Abstract
Objective
Head and trunk control is essential for enhancing engagement and participation by improving visual integration, respiration, oromotor skill, arm control, and self-care. Our study protocol aims to investigate the effect of novel Head And Trunk Control Rehabilitation (HATCoRe) device on promoting head and trunk control in children with Cerebral Palsy (CP).
Method
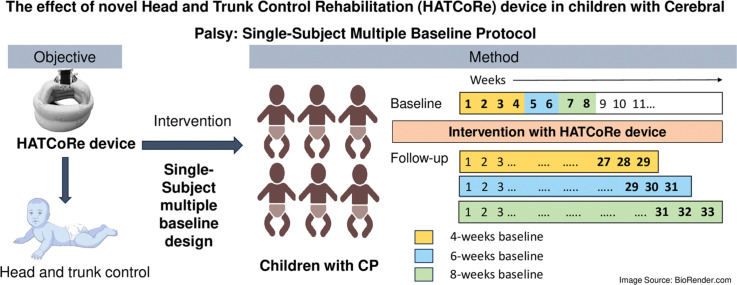
This single subject multiple baseline design trial will include six children with CP aged two to 10 years, exhibiting delayed head and trunk control, having Gross Motor Function Classification System level IV and V will be enrolled. Participants will be randomly assigned to 4-, 6-, or 8-weeks baseline phases followed by 16-week intervention phase with HATCoRe device, for 30–45 min thrice weekly; and follow-up phase of 9 weeks. The study will span for 29 to 33 weeks. An experienced pediatric physiotherapist, blinded to the baseline duration, will assess the outcome measures through 15 to 19 observations. Structured visual analysis will be used supplemented with the celeration line approach to detect statistically significant change.
Impact
HATCoRe device can enable health-care professionals to objectively measure head movement trajectories in children with CP. If proven effective, clinicians may utilize this device to create interactive and child engaging sessions.
Keywords: Developmental delay, Physiotherapy, Technology-based intervention
Method name: Single subject multiple baseline design study protocol
Graphical abstract
Specifications table
| Subject area: | Neuroscience |
| More specific subject area: | Pediatric Physiotherapy |
| Name of your protocol: | The effect of novel Head and Trunk Control Rehabilitation (HATCoRe) Device in children with Cerebral Palsy: Single-Subject Multiple Baseline Protocol |
| Reagents/tools: | This study will follow the guideline outlined in the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) -guidelines, a standard reporting guideline for the clinical trials [1] and the Single-Case Reporting guideline In Behavioural interventions (SCRIBE) guide, a recommended reporting guideline for the single case experimental design [2] |
| Experimental design: | A Non-Concurrently Single Subject Multiple Baseline design wherein data are evaluated by comparing each participant's performance, utilizing a visual analysis method, which is a traditional method that serves as the gold standard for assessing quantitative procedures. |
| Trial registration: | CTRI/2021/09/036,934 CTRI/2022/03/041,269 |
| Ethics: | Inclusion of the participants will occur following the provision of Participant Information Sheet and obtaining their written informed consent. Participants have the option to withdraw from the study at any time without facing consequences. |
| Value of the Protocol: |
|
Description of protocol
Introduction
A critical component in predicting ambulatory status in children with CP is head and trunk control, which is established as a cornerstone of postural control [3]. Postural control is vital for stability and forms the foundation for movement by correcting and maintaining the alignment of head, trunk and limbs [4]. Normal motor skill development required mature postural control, as an immature postural system can limit coordinated arm and hand movements, and reflex inhibition in various behaviors [5]. According to the Systems Theory of Motor Control and Dynamic Systems Theory [6], postural control is the result of a complex interaction of musculoskeletal and neurological systems known as the “postural control system,” which is organized by task and environment [7,8]. Moreover, mastery of head control precedes the development of pivotal skills such as visual hand regards, reaching, grasping and manipulation of objects [9,10], as well as, oromotor skills [11], sitting [12], and walking [13]. Furthermore, head control plays a vital role in visual orientation and balance, owing to the position of the eyes and vestibular systems. Hence, delayed head and trunk control has an impact on various areas of a child's life [14], including eye-hand coordination, gaze refinement, motor planning, and fine manipulation [15,16], all addressed as physiotherapy treatment goals.
Postural control occurs as a result of cranial-caudal progression starting approximately 2 or 3 months of age, establishing head stability on the trunk [17,18]. This upright/midline position is recognized as a basic level of head control [19], that improves over the first year and beyond [20]. A dynamically stable head may result in less biomechanical disturbance of the trunk and arms, allowing for better visual function and postural control [15]. Controlling the head and trunk is crucial for integrating vision, respiratory functions, oromotor skill, and arm control, thereby improving quality of life as per “United Nations Sustainable Developmental Goals 3 – Good Health and Well-being” [21]. Typically, the postural control develops from the neck and progresses down to the upper thorax, thereby facilitating independent sitting around 6 months of age [22]. Trunk control is crucial for providing primary stability to the joint proximally, mainly through the activation of extrapyramidal and anterior corticospinal tract, consequently facilitating accurate performance of fine motor tasks in the distal extremities via the lateral corticospinal tract [23]. This progression allows the infants to explore the environment and develop further physical and cognitive skills [24]. Hence, the ability to control the head and trunk not only enhances the postural control but also fosters independence and engagement in daily tasks for children with CP, ultimately improving their quality of life [25].
Children with CP require long-term rehabilitation, which can put a strain on both family and healthcare system. Many studies have incorporated both conventional (exercises, NDT) [26] and recent treatment approaches (Headpod device [25], treadmill training [27], VR [28], ICP [29], robotics [30], hippotherapy [31]) for rehabilitation of children with CP. However, these often focuses on the body structure and function component of ICF, and little attention provided to the contextual factors (for instance; familial involvement and recreational activities) [25,29,32]. Therefore, incorporating fun and interactive strategies during exercise sessions based on “The F-words in Childhood Disability" may be helpful [33]. Aligned with the ICF model, this approach focuses on ‘function’, ‘family’, ‘fitness’, ‘fun’, ‘friendship’, and ‘future’ [34]. However, challenges in implementing technology-based interventions, like lack of infrastructure and expensive equipment, act as barriers for parents in utilizing these interventions in rehabilitation practices [35].
Therefore, the need of the hour is a cost-effective, user-friendly technology-based intervention, for improving head and trunk control in children with CP. As a result, a team of professional physiotherapists and engineers invented the “Head And Trunk Control Rehabilitation (HATCoRe) device,” which is an indigenous technology-based intervention aimed at improving head and trunk control in children with developmental delays.
The HATCoRe device is a patent pending device (Reference number 202,343,006,825) which has an integration of hardware and software. The hardware includes a motion sensor and microcontroller and a Bluetooth transreceiver housed in a small box, powered by a rechargeable lithium-ion battery with charging protection circuit. The motion sensor measures the head position in X, Y and Z axes relative to a reference point in each plane, transmitting data wirelessly to an android application for auditory and visual feedback. The box will be attached to a commercially available cap, worn by the child, during intervention (Fig. 1). The android application will be installed on an android device (android phone, smart TV, tablet), connecting wirelessly to the hardware. The device combines user-preferred auditory and visual stimuli to aid head and trunk control, with exercise data stored for analysis in the android device. (Fig. 2).
