Abstract
Objectives
To characterize variations in real‐world treatment patterns in multiple myeloma (MM) in Portugal over a 5‐year period.
Methods
A retrospective cohort multicenter study using secondary data of national hospital drug consumption database from 11 Portuguese public hospitals between 2017 and 2022.
Results
Number of MM‐treated patients increased 53% over 5 years (from 825 to 1266 patients). Constant slight predominance of male patients (55%), 82% over 60 years old (median age, 70 years), and half of newly diagnosed patients were transplant‐eligible. The highest growth rate was in second‐line treatments, with a sixfold increase in patients in fourth‐line or beyond. First‐line treatment pattern remained stable both in transplant‐eligible (bortezomib, cyclophosphamide and dexamethasone (VCd_, bortezomib, thalidomide and dexamethasone (VTd), and bortezomib, lenalidomide and dexamethasone (VRd)) and noneligible patients (bortezomib, melphalan and prednisolone (VMP), VCd, and lenalidomide, dexamethasone (Rd)). Maintenance therapy increased from 5% to 16%, shifting from thalidomide to lenalidomide. Second and third lines were dominated by daratumumab‐based regimens after 5 years. No standard of care in fourth‐line treatment. Treatment duration increased in transplant‐eligible due to maintenance therapy and in noneligible due to fourth‐line treatments. Patients moved from first‐ to second‐line more rapidly over time.
Conclusions
There was an increase in MM patients reaching advanced treatment lines and significant changes in the treatment patterns, driven by access to more effective frontline treatments and longer duration of treatment.
Keywords: evolution, landscape, multiple myeloma, Portugal, treatment
1. INTRODUCTION
Multiple myeloma (MM) is a clonal plasma cell neoplasm representing about 1% of all cancers and 10% of all hematological malignancies worldwide [1].
In high‐income countries, the crude incidence of MM is increasing, whereas the mortality rate has fallen [2]. In 2022, around 50,092 new cases of MM were diagnosed in Europe, and 31,969 persons died of the disease. The crude and age‐standardized MM incidence were 6.7 and 2.8 new cases/100,000, respectively. In Portugal, the incidence was higher than in Europe, with crude and age‐adjusted incidence of 9.8 and 3.4/100,000, respectively [3].
MM affects mainly elderly people, with a median age at diagnosis around 66–70 years [4], and is slightly more prevalent in men than women [1]. MM is a high‐burden disease [5] and remains essentially incurable. However, the treatment landscape for its clinical management has evolved quite significantly over the past years. Indeed, MM has become a focal point of clinical attention due to the notable advancements in treatment options [6]. The introduction of high‐dose chemotherapy plus autologous stem cell transplant (ASCT) as well as the approval and use of novel agents, such as proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), histone deacetylase inhibitors, and monoclonal antibodies (mAbs) used in therapeutic combinations (duplets, triplets, or quadruplets) has dramatically improved patient survival outcomes namely response rate, progression‐free survival, and overall survival (OS) [7, 8] in both newly diagnosed and relapsed/refractory settings. A large‐scale real‐world study based on the data from the European Medicines Agency (EMEA) network showed that the median OS increased from 2.9 years in the 2010–2014 cohort to 4.5 years in the 2015–2020 cohort [9].
The European Society for Medical Oncology (ESMO) treatment guidelines for MM were first updated in 2017 [10] and more recently in 2021 [11] to account for the increasing number of treatment options available following the approval of therapies across the treatment pathway.
In Portugal, recommendations for managing older and frail patients with MM in routine clinical practice were released in 2020 [12], and the MM treatment guidelines of the Portuguese Group of MM in 2023 [13].
In Europe, treatment strategies are heterogenous, as drug availability varies across countries and affects access to novel treatments. From 2017 to 2022, about 15 innovative agents or regimens were approved and reimbursed in Portugal, both in frontline and in advanced treatment lines, expanding the treatment options available for MM. Currently, approved and reimbursed therapeutic options for MM in Portugal practically overlap the European setting with access delays due to approval decisions.
Given the evolving and complex MM treatment landscape, updated real‐world evidence around treatment patterns and pathways is essential to understanding whether clinical practice is adopting innovation and effectively contributing to improved outcomes for MM patients.
This large‐scale national study was conducted from 2017 to 2022 across 11 Portuguese public hospitals. The primary objective was to characterize real‐world treatment patterns in MM. The secondary objectives were to analyze temporal changes in the number of MM patients, their characteristics, and their treatment duration.
What is the NEW aspect of your work?
This real‐world characterization of treatment patterns of multiple myeloma (MM) patients in Portugal between 2018 and 2022 across all treatment lines showed drastically evolving changes in the uptake of different regimens over time.
What is the CENTRAL finding of your work?
A notable transformation of the treatment landscape driven by patient access to therapeutic innovation in earlier lines of treatment, significant increase in the number of patients under treatment, a greater number of patients reaching advanced treatment lines, and an increase in the treatment duration.
What is (or could be) the SPECIFIC clinical relevance of your work?
