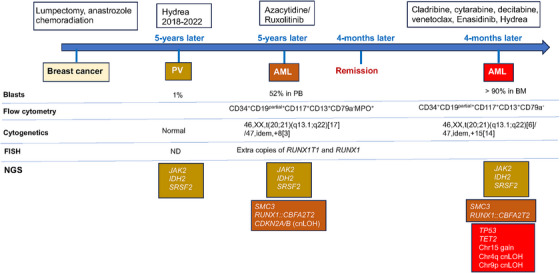
Timeline of the patient's clinical course including disease progression, treatment, cytogenetic/FISH, immunophenotype by flow cytometry, and gene alterations by NGS (cnLOH, copy neutral loss of heterozygosity).
FIGURE 2.
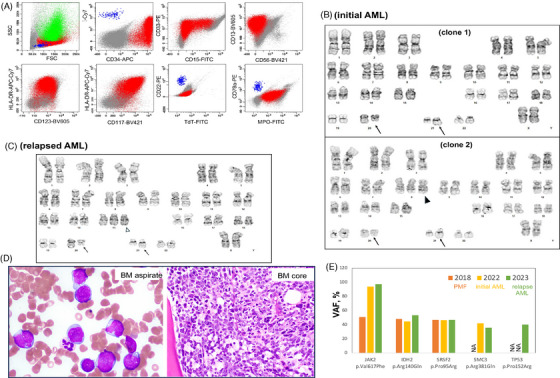
Clinicopathological and molecular characteristics of RUNX1::CBFA2T2 rearranged AML transformed from JAK2 mutated primary myelofibrosis. (A) Flow cytometric immunophenotype of bone marrow aspirate. Myeloblasts (in red) are medium sized to large with low side scatter, positive for CD34 (bright), CD33, CD13, HLA‐DR, CD117, and MPO, partially positive for CD19 and CD15, and negative for CD22, CD79a, or TdT. Color code: green, granulocytes; blue, normal B cells. (B) and (C) The abnormal karyotype at initial AML (C): clone 1 with t(20;21)(q13.1;q22) (arrow), and clone 2 with additional trisomy 8 (arrowhead); and at relapsed AML (D): second clone with additional trisomy 15 (arrowhead). (D) The morphology of myeloblasts in relapsed AML: medium‐sized to large blasts in BM aspirate (Wright–Giemsa stain, 1000×) and core biopsy stain (hematoxylin and eosin stain, 200×). (E) The variant allele frequency (VAF%) of gene mutations detected by NGS. ND, not detected.
TABLE 1.
Literature review on RUNX1::CBFA2T2 rearranged AML.
| Cases | Diagnosis | Age/sex | Immunophenotype | Cytogenetics | Cooperative gene alterations | F/U | Survival |
|---|---|---|---|---|---|---|---|
| Patient 111 | de novo AML | 1.5/M | NA | 47,XY,+8,t(14;20)(q11.2;q11.2),del(21)(q21q2) | No FLT3‐ITD (NA on other genes) | NA | Alive |
| Patient 212 | de novo AML | Infant/NA | NA | NA | NA | NA | NA |
| Patient 38 | de novo AML | 69/M | NA | 46,XY,ins(21;20)(q22.12;q13.33)[5]/46,indem,del(7)(q22)[7]/46,XY[4] | NA | NA | Dead |
| Patient 413 | Extramedullary relapse (post‐transplant for AML FIP1L1::PDGFRA) | 25/M | Monocytic diff: CD45−CD34−CD117− CD19−CD33+CD64+ HLADR+ CD56+CD15+ CD13+ CD11c+MPO+ | NA | FIP1L1::PDGFRA (no other gene alterations reported by NGS) | 280 days a | NA |
| Patient 5 (our patient) | AML from PMF (breast cancer) | 64/F | Myeloblast diff: CD45+CD34bgt+CD19partial+ CD15partial+CD13+CD117+ CD33+HLA‐DR+/MPO+ | 46,XX,t(20;21)(q13.1;q22)[17]/47,dem,+8[3] | JAK2, IDH2, SRSF2, SMC3, TP53, TET2 | 4 years b | Dead |
Abbreviations: AML, acute myeloid leukemia; bgt, bright; diff, differentiation; F, female; F/U, follow‐up; M, male; NA, not available; PMF, primary myelofibrosis.