Fig. 1.
Cap with HATCoRe device.
Fig. 2.
HATCoRe application with exercise module.
Therefore, the objective of the study protocol is to investigate the effect of HATCoRe device on promoting head and trunk control in children with CP, utilizing a single subject multiple baseline design. This study design would be the most appropriate for this study protocol as we can expect variability in functioning majorly in this population. As a pragmatic trial, it efficiently establishes a robust cause-and-effect relationship among the intervention and behavioral changes, emphasing personalized, individual-based care [36]. We hypothesize that the innovative and interactive HATCoRe device can assist children with developmental delays train their head and trunk musculature, resulting in improved head and trunk control.
Materials and method
This protocol outlines the approach for implementing a Single Subject Multiple Baseline Design, chosen for its ability to assess causal relationships through multiple baseline-treatment comparisons, with numerous data points. The phase transitions can occur at different points in time, in terms of calendar date, duration of baseline, and the number of sessions [37]. This study will follow the guideline outlined in the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines, a standard reporting guideline for the clinical trials [1] and the Single-Case Reporting guideline In Behavioural interventions (SCRIBE) guide, a recommended reporting guideline for the single case experimental design [2].
Trial design and setting
The trial employs a Non-concurrent Single Subject Multiple Baseline Design across participants with 4-, 6- and 8-weeks baseline phases using blocked randomization for participation allocation. Participant will be divided into three blocks of two and pseudorandom number generator will be used for sequence generation. The screening, assessment, and intervention will be carried out at the physiotherapy department in a tertiary care hospital in Southern India.
Trial recruitment and allocation
A blinded investigator will assess participants for inclusion using a screening form. A participant information sheet detailing the intervention, procedure, and potential outcomes will be provided. Parent will be required to provide written informed consent, and assent for the participants over seven years of age, assent from children will be sought.
Study participants
The sample will include six children diagnosed with CP (1) between two and 10 years, (2) having delayed head and trunk control, (3) unable to hold their head upright against gravity for less than a minute with support, (4) requiring adapting seating to support their posture adequately, (5) having functional vision and hearing, (6) Gross Motor Function Classification System level IV and V who typically exhibit poor head and trunk control and (7) grade of 0 to 12 in the Head Control Scale (HCS).
Criteria for the exclusion of the participants are children who (1) have established genetic disorders, (2) have undergone surgical procedures, (3) are medically unstable, (4) have history of epilepsy, (5) have auditory and visual hypersensitivity.
Sample size
The estimated sample size for the study is six. This study design usually suggests smaller sample size, as it involves multiple baselines measurements [38]. Opting for this sample size increases the likelihood of detecting changes in head and trunk control after treatment, indicating the ability to capture evidence of a treatment effect. In Single Subject Experimental Design (SSED), the determination of sample size is based not on the number of participants, but on the quantity of data points necessary per participant to detect significant changes in the outcomes. The power in SSED stems from the frequency of repeated measures rather than the number of participants involved [39]. Although no specific formula exists, consideration of factors such as baseline stability, number of data points, effect sizes, and statistical analysis may assist in determining an appropriate number of data point to detect significant changes in the outcome measures [40]. Moreover, to strengthen the internal validity of the study, the length of baseline of the participants will be randomized via blocked randomization sequence with pseudo random number generator for sequence generation as described in Table 1.
Table 1.
Blocked Randomization Sequence generated by pseudorandom number generator.
| Participant | Baseline Length |
|---|---|
| 1 | C |
| 2 | A |
| 3 | B |
| 4 | C |
| 5 | B |
| 6 | A |
A = 4-weeks baseline.
B = 6- weeks baseline.
C = 8-weeks baseline.
Procedure
Participant will receive an information sheet and written informed consent will be sought. Functional vision and hearing screening will be conducted. Two video cameras will record the sessions for assessing parameters obtained from the HATCoRe device and parameters obtained from the recorded videos.
Randomization
Randomization will be achieved through a block randomization process, organizing participants into three blocks of two. The participants will be allotted to 4-, 6-, or 8-weeks baseline phases, accordingly. The randomization sequence will be generated using a pseudorandom number generator and the sample function of the R statistical software, which will help in improving the study's internal validity [41].
Demographic information and baseline outcome assessments
Demographic information will be collected and detailed evaluation of the child will be conducted using a proforma. Baseline assessments including HCS, Segmental Assessment of Trunk Control (SATCo), Global Perceived Effect (GPE) scale, and Gross Motor Function Measure (GMFM)−66, will be documented before the intervention. Outcome measures will be periodically evaluated during both the intervention phase and follow-up phase, as detailed later in the manuscript.
Intervention
At the beginning of each session, the child wears the cap with the HATCoRe device. The software creates a patient profile with demographic details, medical history, and desired auditory and visual stimuli as cartoon images, Graphics Interchange Format (GIF) and short rhymes/songs. The HATCoRe hardware connects to the software via Bluetooth. Based on sensor data (X, Y, Z axes values) from the hardware, initial calibration is done manually, depending on the plane of exercise module (sagittal, transverse and frontal). Calibration involves three reference points: “starting point” of head raise, the “optimal position” (head aligned with the neck and trunk in upright position), and the “ending point” of neck movement in the chosen plane (sagittal, transverse and frontal) Each plane has specific reference points such as for the “starting position” as zero for chin touching torso in sagittal, right ear lobe touching right shoulder in frontal for frontal, and chin touching right shoulder in right neck rotation for transverse plane. The optimal position is when the head aligns with the neck and trunk.
An android tablet will be placed at approximately 100 cm from the participant on a stand. At the start of a session, an auditory stimulus prompts the child to initiate head movement, with the expectation that the child will follow the auditory stimulus and lift the head. As the child raises the head, a visual stimulus will gradually appear on the screen, acting as an additional stimulus. When the child reaches an optimal position, the auditory stimulus will be changed to auditory feedback of the child's preference (example; clapping), and the visual stimulus will be converted to dynamic display (example: cartoon GIF), to maintain the aligned head position.
The device has undergone initial testing with five physiotherapist experts, to gain feedback for reiteration of device. Suggestions included a full-screen view of visual stimulus, continuous looped auditory stimuli until the optimal position, more auditory stimulus options, and use of colorful and contrasting GIF images. The suggestions were incorporated, and the software was iterated. Following this iteration process, five typical and five atypical children will be recruited to standardize the parameters obtained from the device (number of head lifts, sustenance time of head lift, degree of cervical movement); parameters obtained from the participants (enjoyment, reaction time, comfort, and attractiveness of the stimulus); and parameters obtained from the video (no. of attempts to lift the head, successful attempts of head lift, total duration of cap worn by the participants) for assessment and intervention of children with developmental delay using the HATCoRe device. After observing the challenges faced during the trial, modifications in the device or protocol if required, will be made.