Our study demonstrates there has been advances in MM treatment and access to innovation has been adopted which in the end will translate into better care and health outcomes for the patients. The characterization of real‐world treatment patterns in MM may be an important contribute for informed decisions like a future review and update of the therapeutical guidelines, to optimize healthcare and outcomes.
2. METHODS
2.1. Study design and population
A national, observational, multicenter, retrospective, cohort study was conducted using secondary data of patients treated for MM in the Portuguese public hospital setting.
Data were extracted from the longitudinal retrospective hospital drug‐related consumption database of IQVIA Consulting [5] (derived from hospital pharmacy drug management electronic systems) of a panel of 11 Portuguese public hospitals (Table S1). This database only collects data on hospital drug consumption dispensed through hospital pharmacies (inpatient/outpatient) and basic demographic data (age and gender). No further data, including disease diagnosis, are available.
In this study, MM patients were identified by the consumption of MM‐specific treatment. A list of valid MM regimens across the cohort period was predefined by a clinical expert (Table S2). All patients of any age who received a valid MM regimen at any time during the cohort period were included in the study. Additional rules were established to avoid including patients treated with regimens labeled for conditions other than MM (Tables S2 and S3).
The classification of each regimen as first‐line (1L), second‐line (2L), third‐line (3L), or fourth‐line onward (4L+) was not directly available in the database. Thus, lines of treatment were defined based on the cumulative number of switches that each patient experienced over time, considering a list of consistency rules predefined by a clinical expert (Table S3).
Data were pseudoanonymized by an automatic nonidentifiable encryption at hospitals before being sent to IQVIA.
The study was conducted in compliance with the Portuguese law and the European Regulation 2016/679 and subsequent amendments.
2.2. Data collection
The patient‐level dataset of this study included a unique pseudoanonymized patient code attributed by each hospital, hospital name, patient birthdate, gender, international nonproprietary name, drug brand, pack size, number of dispensed units, dispensation date (month and year), and cost center (e.g., hospital department/ward).
To identify patients that may have been treated in more than one hospital, birthdate, gender, and consumption dates were checked between hospitals.
2.3. Classification of patients
The following definitions were used to classify patients in each year of the cohort period:
Naïve: a patient not treated for MM in the previous 5 years.
Switch: a patient that started a MM treatment different from the previous one.
Drop‐out: a patient receiving no treatment for at least 3 months consecutively.
The same patient may have been classified in more than one category within each year of the cohort period considering the dynamics in his/her clinical condition.
Stem cell transplant (SCT) eligibility was not directly available in the database. Therefore, a patient was classified as SCT eligible (SCT‐E) if treatment‐naïve and under the age of 66, or between 66 and 70 years old with consumption of four to six cycles of DaraVTd, VTd, VRd, or VCd (induction treatment). Patients not meeting these criteria were classified as SCT noneligible (SCT‐NE).
2.4. Outcomes and statistical analysis
The study included two cohorts of patients: cohort 1 from September 2017 to August 2022 and cohort 2 from September 2012 to August 2017. Each patient may have been included in more than one cohort.
Cohort 1 was considered for the descriptive statistical analysis of the number of MM patients (overall cohort and by gender, age group, naïve/switch, and SCT eligibility) and percentage of patients by regimen and line of treatment at each year: year 1 (Sep 2017–Aug 2018), year 2 (Sep 2018–Aug 2019), year 3 (Sep 2019–Aug 2020), year 4 (Sep 2020–Aug 2021), and year 5 (Sep 2021–Aug 2022).
Cohort 2 was used to compare modified time to the next treatment (mTTNT) between cohorts. mTTNT, expressed in months, was defined as the time from the start of a treatment line to the start of the next treatment line in patients with at least one switch without censoring rules. Kaplan–Meier curves were used to summarize mTTNT, stratified by SCT‐E and SCT‐NE patients. Among SCT‐E patients, mTTNT was also calculated separately for patients switching from 1L to maintenance therapy (1L > 1m) and from maintenance therapy to 2L (1m > 2L).
Missing data were not imputed, and valid cases were reported for each analysis. No formal sample size was calculated, as this was a population‐based descriptive study, not hypothesis‐driven. Statistical analyses were performed using SAS 9.4 (SAS Institute).
3. RESULTS
3.1. Number of MM patients over time
This study included a total of 961 unique MM patients between 2017 and 2022. The cumulative number of MM patients showed a steady increase, rising from 825 patients in year 1 to 1266 patients in year 5 (Figure 1A), corresponding to a 5‐year growth of 53%, or an average annual growth of 10%.
FIGURE 1.
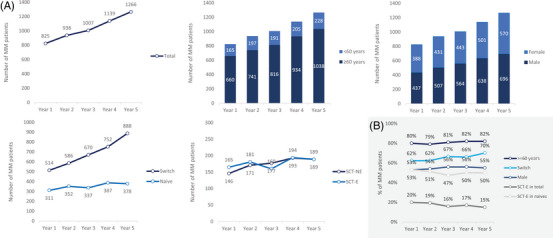
(A) Number of multiple myeloma (MM) patients, overall and stratified by characteristics, and (B) percentage of patients by characteristic by year. 1L, first‐line; 2L, second‐line; 3L, third‐line; 4L, fourth‐line; SCT‐E, eligible; SCT‐NE, noneligible.