Interval from transplant to extramedullary AML relapse.
Interval from PMF to AML.
The patient was treated with azacytidine/ruxolitinib and achieved remission after 4 months. Her AML relapsed 5 months later at which time she was treated with cytarabine, venetoclax, and enasidenib. Repeat BM evaluation showed persistent AML with 90% blasts (Figure 2D). Flow cytometric analysis revealed 69% neoplastic myeloblasts with a similar immunophenotype to prior analysis. Cytogenetic studies identified recurrent abnormal karyotype with an additional clone with trisomy 15 (Figure 2C). NGS detected recurrent gene alterations with additional alterations in TP53 (p.P152R, 40.2%) and TET2 (p.M906V) (Figure 2E). These results indicate continued clonal evolution at relapse. The patient expired 13 months after initial diagnosis of AML.
4. DISCUSSION
AML with RUNX1::CBFA2T2 fusion is extremely rare, with only four patients reported in the literature (Table 1) and with largely unknown clinicopathological and genomic features [8, 11–13]. A summarized analysis, including our patient, suggests that RUNX1::CBFA2T2 rearranged AML is characterized by a male predominance and variable age of onset ranging from infancy to elderly. Three patients were diagnosed as de novo AML and two patients as secondary AML. Patient 4 had an extramedullary AML relapse post‐transplant of FIP1L1::PDGFRA rearranged AML. Our patient (Patient 5) had an AML transformed from JAK2 mutated primary myelofibrosis. The prognosis of RUNX1::CBFA2T2 rearranged AML is largely unknown; two patients were deceased, and our patient died 13 months after AML diagnosis.
The immunophenotypic features of this subtype of AML were only available in two patients, which showed distinctly different profiles. Patient 4 had monocytic differentiation (CD34−/CD117−/CD19−/monocytic markers+) and presented with extramedullary relapsed AML harboring both FIP1L1::PDGFRA and RUNX1::CBFA2T2 fusions (without other gene alterations) [13]. Our patient had myeloblast differentiation (CD34bright+/CD117+/CD19partial+/MPO+) and presented as transformed AML (from JAK2 mutated PMF) harboring RUNX1::CBFA2T2 fusion with other cooperative gene alterations. It is unknown whether these different immunophenotypes are associated with extramedullary infiltration and/or cooperative gene alterations. Nonetheless, our case presented with an immunophenotype resembling AML with RUNX1::RUNX1T1 and with RUNX1::CBFA2T3, such as bright CD34 expression and aberrant expression of CD19 on myeloblasts [1–3, 9]. Differentiating between these CD19(+) RUNX1 rearranged AML requires cytogenetic and molecular studies. Additionally, CD19 expression raises the differential diagnosis of mixed phenotype acute leukemia (MPAL). Evaluation of other B lineage markers, such as CD20, CD22, and CD79a, is important to distinguish AML from MPAL.
Our case study is the first to reveal the landscape of cooperative gene alterations in RUNX1::CBFA2T2 rearranged AML. A recent case of RUNX1::CBFA2T2 and FIP1L1::PDGFRA rearranged AML did not report any additional gene alterations [13]. Our case of RUNX1::CBFA2T2 rearranged AML transformed from JAK2/IDH2/SRSF2 mutated PMF demonstrates cooperative gene alterations in signaling pathways (JAK2), epigenetic/chromatin and cell cycle regulations (SMC3, TET2, and CDKN2A/B), and tumor suppressor genes (TP53), along with chromosomal abnormalities during development and progression of AML. This genomic alteration landscape is similar to that of AML with RUNX1::RUNX1T1 [5, 6], and provides insight into the pathogenesis of this rare AML subtype.
In conclusion, this is the first case study to describe the clinicopathological and genomic features of RUNX1::CBFA2T2 rearranged AML transformed from JAK2 mutated PMF and demonstrates that the immunophenotype and the landscape of cooperative gene alterations resemble those in RUNX1::RUNX1T1 rearranged AML. Future studies on larger cohorts of patients are needed to further characterize the clinicopathologic and molecular landscape of this disease, which in turn may guide evidence‐based treatment rationales for improved clinical management.