The study will adhere to the What Works Clearinghouse standards for single-case design, involving baseline, intervention, and follow-up phases in the single subject multiple baseline study across six participants [42].
During the baseline phase, no intervention will be provided, and they will continue their routine home exercises. Video with two cameras will be captured to acknowledge the head and trunk movements. This phase will last for “4-, 6-, and 8- weeks” for each participant simultaneously, via blocked randomization represented in Table 1. Individual participants will have different starting points to eliminate confounding factors.
The intervention phase will span 16 weeks, involving three sessions per week lasting 30–45 min, approximately. The participants enter the phase upon completing their randomized baseline phase. The initial “0–2 weeks” will involve the participant sitting on the mother's lap (‘A’) wearing cap with the HATCoRe device attached on it, then the system will be switched on. Initially, familiarization time will be provided with the HATCoRe device to the child. The child's “profile” will be customized on HATCoRe application with demographics and basic medical history. Mother will select the desired visual and auditory stimuli for the session. The device will be calibrated for the “starting point”, “optimum”, and “ending point” of Cervical Range of Motion (CROM) of the child, such that their desired auditory stimulus is played while the child places the head at the “starting point”, and the desired visual stimulus will be gradually displayed on the screen. Upon reaching the “optimal point” (head in line with neck and trunk), the image will be perfectly fitting the display and will be converted to a GIF image, and another auditory feedback will be provided.
In subsequent weeks, the procedure will continue with “A” and “participant lying in inclined position to 45° (‘B’)” for “2–4 weeks”. Similarly, for “4–6″ weeks, “A + B + participant lying supine (‘C’)” and for “6–8″ weeks, “A + B + C + participants lying prone (‘D’)”. For the rest of the sessions, i.e., “8–16″ weeks, the procedure will be carried out in all the positions, i.e. A + B + C + D to document the intervention results.
Video will be captured with two video cameras to monitor the child's performance for the “parameter obtained from the device” and “parameter obtained from the video”. For the ease of understanding, the progression of intervention is mentioned in Table 2.
Table 2.
Progression of the intervention.
| 0–2 weeks | Participant will be intervened in the mother's lap (A) |
| 2–4 weeks | A + Participant will be intervened in an inclined position to 45° (B) |
| 4–6 weeks | A + B + Participant will be intervened in supine position (C) |
| 6–8 weeks | A + B + C + Participant will be intervened in prone position (D) |
| 8–16 weeks | A + B + C + D |
In the follow-up, intervention will not be provided. Video recording will document the head and trunk movements, aiming to prevent them from returning to baseline after the intervention is completed. To ensure comprehensive reporting, the Template for intervention description and replication (TIDieR) checklist will be used (Table 3) [43].
Table 3.
Template for intervention description and replication (TIDieR) checklist for HATCoRe device intervention.
| TIDieR Items | Description of Items |
|---|---|
| Name of the intervention for experimental/ comparator group | HATCoRe device training |
| Rationale | Principle of motor learning and neuroplasticity (participation, variability, inactivity, feedback) |
| Materials used in the intervention | HATCoRe device (hardware), HATCoRe application (software), strap to attach the device to the cap, android device (tablet), tablet holder, chair, mattress, wedge, pillow |
| Intervention Procedure | Frequency of the intervention: Thrice per week |
| Intervention: Provided with the HATCoRe device | |
| Time: After completion of the baseline phase, intervention will be provided for 30–45 min per session | |
| Type: Progression of the intervention to different positions such as; sitting, prone on the wedge, supine and prone, to train various cervical ROM exercises | |
| Provider | The intervention will be provided by a physiotherapist who is trained by the supervisors of the team |
| Mode of intervention delivery | Individual therapy sessions |
| Setting of intervention | Study screening, interventions, and assessments will be conducted at the physiotherapy departments of tertiary care hospital located in Southern India |
| Dosage | 30 to 45 min per session, 3 times per week, for 16 weeks |
| Tailoring | The profile of the individual participant will be set according to the child's preference, i.e., the mother will be asked for the child's desired auditory and visual stimuli to be used during the session. If the child does not respond during the session with the chosen auditory and visual stimuli, the profile can be reset during the session for his desired stimuli |
| Modifications | If the participant does not respond to a profile or the one exercise session (flexion, extension, lateral flexion, rotations), the training of cervical ROM can be provided as per the participants desire |
| Fidelity assessment | The outcome measures will be assessed by a blinded investigator who is experienced in pediatric handling, as well as by the other investigator (supervisor) to determine the Interrater Reliability of the outcome measures for 1 to 2 participants. A guide to evaluate the video will be provided to the blinded investigator for the assessment of the parameters obtained from the participant and the video. And the videography of the session will be provided to the blinded investigator to rate the outcome measures |
Participant withdrawal
If any adverse events/effects occur during the study or if a participant/parent withdraws consent, they can discontinue participation from assigned intervention without facing consequences.
Harms
If participants experience any study-related harm, the principal investigator will ensure that appropriate medical care is provided. Adverse effects will be reported to the review board and ethics committee, and compensation for such effects will be covered by the principal investigator (SS).
Outcome measures
Primary outcome measures
-
(1)
HCS:
Jodi Thomas et al. introduced the new scale for assessing head control in four postures on a 0–4 scale: “prone”, “supine”, “pull to sit”, and “supported sitting”. Inter-rater reliability measured by Fleiss's weighted kappa coefficient was consistently high, ranging from 0.68 to 0.91. The study concludes that the HCS is valuable and user-friendly tool beneficial to therapeutic practise [44].
-
(2)
SATCo:
SATCo systematically assesses trunk control through static, active, and reactive components. Inter-rater and intra-reliability are high (ICC > 0.84 and >0.98), and concurrent validity with the Alberta Infant Motor Scales is strong 0.86 to 0.88. Therefore, SATCo provides clinicians with reliable and valid measure in evaluating trunk control [45].
Secondary outcome measures
-
(3)
GMFM-66:
The GMFM-66 comprises 66 items from the original 88, effectively captures gross motor function of children with CP. The items, arranged by difficulty level on a 0 to 100 scale, provide insights for goal setting [46]. A software tool called as Gross Motor Ability Estimator (GMAE) will be used to score the GMFM [47]. The ICC for GMFM 66 for inter-rater reliability is 0.93 and intra-rater reliability is 0.99–1.00 for the total score [46].
-
(4)
GPE Scale:
GPE scale is a widely used tool for assessing patients' perceptions of their condition in research and therapy. GPE assesses global change in the patient's primary complaint, demonstrated excellent reproducibility (ICC values of 0.90–0.99), and test–retest reliability [48].
-
(5)
Parameters obtained from the device:
-
(i)
Degree of CROM (): It is the CROM across flexion, extension, right and left lateral flexions, and right and left rotations.