The characteristics of MM patients remained relatively similar over the 5‐year period (Figure 1B) with only modest fluctuations. By year 5, most patients were 60 years or older (82%), with a median age of 70 and approximately half were male (55%), the majority were switches (70%), and 15% were SCT‐E (50% of naïve patients).
3.2. Treatment lines over time
The number of MM patients increased over the 5‐year period across all treatment lines, but at a different rate (Figure 2A). The yearly rate of change in 2L was higher than in other lines perceived by a steeper slope. The number of patients in 2L and in 3L doubled, while those in 4L+ increased sixfold.
FIGURE 2.
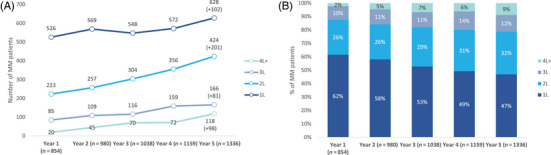
(A) Number and (B) percentage of multiple myeloma (MM) patients by treatment line by year. Note: A patient may have been counted in more than one treatment line if a switch occurred. 1L, first‐line; 2L, second‐line; 3L, third‐line; 4L, fourth‐line.
Figure 2B depicts the increasing weight of patients in 2L (from 26% to 32%) and 4L+ (from 2% to 9%). The ratio of patients in 1L:4L+ dramatically changed from 26:1 in year 1 to 5:1 in year 5.
The maximum number of therapy lines observed for a patient was six, both in years 1 and 5.
3.3. Treatment regimens per treatment line over time
Figure 3 describes the percentage of patients treated by regimen in year 1 and year 5.
FIGURE 3.
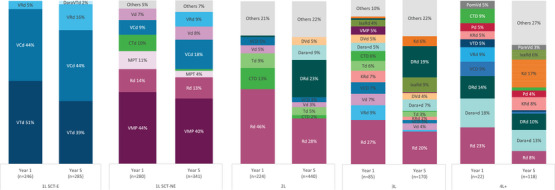
Percentage of multiple myeloma (MM) patients by treatment regimen in year 1 (Sep 2017–Aug 2018) and year 5 (Sep 2021–Aug 2022; excluding maintenance). Note: Percentages may not sum 100% due to rounding; regimens used in less than 5% both in year 1 and 5 were grouped in “Others”; labels from treatment regimens with less than 2% are not displayed. 1L, first‐line; 2L, second‐line; 3L, third‐line; 4L, fourth‐line; SCT‐E, eligible; n, number of patients treated per year; SCT‐NE, noneligible.
In the 1L SCT‐E subgroup, there were no marked changes over time, with patients being mainly treated with VCd (44% both in years 1 and 5) and VTd (51% in year 1 and 39% in year 5). Following national reimbursement in March 2022, DaraVTd appeared as a new alternative for 2% of the patients in year 5.
The number of patients in maintenance therapy after 1L increased fivefold from year 1 (n = 40, 7% of 1L patients, 5% of all patients) to year 5 (n = 204, 33% of 1L patients, 16% of all patients). During this period, lenalidomide became the most common maintenance treatment, increasing from 43% in year 1 to 76% in year 5, whereas thalidomide decreased significantly from 55% to 15% (data not shown).
In 1L SCT‐NE patients, VMP remained the most common regimen (44% in year 1 and 40% in year 5). There was a very modest increase in the percentage of patients treated with VCd and VRd (+9% of patients, each) and a decrease with melphalan, prednisolone and thalidomide (MPT) (−7%) and CTd (−9%).
The treatment landscape in 2L evolved considerably over the 5‐year period. Use of Rd decreased from 46% to 28% and CTd lost relevance, dropping from 13% to 2%. Daratumumab‐based regimens (DRd, Dara + d, DVd, Dara + Td, and Dara + Kd) became dominant in 2L with a substantial increase from 2% in year 1 to 42% in year 5, with DRd alone increasing from 1% to 23%.
In 3L, Rd was the most common regimen treating 27% of the patients in year 1 and 20% in year 5. The percentage of patients treated with daratumumab‐based regimens (DRd, Dara + d, Dara + Td, Dara + Kd, DVd) increased significantly from 11% to 35%. DRd was used in 1% of the patients in year 1 but was the second most common regimen in year 5 (19%).
The considerable diversity of treatment regimens among 4L+ patients showed a heterogeneous treatment pattern. Rd, which was the most common regimen in year 1 (23%), decreased markedly in year 5 (8%). On the other hand, daratumumab‐based (32% both in year 1 and year 5) and carfilzomib‐based regimens (5% in year 1 and 25% in year 5) emerged as the most common treatments.