AUTHOR CONTRIBUTIONS
Lina Han and Weina Chen conceptualized the case study and wrote the original draft. All authors critically revised the manuscript and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
FUNDING INFORMATION
The authors received no specific funding for this work.
ETHICS STATEMENT
All procedures performed in this case study were part of the clinical management in accordance with the ethical standards.
CLINICAL TRIAL REGISTRATION
The authors have confirmed clinical trial registration is not needed for this submission.
PATIENT CONSENT STATEMENT
Patient consent statement was waived per approved protocol (IRB STU 122013‐023).
Han L, Koduru P, Cantu M, Fuda F, Chen W. RUNX1::CBFA2T2 rearranged acute myeloid leukemia transformed from JAK2 V617F mutated primary myelofibrosis. eJHaem. 2024;5:1330–1334. 10.1002/jha2.985
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han SY, Mrozek K, Voutsinas J, Wu Q, Morgan EA, Vestergaard H, et al. Secondary cytogenetic abnormalities in core‐binding factor AML harboring inv(16) vs t(8;21). Blood Adv. 2021;5(10):2481–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al‐Harbi S, Aljurf M, Mohty M, Almohareb F, Ahmed SOA. An update on the molecular pathogenesis and potential therapeutic targeting of AML with t(8;21)(q22;q22.1);RUNX1‐RUNX1T1. Blood Adv. 2020;4(1):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krauth MT, Eder C, Alpermann T, Bacher U, Nadarajah N, Kern W, et al. High number of additional genetic lesions in acute myeloid leukemia with t(8;21)/RUNX1‐RUNX1T1: frequency and impact on clinical outcome. Leukemia. 2014;28(7):1449–1458. [DOI] [PubMed] [Google Scholar]
- 6. Faber ZJ, Chen X, Gedman AL, Boggs K, Cheng J, Ma J, et al. The genomic landscape of core‐binding factor acute myeloid leukemias. Nat Genet. 2016;48(12):1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis JN, McGhee L, Meyers S. The ETO (MTG8) gene family. Gene. 2003;303:1–10. [DOI] [PubMed] [Google Scholar]
- 8. Guastadisegni MC, Lonoce A, Impera L, Di Terlizzi F, Fugazza G , Aliano S , et al. CBFA2T2 and C20orf112: two novel fusion partners of RUNX1 in acute myeloid leukemia. Leukemia. 2010;24(8):1516–1519. [DOI] [PubMed] [Google Scholar]
- 9. Liu H, Wang SA, Schlette EJ, Xu J, Jorgensen JL, Cameron Yin C, et al. Myeloid neoplasms with t(16;21)(q24;q22)/RUNX1‐RUNX1T3 mimics acute myeloid leukemia with RUNX1‐RUNX1T1. Ann Hematol. 2018;97(10):1775–1783. [DOI] [PubMed] [Google Scholar]
- 10. Lavallee VP, Lemieux S, Boucher G, Gendron P, Boivin I, Armstrong RN, et al. RNA‐sequencing analysis of core binding factor AML identifies recurrent ZBTB7A mutations and defines RUNX1‐CBFA2T3 fusion signature. Blood. 2016;127(20):2498–2501. [DOI] [PubMed] [Google Scholar]
- 11. Shiba N, Yoshida K, Hara Y, Yamato G, Shiraishi Y, Matsuo H, et al. Transcriptome analysis offers a comprehensive illustration of the genetic background of pediatric acute myeloid leukemia. Blood Adv. 2019;3(20):3157–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bolouri H, Farrar JE, Triche T Jr, Ries RE, Lim EL, Alonzo TA, et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age‐specific mutational interactions. Nat Med. 2018;24(1):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Panda D, Pathak A, Tejwani N, Pandey P, Mehta A. Acquired RUNX1::CBFA2T2 fusion at extramedullary relapse in a patient of PDGFRA rearranged acute myeloid leukemia post allogenic HSCT. Cytometry B Clin Cytom. 2023;104(5):404–406. [DOI] [PubMed] [Google Scholar]
- 14. Zheng R, Fuda F, Gagan JR, Weinberg OK, Koduru P, Cantu M, et al. Genomic heterogeneity within B/T mixed phenotype acute leukemia in a context of an immunophenotype. Leuk Res Rep. 2024;21:100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