-
(ii)
Sustenance time of head lift (sec/min): The amount of time the participant sustains the head aligned over the neck and thoracic spine.
-
(6)
Parameters obtained from the video:
-
(i)
No. of attempts to lift the head (N =): It is the number of times the participant attempts to lift the head up, upon the auditory stimulus.
-
(ii)
Successful attempts of head lift (N =): The number of times, the participant aligns their head with their neck and trunk.
-
(iii)
Total duration of cap worn by the participants (sec/min): The total duration of time, participant wills to put the cap on, in seconds or minutes.
Among the outcome measures, the HCS and SATCo are the primary outcome measures, whereas, GMFM-66, GPE scale, parameters obtained from the device and video are the secondary outcome measures.
Outcome assessment
There are different time points of evaluation for the baseline, intervention, and follow-up/maintenance phases of the study design.
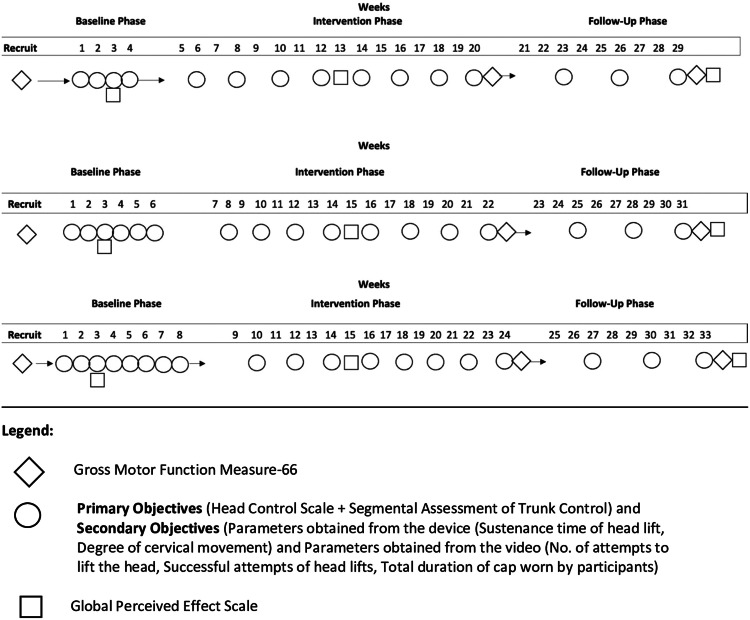
During the baseline phase, a blinded evaluator will weekly assess outcome measures (HCS, SATCo, parameters obtained from the device and parameters obtained from the video) based on participant's baseline phases. (Table 4). At 3rd week, GPE scale will be assessed from the parents. GMFM-66 will be evaluated post-recruitment.
Table 4.
Guide to evaluate the video.
| Enjoyment | Reaction time | Comfort | Attractiveness |
|---|---|---|---|
| “Visual Analogue Scale” with facial expressions to depict the amount of enjoyment will be used to grade the participant's enjoyment during the session | The amount of time taken by the participants to respond towards the auditory stimulus, in seconds or in minutes | The amount of time taken by the participants to respond towards the auditory stimulus, in seconds or in minutes | The amount of time taken by the participants to respond towards the auditory stimulus, in seconds or in minutes |
“Enjoyment” will be rated by the participant's mother.
For the intervention phase, outcome measures; will be assessed fortnightly (8 times), and GPE will be evaluated mid-intervention, GMFM-66 assessment follows immediately after the intervention phase.
In the follow-up, outcome measures will be assessed once in three weeks for nine weeks (3 times). At the end of this phase, GPE scale will be assessed from parents to determine the goals achieved (In total 3 times). GMFM-66 will be evaluated after the follow-up phase (In total 3 times).
Outcome measures will be assessed by an experienced pediatric physiotherapist serving as a blinded investigator. Outcomes measures including HCS, SATCo, parameters obtained from the device and parameters obtained from the video will be assessed for a total of 19, 17 and 15 times for the 8-, 6-, and 4- weeks of baseline phases respectively, the GPE and GMFM-66 will be assessed thrice.
The schedule for evaluation of outcome measures is mentioned in Table 5 and Fig. 3. The timeline of outcome assessment is mentioned in Table 6.
Table 5.
Schedule for assessment of outcome measures.
| Outcome Measures | Baseline phase (4-, 6-, 8- weeks) | Intervention Phase (16 weeks) | Follow-up Phase (9 weeks) | No. of observations |
|---|---|---|---|---|
| HCS | Weekly once | Fortnightly | Once in three weeks | 19 (8-weeks baseline) |
| 17 (6-weeks baseline) | ||||
| 15 (4 weeks-baseline) | ||||
| SATCo | Weekly once | Fortnightly | Once in three weeks | 19 (8-weeks baseline) |
| 17 (6-weeks baseline) | ||||
| 15 (4 weeks-baseline) | ||||
| Sustenance time of head lift (sec/min) | Weekly once | Fortnightly | Once in three weeks | 19 (8-weeks baseline) |
| 17 (6-weeks baseline) | ||||
| 15 (4 weeks-baseline) | ||||
| Degree of cervical movement (°) | Weekly once | Fortnightly | Once in three weeks | 19 (8-weeks baseline) |
| 17 (6-weeks baseline) | ||||
| 15 (4 weeks-baseline) | ||||
| No. of attempts to lift the head (N =) | Weekly once | Fortnightly | Once in three weeks | 19 (8-weeks baseline) |
| 17 (6-weeks baseline) | ||||
| 15 (4 weeks-baseline) | ||||
| Successful attempts of head lift (N =) | Weekly once | Fortnightly | Once in three weeks | 19 (8-weeks baseline) |
| 17 (6-weeks baseline) | ||||
| 15 (4 weeks-baseline) | ||||
| Total duration of cap worn by the participants (sec/min) | Weekly once | Fortnightly | Once in three weeks | 19 (8-weeks baseline) |
| 17 (6-weeks baseline) | ||||
| 15 (4 weeks-baseline) | ||||
| GPE | 3rd week baseline | At mid-intervention | End of follow-up | 3 |
| GMFM-66 | After recruitment | After intervention | End of follow-up | 3 |
Fig. 3.
Schedule for assessment of the outcome measures.
Table 6.