3.4. Drop‐out and switch rates over time
In 1L, the drop‐out rate remained stable over time, while the switch rate increased from 23% in year 1 to 36% in year 5. In 2L, the drop‐out rate decreased markedly from 32% in year 1 to 17% in year 5, accompanied by a modest decrease in the switch rate (26%–18%). In 3L, the drop‐out decreased from 26% to 19%, and the switch rate increased substantially from 13% to 30% (Figure 4).
FIGURE 4.
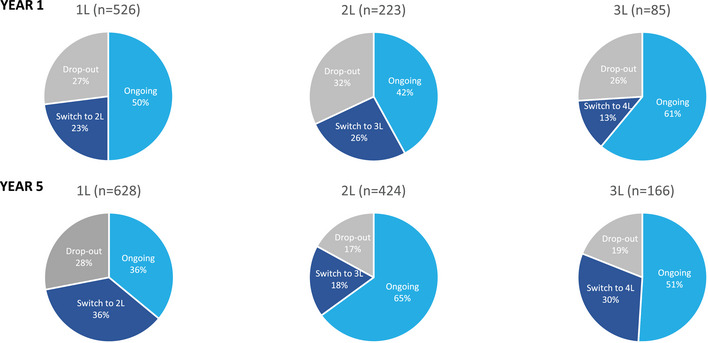
Drop‐out and switch rates in year 1 and year 5 (switches to maintenance not considered). 1L, first‐line; 2L, second‐line; 3L, third‐line; 4L, fourth‐line; n, number of patients in each treatment line in year 1 and year 5.
3.5. Modified time to next treatment
mTTNT until 4L was compared between the most recent cohort 2017–2022 (n = 961) and the preceding cohort 2012–2017 (n = 389), stratified for SCT‐E (Figure 5A) and SCT‐NE patients (Figure 5B). The average mTTNT for each line was summed to estimate the total mTTNT until 4L.
FIGURE 5.
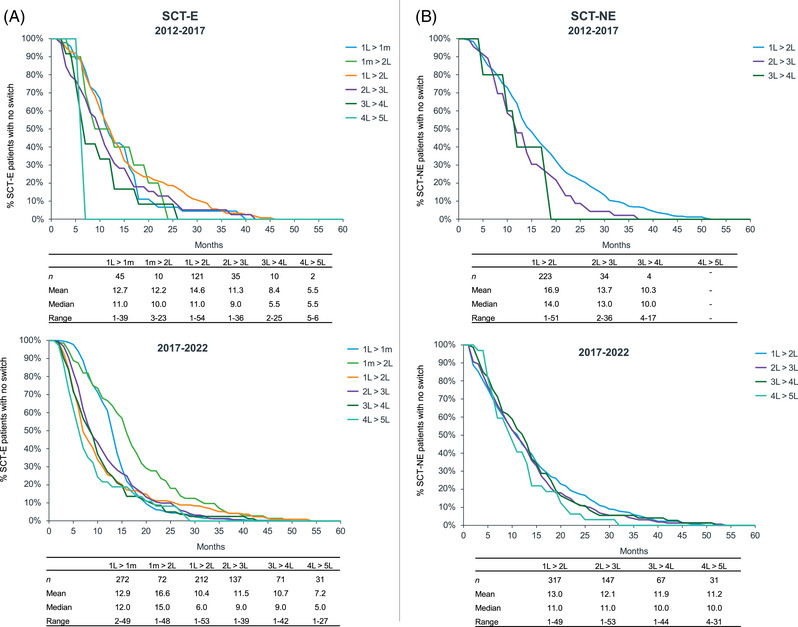
Time to the next treatment (TTNT) for (A) SCT‐E patients and (B) SCT‐NE patients: 2017–2022 versus 2012–2017. 1m, maintenance therapy; 1L, first‐line; 2L, second‐line; 3L, third‐line; 4L, fourth‐line; SCT‐E, eligible; SCT‐NE, noneligible.
In SCT‐E with maintenance, there was an increase of 9 months between cohorts in the total mTTNT (from 50 to 59 months). This increase was driven by an additional 4 months in maintenance treatment and an increase of 2 months in both 3 and 4L.
In SCT‐E without maintenance, the total mTTNT did not change between cohorts (40 months). However, the time in 1L decreased by 4.6 months, offset by an increase of 2 months in 3 and 4L each.
In SCT‐NE, the total mTTNT increased 7 months between cohorts (from 41 to 48 months) due to an increase of 11 months in 4L partially offset by a reduction of 4 months in 1L.
4. DISCUSSION
The main objective of our study was to characterize treatment patterns in MM in patients treated at public hospitals, mainly to understand how evolving innovation over the last years has been adopted in clinical practice in Portugal. Secondary data related with hospital drug consumption were retrieved from a panel of 11 national hospitals.
Our study showed a sustained rise in the number of MM‐treated patients throughout 2017–2022. This rise can be primarily explained by the improved survival rates due to treatment advances, as evidenced by the exponential increase in the number of switch patients. Additionally, the aging Portuguese population (+9.3% people aged 65+ years between 2018 and 2022 [14]) may have also contributed for the smooth increase in the number of naïve patients.