Timeline of Outcome Assessment.
| 1. Baseline Phase | 4 - weeks | 6 - weeks | 8 - weeks | |||||
|---|---|---|---|---|---|---|---|---|
| Outcome measures | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 |
| Head Control Scale (HCS) | ||||||||
| Segmental Assessment of Trunk Control (SATCo) | ||||||||
| Parameters Obtained from the device | ||||||||
| Sustenance time of head lift (sec/min) | ||||||||
| Degree of cervical movement (°) | ||||||||
| Parameters obtained from the video | ||||||||
| No. of attempts to lift the head (N =) | ||||||||
| Successful attempts of head lifts (N =) | ||||||||
| Total duration of cap worn by participants (sec/min) | ||||||||
| Global Perceived Effect (GPE) Scale | Week 3 | |||||||
| 2. Intervention Phase: (16-weeks of intervention after Baseline Phase) | ||||||||
| Outcome Measures | Post Baseline | |||||||
| Day 14 | Day 28 | Day 42 | Day 56 | Day 70 | Day 84 | Day 98 | Day 112 | |
| Head Control Scale (HCS) | ||||||||
| Segmental Assessment of Trunk Control (SATCo) | ||||||||
| Sustenance time of head lift (sec/min) | ||||||||
| Degree of cervical movement (°) | ||||||||
| No. of attempts to lift the head (N =) | ||||||||
| Successful attempts of head lifts (N =) | ||||||||
| Total duration of cap worn by participants (sec/min) | ||||||||
| Global Perceived Effect (GPE) Scale | At Mid-Intervention Phase (Post-Baseline Phase) Day 63 | |||||||
| 3. Follow-up Phase: | ||||||||
| Outcome Measures | 3rd week Post-intervention | 6th week Post-intervention | 9th week Post-intervention | |||||
| Head Control Scale (HCS) | ||||||||
| Segmental Assessment of Trunk Control (SATCo) | ||||||||
| Sustenance time of head lift (sec/min) | ||||||||
| Degree of cervical movement (°) | ||||||||
| No. of attempts to lift the head (N =) | ||||||||
| Successful attempts of head lifts (N =) | ||||||||
| Total duration of cap worn by participants (sec/min) | ||||||||
| Global Perceived Effect (GPE) Scale | At the end of follow-up phase 9th week post intervention | |||||||
| Pre and Post Score of Gross Motor Function Measure-66 (GMFM-66): | ||||||||
| Outcome measures | Pre-Score (After recruitment) | Immediately after intervention | Post-Score (After follow-up phase) | |||||
| i. GMFM – 66 | ||||||||
Statistical analysis
In SSED, data are evaluated by comparing the participant's performance during the baseline and intervention phases [49]. Visual analysis, a traditional method in SSED [50], serves as the gold standard for assessing quantitative procedures [51]. Data collected on the parameters obtained from participants and the outcome measures will be entered into MS-Excel for graphical representation (line graphs). The visual analysis parameters will include level, trend, variability, immediacy of effect and Percentage of Non-Overlapping Difference (PND). The celebration line, a supplementary statistical method that assesses the proportion of data points falling above & below the median line, will also employed [52]. Changes in head and trunk movement due to intervention are determined by mean score changes based on visual inspection criteria outlined in Table 6, and celebration line calculations. The assessment may consider past research regarding a minimum clinically important difference (MCID) for the head and trunk movement, in case some participant demonstrate unstable baseline [53,54]. Results on all measures will be tabulated and descriptive statistics including measures of central tendency will be presented for each of the visual inspection and the statistical analysis.
Timeline
Participant recruitment has been carried out tentatively from June 2023. Data analysis and report writing will be conducted tentatively from July 2024 onward.
Data collection and management
The collected data will be securely stored at the main study center to protect participant confidentiality. Authorized personnel from the ethics committee or regulatory bodies may review study records, including participant's detail. The HATCoRe device collects data, including the demographics, brief medical history, patient profile, and time spent during each trial and session, from the system processor. The data from the device will undergo anonymization and managed exclusively by the primary investigator (SS) with no disclosure to third parties.
Data monitoring and auditing
The local ethical committee will oversee data security. Any adverse events or protocol deviations will be reported to the Institutional Ethics Board, and no formal audit is planned for this clinical trial.
Dissemination
A dissemination strategy will be formulated in the early trial phase, with findings updated in the Clinical Trial Registry of India, national/ international conferences and peer-reviewed journals. The participants/parent will also receive result dissemination. Authorship will be adhered to the International Committee of Medical Journal Editors (ICMJE) recommendations.
Ethics approval
Participants will receive information about the intervention, including benefits and potential risks through the participant information sheet. The trial will begin once the written informed consent is obtained from parents and assent from children older than seven years old. The study protocol has been approved by the Institutional Ethics Committee, Kasturba Medical College and Kasturba Hospitals, Manipal Academy of Higher Education, Manipal, Karnataka, India, IEC reference Number – 373/2021 and 650/2021.
Protocol amendments
Any amendments to the study protocol in later stages, will be communicated to institutional review board, ethics committee, study participants, and trial registry with the ethics committee amendment form attached.
Informed consent
Inclusion of the participants will occur following the provision of Participant Information Sheet and obtaining the written informed consent from the parents. If the child is more than 7 years of age, then the assent will be sought. Participants have the option to withdraw from the study at any time without facing consequences.
Discussion
Head control is vital for integrating vision, regulating respiratory, and deglutition, impacting the development of higher-level motor skills crucial for child's independence and quality of life [44,55].
Trunk control, an integral component of postural control, contributes to trunk stability/mobility, fostering anti-gravity control in infancy. This control is often compromised in children with CP, hindering independent engagement [56]. A stable trunk facilitates infants to orient themselves in their environment, promoting the development of cognitive, social and communication abilities [57].
Various interventions, from traditional physiotherapy (active/passive) to technology-based (manual/digital) interventions [58], prove effective in enhancing head and trunk control in children with CP [59]. Interventions such as; ICP, headpod, and HeadUp device [32,25,60] have been useful in gaining the attention of the children, however, some are too expensive for the stakeholders, while, some lack the component of variability and feedback following the interventions.
Moreover, improving head control necessitates the activation and strengthening of muscles in the neck and upper thorax to stabilize and facilitate head movement, This process contributes to the overall postural stability and trunk control by providing a stable base of support [61]. Consequently, interventions focused on improving head control may indirectly benefit trunk control by targeting these muscle groups. Hence, recognizing the interdependent connection between the head and trunk emphasizes the significance of incorporating exercises for both components to enhance static and dynamic postural control [22]. This trial aims to address these shortcomings by developing an affordable, attention-grabbing, and innovative technology-based intervention, aimed at improving head control and trunk control. Additionally, the evidence generated from this study may contribute valuable insights to the benefits of utilizing child-friendly stimuli over routine treatment approaches.
Potential impact and significance
The HATCoRe device, innovated by a dedicated team of physiotherapists and engineers, uniquely focuses on enhancing head and trunk control in children with CP through visual and auditory stimuli. If proven effective, re/habilitation via HATCoRe device could be commercialized for parents/stakeholders, who seek an easily applicable home-based rehabilitation device.
Our study is the first clinical trial aimed at standardizing the parameters (device, participants, and video) for assessment and intervention for promoting head and trunk control in children with developmental delay using HATCoRe device. Pilot testing of the device and its parameters will guide in reiterations in hardware and software of the device, which will be updated subsequently during the study trial. After the modifications/reiterations, we aim to investigate the effect of HATCoRe device on promoting head and trunk control in children with CP by incorporating the Single Subject Multiple Baseline Design across participants.
Strength and weaknesses
The Single Subject Experimental Design (SSED) is a valuable choice for evaluating intervention effectiveness, offering advantages over group-level designs by providing a more accurate individual impact assessment [62,63]. Moreover, randomizing participant baselines will further strengthen our study, making it efficient for examining novel technology-based interventions [64,65]. Irrespective of potential 29 to 33-weeks visits, completion of all phases can be a hidden strength, generating numerous data to deem our intervention with the HATCoRe device as effective.
Potential contribution to the field
As a future scope, the device can be provided to the parents of children with CP and train them to use it as a home-based rehabilitation.
Consent for publication
All authors have reviewed the final manuscript and agreed to its publication.
Clinical trial registration
Clinical Trial Registration of India, registration number: CTRI/2021/09/036,934, registered on September 20, 2021 (Phase I of the study), and registration number: CTRI/2022/03/041,269, registered on March 22, 2022 (Phase II of the study) (Table 7).
Table 7.
Visual Inspection of Single Subject Experimental Design.
| Level | Trend | Variability | Immediacy of effect | Percentage of Non-Overlapping Data | Celeration Line |
|---|---|---|---|---|---|
| Median/mean of all data points of the outcome measures, that allows to compare the changes in the levels between the phases | The tendency of the data points of the outcome measures to go upwards or downwards within a phase, to determine the progression or decline in the data points of the outcome measures between the phases | Spread/fluctuation of data point of outcome measures within a phase, to determine the changes between the phases | It helps to compare the extent to which the level, trend, and variability of the last three data points of the outcome measures in one phase are discriminably different from the first three data points of outcome measures in the next | Percentage of data points of outcome measures during “intervention” surpassing extreme values in the baseline According to Scruggs et al., < 50 %: Ineffective 50 to 70 %: Questionable effectiveness 70 to 90 %: Effective intervention > 90%: Very Effective Intervention |
Calculates proportion of data points of outcome measures, falling above & below the median line, to find out if the intervention is effective. |
CRediT authorship contribution statement
Shristi Shakya: Conceptualization, Methodology, Investigation, Visualization, Writing – original draft, Writing – review & editing. Sivakumar Gopalakrishnan: Conceptualization, Project administration, Software, Resources, Writing – review & editing. Dana Anaby: Conceptualization, Supervision, Writing – review & editing. Shamanth Madapura S.: Software, Project administration, Writing – review & editing. Harikishan Balakrishna Shetty: Software, Project administration, Writing – review & editing. Hitesh Hasmukhlal Shah: Supervision, Writing – review & editing. V.S. Venkatesan: Validation, Supervision, Writing – review & editing. Bhamini Krishna Rao: Supervision, Methodology, Project administration, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding details
This research did not receive funding from public, commercial, or not-for-profit sectors.
Acknowledgments
The authors express gratitude to the directors of EKAM Rehab Tech Private Limited Company for provision of the HATCoRe device and all necessary equipments to carry out the research, as well as the Department of Biomedical Engineering, Manipal Institute of Technology, and Manipal Academy of Higher Education, Manipal, India for the technical support. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2024.102649.
Appendix. Supplementary materials
Data availability
No data was used for the research described in the article.
References
- 1.Chan A.W., Tetzlaff J.M., Altman D.G., Laupacis A., Gøtzsche P.C., Krleža-Jerić K., et al. SPIRIT 2013 Statement: defining Standard Protocol Items for Clinical Trials. Ann. Intern. Med. 2013;158(3):200. doi: 10.7326/0003-4819-158-3-201302050-00583. Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tate R.L., Perdices M., Rosenkoetter U., McDonald S., Togher L., Shadish W., et al. The Single-Case Reporting Guideline In BEhavioural Interventions (SCRIBE) 2016: explanation and elaboration. Arch. Sci. Psychol. 2016;4(1):10–31. Apr 14. [Google Scholar]
- 3.Saavedra S. Trunk control in cerebral palsy: are we ready to address the elephant in the room? Dev. Med. Child Neurol. 2015;57(4):309–310. doi: 10.1111/dmcn.12614. Apr. [DOI] [PubMed] [Google Scholar]
- 4.Bobath B. 3rd ed. W. Heinemann Medical Books; Aspen Systems Corp; London: Rockville, MD: 1985. Abnormal Postural Reflex Activity Caused By Brain Lesions; p. 113. [Google Scholar]
- 5.Mercuri E., Dubowitz L. Neurological examination of the newborn. Curr. Paediatr. 1999;9(1):42–50. Mar. [Google Scholar]
- 6.Shumway-Cook A., Woollacott M.H. 3rd ed. Lippincott Williams & Wilkins; Philadelphia: 2007. Motor control: Translating Research Into Clinical Practice; p. 612. [Google Scholar]
- 7.Bertenthal B.I., Boker S.M., Xu M. Analysis of the perception-action cycle for visually induced postural sway in 9-month-old sitting infants. Infant. Behav. Dev. 2000;23(3–4):299–315. Mar. [Google Scholar]
- 8.Barela J.A., Godoi D., Freitas P.B., Polastri P.F. Visual information and body sway coupling in infants during sitting acquisition. Infant. Behav. Dev. 2000;23(3–4):285–297. Mar. [Google Scholar]
- 9.Lima-Alvarez C.D.D., Tudella E., Van Der Kamp J., Savelsbergh G.J.P. Early development of head movements between birth and 4 months of age: a longitudinal study. J. Mot. Behav. 2014;46(6):415–422. doi: 10.1080/00222895.2014.929562. Nov 2. [DOI] [PubMed] [Google Scholar]
- 10.Simon A.D.S., Pinho A.S.D., Grazziotin Dos Santos C., Pagnussat A.D.S. Facilitation handlings induce increase in electromyographic activity of muscles involved in head control of cerebral palsy children. Res. Dev. Disabil. 2014;35(10):2547–2557. doi: 10.1016/j.ridd.2014.06.018. Oct. [DOI] [PubMed] [Google Scholar]
- 11.chul Min K, min Seo S, soon Woo H. Effect of oral motor facilitation technique on oral motor and feeding skills in children with cerebral palsy : a case study. BMC Pediatr. 2022;22(1):626. doi: 10.1186/s12887-022-03674-8. Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arafat M., Shoukry K., El-Maksoud G.A., Kilany A. Effect of different levels of segmental trunk stability training on sitting and upper limbs functions in children with bilateral spastic cerebral palsy. Int. J. Health Sci. 2022:10420–10436. Jul 30. [Google Scholar]