Similar trends have been reported in Catalonia, Spain [15], between 2018 and 2022, where there was an increase of 48.4% in MM‐treated patients (53% in our study) and an increase of 24.4% in naïve patients (22% in our study). It should be mentioned that no patient referral policies were implemented during this period that would have justified the increased patient numbers in the included hospitals.
The COVID pandemic impacted these numbers since the increase rate between September 2019 and August 2020 (+7%) is lower than between other consecutive years (+10% to +12%). This has been reported in a previous publication [16].
Despite the increase in the number of MM patients, their characteristics remained identical over time. Our sample characteristics showed a slightly male predominance, predominantly elderly patients, mostly previously treated, with half of naïve eligible for transplant. Age and gender distribution seem to be aligned with previous studies [5, 17, 18]. However, our study showed a lower rate of SCT‐eligible patients (20% in year 1) compared to the SCT rate reported in a previous study performed at a Portuguese Comprehensive Oncology Center (32% in 2012–2015).
The number of patients in 2L increased at a higher yearly rate than in the other treatment lines. Also, it is worth noting the outstanding increase in patients reaching 3 and 4L+, rising from 12% in year 1 to 21% in year 5. This suggests that patients are accessing to more effective agents with manageable toxicities, allowing them to be fit for subsequent treatments and extending their survival.
After 5 years, clinical practice in 1L SCT‐E remained similar with the predominance of bortezomib‐based regimens (VTd, VCd, and VRd). The EHA‐ESMO 2021 clinical practice guidelines recommend induction treatment for naïve SCT‐E patients with VRd or DVTd as first options, or VTd or VCd if first options are unavailable [11]. DVTd was the only innovative frontline treatment reimbursed in 2022 for SCT‐E patients in Portugal, which explains the modest uptake at the end of the cohort period (2%) compared with Europe, where this label was approved earlier. Currently, Portuguese treatment guidelines [13] recommend DVTd or DVRd as the first options and VRD, VTD, or VCD if the first options are unavailable.
In 1L SCT‐NE, EHA‐ESMO 2021 clinical practice guidelines recommend DRd, DVMP, or VRd as the first option, and VMP or Rd if the first option is unavailable. At the end of our cohort period, these daratumumab‐based regimens were being used only by 1% each (included in “Others” in Figure 3), given their late approval in 2022 for SCT‐NE. Portuguese current guidelines [13] recommend DRd or DVMP as the first options and VRd, VMP, or Rd if the first options are unavailable.
The number of patients in maintenance therapy increased between 2017 and 2022, with a clear shift from thalidomide to lenalidomide, which was approved in Portugal in 2021. Maintenance treatment with lenalidomide is licensed and reimbursed in several European countries and is commonly accepted as the standard of care for all patients regardless of risk profile and remission, owing to better survival outcomes [19, 20].
Treatment patterns in 2 and 3L changed considerably, with a predominance of daratumumab‐based regimens in year 5 due to the reimbursement of DRd in 2L+ and daratumumab in 3L+ in 2018, and DVd in 2L+ in 2019. This rise in the use of daratumumab‐based regimens in both 2 and 3L settings, as well as in 4L+, illustrates the significant impact of this mAb on MM treatment. The heterogeneous treatment pattern seen in 4L+ reflects the lack of a standard of care, with more than 26 different regimens in year 5. Carfilzomib‐ and daratumumab‐based regimens emerged as the most relevant regimens in 2022. The regimen Kd not used in year 1 was preferred in year 5, likely due to its reimbursement in Portugal for 2L+ in 2020.
Over time, the rate of switches in 1L increased. This may have occurred due to more effective 2L regimens in year 5, prompting earlier or more frequent decisions to switch from 1 to 2L. On the other hand, these same innovative regimens in 2L may have improved/prolonged response times, decreasing drop‐out and switch rate over time as patients remained in 2L longer. In 3L, there was an increase in the switching rate, which may be due to the availability of more effective treatments in 4L+.
MM is a chronic disease that tends to further relapse with a progressively shorter TTNT [21] which is fully captured in our study in both cohorts. The comparative analysis of mTTNT between the two cohorts (2012–2017 vs. 2017–2022) reveals extended treatment durations in the more recent cohort. In SCT‐E, there was an increase in mTTNT in patients undergoing maintenance treatment, likely due to the switch from thalidomide to lenalidomide. In SCT‐NE, mTTNT in 4L increased markedly due to the novel agents. In SCT‐E not undergoing maintenance and in SCT‐NE, mTTNT in 1L decreased by 4 months, likely related to the higher switch rate mentioned previously and underscores the importance of developing robust treatment protocols for patients who are not eligible for SCT. Although the mean mTTNT in 2 and 3L remained stable, the maximum observed mTTNT in these lines is higher in year 5.