- 13.Einspieler C., Marschik P.B., Prechtl H.F.R. Human motor behavior: prenatal origin and early postnatal development. Z Für Psychol. J. Psychol. 2008;216(3):147–153. Jan. [Google Scholar]
- 14.Mital M.A., Belkin S.C., Sullivan M.A. An approach to head, neck and trunk stabilization and control in cerebral palsy by use of the milwaukee brace. Dev. Med. Child Neurol. 2008;18(2):198–203. doi: 10.1111/j.1469-8749.1976.tb03629.x. Nov 12. [DOI] [PubMed] [Google Scholar]
- 15.Bertenthal B., von Hofsten C. Eye, head and trunk control: the foundation for manual development1the responsibility was shared equally between the authors.1. Neurosci. Biobehav. Rev. 1998;22(4):515–520. doi: 10.1016/s0149-7634(97)00038-9. Mar. [DOI] [PubMed] [Google Scholar]
- 16.Pelz J., Hayhoe M., Loeber R. The coordination of eye, head, and hand movements in a natural task. Exp. Brain Res. 2001;139(3):266–277. doi: 10.1007/s002210100745. Aug 1. [DOI] [PubMed] [Google Scholar]
- 17.Assaiante C. Development of locomotor balance control in healthy children. Neurosci. Biobehav. Rev. 1998;22(4):527–532. doi: 10.1016/s0149-7634(97)00040-7. Mar. [DOI] [PubMed] [Google Scholar]
- 18.Thelen E., Corbetta D., Spencer J.P. Development of reaching during the first year: role of movement speed. J. Exp. Psychol. Hum. Percept. Perform. 1996;22(5):1059–1076. doi: 10.1037//0096-1523.22.5.1059. [DOI] [PubMed] [Google Scholar]
- 19.Lee H.M., Galloway J.C. Early intensive postural and movement training advances head control in very young infants. Phys. Ther. 2012;92(7):935–947. doi: 10.2522/ptj.20110196. Jul 1. [DOI] [PubMed] [Google Scholar]
- 20.Woollacott M.H., Shumway-Cook A. Changes in posture control across the life span—a systems approach. Phys. Ther. 1990;70(12):799–807. doi: 10.1093/ptj/70.12.799. Dec 1. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . World Health Organization; Geneva: 2018. World Health Statistics 2018: Monitoring Health For the SDGs, Sustainable Development Goals [Internet]https://apps.who.int/iris/handle/10665/272596 [cited 2021 Dec 28]. Available from: [Google Scholar]
- 22.won Shin J, bin Song G, Ko J. The effects of neck and trunk stabilization exercises on cerebral palsy children's static and dynamic trunk balance: case series. J. Phys. Ther. Sci. 2017;29(4):771–774. doi: 10.1589/jpts.29.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiou S.Y., Strutton P.H., Perez M.A. Crossed corticospinal facilitation between arm and trunk muscles in humans. J. Neurophysiol. 2018;120(5):2595–2602. doi: 10.1152/jn.00178.2018. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adolph K.E., Franchak J.M. The development of motor behavior. WIREs Cogn. Sci. 2017;8(1–2):e1430. doi: 10.1002/wcs.1430. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown J.E., Thompson M., Brizzolara K. Head control changes after headpod use in children with poor head control: a feasibility study. Pediatr. Phys. Ther. 2018;30(2):142–148. doi: 10.1097/PEP.0000000000000492. Apr. [DOI] [PubMed] [Google Scholar]
- 26.Park M., Kim J., Yu C., Lim H. The effects of neurodevelopmental treatment-based trunk control exercise on gross motor function and trunk control in children with developmental disabilities. Healthcare. 2023;11(10):1446. doi: 10.3390/healthcare11101446. May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flores M.B., Da Silva C.P. Trunk control and gross motor outcomes after body weight supported treadmill training in young children with severe cerebral palsy: a non-experimental case series. Dev. Neurorehabilit. 2019;22(7):499–503. doi: 10.1080/17518423.2018.1527862. Oct 3. [DOI] [PubMed] [Google Scholar]
- 28.Barton G.J., Hawken M.B., Foster R.J., Holmes G., Butler P.B. The effects of virtual reality game training on trunk to pelvis coupling in a child with cerebral palsy. J. NeuroEng. Rehabil. 2013;10(1):15. doi: 10.1186/1743-0003-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velasco M.A., Raya R., Muzzioli L., Morelli D., Otero A., Iosa M., et al. Evaluation of cervical posture improvement of children with cerebral palsy after physical therapy based on head movements and serious games. Biomed. Eng. Online. 2017;16(S1):74. doi: 10.1186/s12938-017-0364-5. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santamaria V., Khan M., Luna T., Kang J., Dutkowsky J., Gordon A.M., et al. Promoting functional and independent sitting in children with cerebral palsy using the robotic trunk support trainer. IEEE Trans. Neural Syst. Rehabil. Eng. 2020;28(12):2995–3004. doi: 10.1109/TNSRE.2020.3031580. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shurtleff T.L., Engsberg J.R. Changes in trunk and head stability in children with cerebral palsy after hippotherapy: a pilot study. Phys. Occup. Ther. Pediatr. 2010;30(2):150–163. doi: 10.3109/01942630903517223. Apr. [DOI] [PubMed] [Google Scholar]
- 32.Sandlund M., Mcdonough S., Häger-Ross C. Interactive computer play in rehabilitation of children with sensorimotor disorders: a systematic review. Dev. Med. Child Neurol. 2009;51(3):173–179. doi: 10.1111/j.1469-8749.2008.03184.x. Mar. [DOI] [PubMed] [Google Scholar]
- 33.Soper A.K., Cross A., Rosenbaum P., Gorter J.W. Knowledge translation strategies to support service providers’ implementation of the “F-words in Childhood Disability. Disabil. Rehabil. 2021;43(22):3168–3174. doi: 10.1080/09638288.2020.1729873. Oct 23. [DOI] [PubMed] [Google Scholar]
- 34.Rosenbaum P., Gorter J.W. The ‘F-words’ in childhood disability: i swear this is how we should think! Child Care Health Dev. 2012;38(4):457–463. doi: 10.1111/j.1365-2214.2011.01338.x. Jul. [DOI] [PubMed] [Google Scholar]
- 35.Burdea G.C. Virtual rehabilitation–benefits and challenges. Methods Inf. Med. 2003;42(5):519–523. [PubMed] [Google Scholar]
- 36.Barlow D.H., Nock M., Hersen M. 3rd ed. Pearson/Allyn and Bacon; Boston: 2009. Single Case Experimental designs: Strategies For Studying Behavior For Change; p. 393. [Google Scholar]
- 37.Slocum T.A., Pinkelman S.E., Joslyn P.R., Nichols B. Threats to internal validity in multiple-baseline design variations. Perspect. Behav. Sci. 2022;45(3):619–638. doi: 10.1007/s40614-022-00326-1. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romeiser-Logan L., Slaughter R., Hickman R. Single-subject research designs in pediatric rehabilitation: a valuable step towards knowledge translation. Dev. Med. Child Neurol. 2017;59(6):574–580. doi: 10.1111/dmcn.13405. Jun. [DOI] [PubMed] [Google Scholar]