To our knowledge, our study is the first to be conducted in the Portuguese setting to analyze trends until 2022. We found a single‐center study [17] performed in a Comprehensive Oncology Center which characterized MM treatment patterns for a pool of 187 patients diagnosed and/or treated between 2012 and 2015, a multicentric study [22] including 386 MM patients above 74 years old and treated between 2009 and 2016, and a nation‐wide study [5] including 1941 MM patients treated in 2016 but not describing treatment patterns. Since the MM treatment landscape has been rapidly evolving, the data published in these studies several years ago are not directly comparable to these results.
Retrospective studies conducted in Europe show that treatment patterns for MM vary widely across European countries due to local guidelines, recommendations, or reimbursement limitations, indicating that homogeneity between the European Union (EU) member states cannot be assumed [18, 23, 24, 25, 26].
A recent real‐world study [18] included 2179 MM patients from France, Germany, Italy, Spain, and the United Kingdom between May and November 2021. The distribution of patients by treatment line contrasted significantly with our study (18% in L1, 23% in L2, 29% in L3, and 29% in L4+). Similar to our study, treatments became more diverse as lines of therapy increased. Patients on 1L mainly received bortezomib‐ (58%), daratumumab‐ (24%), or lenalidomide‐based regimens (15%), but analysis was not stratified for SCT‐E and SCT‐NE. In 2L, daratumumab‐based regimens were most frequently used (50% vs. 42% in our study). In 3L, patients started to receive more diverse regimens, with equally frequent use of IxaRd, DRd, DVd, and Pd (10%–12% each). Overall, 41 unique treatment regimens were used in 3L (vs. at least 25 in our study). In 4L, 36% of patients were treated with anti‐CD38 regimens, and the use of B‐cell maturation antigen targeting regimens was observed for the first time (6%). Overall, it is perceived that innovation reached 1L patients earlier in other European countries compared to Portugal.
An observational study [24] described the current standard of care in France, Germany, Spain, and Italy for SCT‐E MM patients, recording the evolution of frontline treatment during 2017–2020/2021. Induction regimens included VRd (28.7%), VTd (29.6%), VCd (7.8%), daratumumab‐based regimens (5.5%), and others. In Portugal, the use of VRd is increasing but still not as common as in this study. Maintenance treatment seems to be increasing as reported by the authors (64.9% planned or ongoing in 20/21) and was dominated by lenalidomide (86.9% in 20/21), aligned with trends seen in our study.
4.1. Strengths and limitations
Our study included 961 unique MM patients between September 2017 and August 2022. According to GLOBOCAN [27], there were 2706 MM patients in Portugal between 2018 and 2022 (5‐year prevalence). Therefore, our study may have included 35.5% of the total number of MM patients in Portugal. According to the same source [27], a total of 994 new cases of MM were diagnosed in 2022 meaning that our study included approximately 38.9% of all new MM cases in Portugal. Since GLOBOCAN estimates for MM prevalence and incidence include other code diseases besides MM, these percentages may be even underestimated. Therefore, our estimates are accurate given the expected low precision error.
Another strength of this study is the inclusion of different types of hospitals, including Central University hospitals and Reference Oncology Centers, where MM patients are treated by Hematologists or Hemato‐Oncologists, from the three most populated regions of the country. Our study did not include all Portuguese transplantation reference centers, which may have contributed to an underestimation of the SCT‐E population. Our study does not represent private hospitals, but treatment patterns are not expected to differ from the public setting.
This large‐sample study included all eligible MM‐treated patients recorded in the hospital consumption database without sampling and therefore minimizing selection bias. Both outpatient and inpatient settings were included, enriching the real‐world characterization of MM patients and clinical practice. The 5‐year period under analysis was chosen for being wide enough to capture changes in MM treatment patterns over time and the use of new agents approved over the last years and for being relevant, considering the scarcity of studies covering this most recent period. The study reflects the most recent data available during the data extraction.
The data source used in this study is subject to extensive data quality control, showing a low rate of missing data, and has been used in previous published studies [5]. Data were limited to drug consumption records and basic demographic characteristics. Therefore, MM patients were not identified through a diagnosis code but rather indirectly by the consumption of MM‐specific therapies. Selection bias was minimized because (1) there are low chances of missing an eligible MM patient given the compulsory registry of all dispensed drugs at hospital pharmacies, and (2) a predefined algorithm designed by a clinical expert with comprehensive validation rules was used to exclude patients treated for indications other than MM.
Other characteristics, such as International Staging System (ISS) stage, Eastern Cooperative Oncology Group (ECOG) status, comorbidities, complications of MM, bone lesions, cytogenetic risk, history of transplants, history of radiation, and history of other cancers, were not available in the database, which limited the comparison of our patients’ characteristics with other studies.
The duplication of patients treated in multiple hospitals was minimized. Nevertheless, it is foreseen that double counting may have been minor since MM referral/transferal between hospitals is not common.
SCT eligibility of naïve patients was indirectly ascertained using a simplified algorithm based on the patient's age and drug consumption, which mirrors the course of induction therapy. Therefore, misclassification bias should be minimal.
First and foremost, this study's findings are particularly important because they demonstrate an increased number of patients achieving more advanced lines of treatment, meaning that patients have access to innovation and improved health outcomes. This study is also important since it may contribute to informed decisions like a future review and update of the therapeutic guidelines to optimize healthcare and outcomes.
5. CONCLUSIONS
This study provides extensive, contemporary, and valuable information on real‐world clinical practice. It emphasizes the drastically evolving treatment landscape in MM over the last 5 years the substantial increase in the number of treated patients, especially those reaching more advanced treatment lines, and the importance of access to therapeutic innovation. The increasing complexity of treatment patterns necessitates continuous real‐world evidence to guide clinical practice and optimize patient outcomes. Future research should focus on long‐term outcomes and the development of personalized treatment strategies to further enhance the management of MM.
AUTHOR CONTRIBUTIONS
All the authors were involved in all phases of the work, including conception and design, data analysis and data interpretation, the writing of the manuscript, and critical revision for intellectual content. All the authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
Rui Bergantim received fees from Janssen, Amgen, BMS, Takeda, Sanofi, and Pfizer for consultancy and/or speaker services, and research funding from Amgen and BMS; Catarina Geraldes received fees from Celgene/BMS, Janssen, Amgen, Takeda, Sanofi, Pfizer, Gilead, and Abbvie for consultancy, speaker services, and/or advisory boards; Cristina João received fees from Janssen, Amgen, BMS, Takeda, Sanofi, Lilly, and Pfizer for consultancy and/or speaker services, and research funding from Janssen, Takeda, and Amgen; Paulo Lúcio received fees from Janssen, Amgen, BMS, Takeda, Sanofi, and MSD for consultancy and/or speaker services; Manuel Neves received fees for consultancy and/or speaker services from Amgen, Janssen, BMS, Sanofi, Pfizer, or Takeda; Susana Santos and Diogo Ramos are employees of Johnson & Johnson Innovative Medicine; Hugo Pedrosa and Miguel Ventura are employees of IQVIA Solutions contracted by Johnson & Johnson Innovative Medicine for developing and implementing the project, including data collection and analysis.
ETHICS STATEMENT
The authors have confirmed ethical approval statement is not needed for this submission.
PATIENT CONSENT STATEMENT
The authors have confirmed patient consent statement is not needed for this submission.
CLINICAL TRIAL REGISTRATION
The authors have confirmed clinical trial registration is not needed for this submission.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to acknowledge Catarina Silva (Health Economics & Outcomes Research Senior Researcher), employee of the Institute for Evidence‐Based Health (Lisbon, Portugal) for medical writing support. This study was funded by Johnson & Johnson Innovative Medicine, Portugal.
Bergantim R, Geraldes C, João C, Lúcio P, Neves M, Trigo F, et al. The evolving treatment landscape of multiple myeloma in Portugal: A nation‐wide retrospective cohort study of real‐world clinical practice. eJHaem. 2024;5:1144–1153. 10.1002/jha2.1035
Contributor Information
Rui Bergantim, Email: rui.bergantim@gmail.com.
Cristina João, Email: cristina.joao@fundacaochampalimaud.pt.
DATA AVAILABILITY STATEMENT
Patient‐level data used for this study cannot be made publicly available due to data access and ownership compliance regulations.
REFERENCES
- 1. Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97(8):1086–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang J, Chan SC, Lok V, Zhang L, Lucero‐Prisno DE 3rd, Xu W, et al. The epidemiological landscape of multiple myeloma: a global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol. 2022;9(9):e670–e677. [DOI] [PubMed] [Google Scholar]
- 3. GLOBOCAN 2022. [Online]. Accessed July 2024. Available from: https://gco.iarc.fr/today/en/dataviz/
- 4. Kazandjian D. Multiple myeloma epidemiology and survival: a unique malignancy. Semin Oncol. 2016;43(6):676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neves M, Trigo F, Rui B, João C, Lúcio P, Mariana N, et al. Multiple myeloma in Portugal: burden of disease and cost of illness. Pharmacoeconomics. 2021;39(5):579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludwig H, Novis Durie S, Meckl A, Hinke A, Durie B. Multiple myeloma incidence and mortality around the globe; interrelations between health access and quality, economic resources, and patient empowerment. Oncologist. 2020;25(9):e1406–e1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Torimoto Y, Shindo M, Ikuta K, Kohgo Y. Current therapeutic strategies for multiple myeloma. Int J Clin Oncol. 2015;20(3):423–30. [DOI] [PubMed] [Google Scholar]
- 8. Pinto V, Bergantim R, Caires HR, Seca H, Guimarães JE, Vasconcelos MH. Multiple myeloma: available therapies and causes of drug resistance. Cancers (Basel). 2020;12(2):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopez‐Muñoz N, Hernández‐Ibarburu G, Alonso R, Sanchez‐Pina JM, Ayala R, Calbacho M, et al. Large‐scale real‐life analysis of survival and usage of therapies in multiple myeloma. J Hematol Oncol. 2023;16(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moreau P, San Miguel J, Sonneveld P, Mateos MV, Zamagni E, Avet‐Loiseau H, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28(Suppl_4):iv52–iv61. [DOI] [PubMed] [Google Scholar]
- 11. Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA‐ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Hemasphere. 2021;5(2):e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. João C, Geraldes C, Neves M, Mariz M, Trigo F. Management of older and frail patients with multiple myeloma in the Portuguese routine clinical practice: deliberations and recommendations from an expert panel of hematologists. J Geriatr Oncol. 2020;11(8):1210–1216. [DOI] [PubMed] [Google Scholar]
- 13. João C, Bergantim R, Santos J, Afonso C, Bernardo P, Coelho H, et al. Multiple myeloma treatment guidelines by the Portuguese group of multiple myeloma. Acta Med Port. 2023;36(7–8):517–526. [DOI] [PubMed] [Google Scholar]
- 14. PORDATA. [Online]. Accessed July 2024. Available from: https://www.pordata.pt/portugal/populacao+residente+total+e+por+grandes+grupos+etarios‐513‐2545
- 15. Garrido‐Alejos G, Saborit‐Canals G, Guarga L, de Pando T, Umbria M, Oriol A, et al. Evolution of pharmacological treatments and associated costs for multiple myeloma in the public healthcare system of Catalonia: a retrospective observational study. Cancers (Basel). 2023;15(22):5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chee A, Low MSY, Vilcassim S, Grigoriadis G, Fedele PL. COVID‐19 unmasks the critical role of primary healthcare providers in the timely diagnosis of multiple myeloma. Intern Med J. 2022;52(5):885–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antunes L, Rocha‐Gonçalves F, Chacim S, Lefèvre C, Pereira M, Pereira S, et al. Real‐world treatment patterns, resource use and cost burden of multiple myeloma in Portugal. Eur J Cancer Care (Engl). 2019;28(4):e13026. [DOI] [PubMed] [Google Scholar]
- 18. Martínez‐Lopez J, Bailey A, Lambert A, Luke E, Ribbands A, Erler‐Yates N, et al. Real‐world treatment patterns, healthcare resource use and disease burden in patients with multiple myeloma in Europe. Future Oncol. 2023;19(31):2103–2121. [DOI] [PubMed] [Google Scholar]
- 19. McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem‐cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson GH, Davies FE, Pawlyn C, Cairns DA, Striha A, Collett C, et al. Lenalidomide is a highly effective maintenance therapy in myeloma patients of all ages; results of the phase III myeloma XI study. Blood. 2016;128(22):1143. [Google Scholar]
- 21. Fazio M, Del Fabro V, Parrinello NL, Allegra A, Markovic U, Botta C et al. Multiple myeloma in 2023 ways: from trials to real life. Curr Oncol. 2023;30(11):9710–9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. João C, Bergantim R, Neves M, Chacim S, Afonso C, Barradas J et al. Multiple myeloma in elderly patients—a Portuguese multicentric real‐life study. Ann Hematol. 2019;98(7):1689–1701. [DOI] [PubMed] [Google Scholar]
- 23. Morè S, Corvatta L, Manieri MV, Olivieri A, Offidani M. Real‐world assessment of treatment patterns and outcomes in patients with relapsed‐refractory multiple myeloma in an Italian haematological tertiary care centre. British journal of haematology. 2023;201(3):432–442. doi: 10.1111/bjh.18658 [DOI] [PubMed] [Google Scholar]
- 24. Weisel K, Wadlund AO, Gungor G, Dergarabetian E, Pacheco C, Masurkar N, Rodriguez‐Otero P. Real‐world study on adoption of standard‐of‐care for transplant‐eligible newly diagnosed multiplemyeloma patients between 2017 and 2020/2021 across France, Germany, Spain, and Italy. Eur J Haematol. 2022;109(4):388–397. [DOI] [PubMed] [Google Scholar]
- 25. Oriol Rocafiguera A, Steinmetz TH, Brescianini A, Gonzalez‐McQuire S, Eugene N, Abbasi A, et al. P15: evolution of multiple myeloma treatment patterns from 2015 through 2019: real‐world evidence from a European database study. Hemasphere. 2022;6(Suppl):19–20. [Google Scholar]
- 26. Lehne M, Kortüm KM, Ramasamy K, Zamagni E, d'Estrubé T, Zhuleku E, et al. Real‐world treatment patterns in patients initiating third‐line therapy for relapsed or refractory multiple myeloma in Germany, Italy, the United Kingdom, France, and Spain. Eur J Haematol. 2024;112(5):701–713. [DOI] [PubMed] [Google Scholar]
- 27. GLOBOCAN 2022. [Online]. Accessed July 2024. Available from: https://gco.iarc.who.int/media/globocan/factsheets/populations/620‐portugal‐fact‐sheet.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Patient‐level data used for this study cannot be made publicly available due to data access and ownership compliance regulations.