- 39.Krasny-Pacini A., Evans J. Single-case experimental designs to assess intervention effectiveness in rehabilitation: a practical guide. Ann. Phys. Rehabil. Med. 2018;61(3):164–179. doi: 10.1016/j.rehab.2017.12.002. May. [DOI] [PubMed] [Google Scholar]
- 40.Hawkins N.G., Sanson-Fisher R.W., Shakeshaft A., D'Este C., Green L.W. The multiple baseline design for evaluating population-based research. Am. J. Prev. Med. 2007;33(2):162–168. doi: 10.1016/j.amepre.2007.03.020. Aug. [DOI] [PubMed] [Google Scholar]
- 41.Levin J.R., Kratochwill T.R. Randomized single-case intervention designs and analyses for health sciences researchers: a versatile clinical trials companion. Ther. Innov. Regul. Sci. 2021;55(4):755–764. doi: 10.1007/s43441-021-00274-z. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hitchcock J.H., Kratochwill T.R., Chezan L.C. What works clearinghouse standards and generalization of single-case design evidence. J. Behav. Educ. 2015;24(4):459–469. Dec. [Google Scholar]
- 43.Hoffmann T.C., Glasziou P.P., Boutron I., Milne R., Perera R., Moher D., et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348(mar07 3) doi: 10.1136/bmj.g1687. Mar 7g1687–g1687. [DOI] [PubMed] [Google Scholar]
- 44.Thomas J., Armstrong-Heimsoth A., Laurent R.S. The Head Control Scale: development, inter-rater reliability, and utility. J. Pediatr. Rehabil. Med. 2019;12(3):295–303. doi: 10.3233/PRM-180574. [DOI] [PubMed] [Google Scholar]
- 45.Butler P.B., Saavedra S., Sofranac M., Jarvis S.E., Woollacott M.H. Refinement, reliability, and validity of the segmental assessment of trunk control. Pediatr. Phys. Ther. 2010;22(3):246–257. doi: 10.1097/PEP.0b013e3181e69490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahasup N., Sritipsukho P., Lekskulchai R., Keawutan P. Inter-rater and intra-rater reliability of the gross motor function measure (GMFM-66) by Thai pediatric physical therapists. J. Med. Assoc. Thail. Chotmaihet. Thangphaet. 2011;94(Suppl 7):S139–S144. Dec. [PubMed] [Google Scholar]
- 47.Beckers L.W., Bastiaenen C.H. Application of the Gross Motor Function Measure-66 (GMFM-66) in Dutch clinical practice: a survey study. BMC Pediatr. 2015;15(1):146. doi: 10.1186/s12887-015-0459-8. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamper S.J., Ostelo R.W.J.G., Knol D.L., Maher C.G., de Vet H.C.W., Hancock M.J. Global Perceived Effect scales provided reliable assessments of health transition in people with musculoskeletal disorders, but ratings are strongly influenced by current status. J. Clin. Epidemiol. 2010;63(7):760–766. doi: 10.1016/j.jclinepi.2009.09.009. Jule1. [DOI] [PubMed] [Google Scholar]
- 49.Kazdin A.E. Single-case experimental designs. Evaluating interventions in research and clinical practice. Behav. Res. Ther. 2019;117:3–17. doi: 10.1016/j.brat.2018.11.015. Jun. [DOI] [PubMed] [Google Scholar]
- 50.Lobo M.A., Moeyaert M., Baraldi Cunha A., Babik I. Single-Case design, analysis, and quality assessment for intervention research. J. Neurol. Phys. Ther. 2017;41(3):187–197. doi: 10.1097/NPT.0000000000000187. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michiels B., Onghena P. Nonparametric meta-analysis for single-case research: confidence intervals for combined effect sizes. Behav. Res. Methods. 2019;51(3):1145–1160. doi: 10.3758/s13428-018-1044-5. Jun. [DOI] [PubMed] [Google Scholar]
- 52.Anaby D., Lal S., Huszczynski J., Maich J., Rogers J., Law M. InterruptedSSpation. Phys. Occup. Ther. Pediatr. 2014;34(4):457–470. doi: 10.3109/01942638.2013.866612. Nov. [DOI] [PubMed] [Google Scholar]
- 53.Wright A., Hannon J., Hegedus E.J., Kavchak A.E. Clinimetrics corner: a closer look at the minimal clinically important difference (MCID) J. Man. Manip. Ther. 2012;20(3):160–166. doi: 10.1179/2042618612Y.0000000001. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaeschke R., Singer J., Guyatt G.H. Measurement of health status. Control. Clin. Trials. 1989;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6. Dec. [DOI] [PubMed] [Google Scholar]
- 55.Dusing S.C., Izzo T.A., Thacker L.R., Galloway J.C. Postural complexity differs between infant born full term and preterm during the development of early behaviors. Early Hum. Dev. 2014;90(3):149–156. doi: 10.1016/j.earlhumdev.2014.01.006. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pin T.W., Butler P.B., Cheung H.M., Shum S.L.F. Relationship between segmental trunk control and gross motor development in typically developing infants aged from 4 to 12 months: a pilot study. BMC Pediatr. 2019;19(1):425. doi: 10.1186/s12887-019-1791-1. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kallem Seyyar G., Aras B., Aras O. Trunk control and functionality in children with spastic cerebral palsy. Dev. Neurorehabilitation. 2019;22(2):120–125. doi: 10.1080/17518423.2018.1460879. Feb 17. [DOI] [PubMed] [Google Scholar]
- 58.Henderson S., Skelton H., Rosenbaum P. Assistive devices for children with functional impairments: impact on child and caregiver function. Dev. Med. Child Neurol. 2008;50(2):89–98. doi: 10.1111/j.1469-8749.2007.02021.x. Feb. [DOI] [PubMed] [Google Scholar]
- 59.Furtado M.A.S., Ayupe K.M.A., Christovão I.S., Sousa Junior R.R., Rosenbaum P., Camargos A.C.R., et al. Physical therapy in children with cerebral palsy in Brazil: a scoping review. Dev. Med. Child Neurol. 2021 doi: 10.1111/dmcn.15067. Oct 2;dmcn.15067. [DOI] [PubMed] [Google Scholar]
- 60.SS Al-azzawi, Khaksar S., Hadi E.K., Agrawal H., Murray I. HeadUp: a low-cost solution for tracking head movement of children with cerebral palsy using IMU. Sensors. 2021;21(23):8148. doi: 10.3390/s21238148. Dec 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spearing EM, Pelletier ES, Drnach M, editors. Tecklin's Pediatric Physical Therapy. 6th ed. Wolters Kluwer Health; Philadelphia: 2022. [Google Scholar]
- 62.Smith J.D. Single-case experimental designs: a systematic review of published research and current standards. Psychol. Methods. 2012;17(4):510–550. doi: 10.1037/a0029312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrington M., Velicer W.F. Comparing visual and statistical analysis in single-case studies using published studies. Multivar. Behav. Res. 2015;50(2):162–183. doi: 10.1080/00273171.2014.973989. Mar 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Backman C.L., Harris S.R., Chisholm J.A.M., Monette A.D. Single-subject research in rehabilitation: a review of studies using AB, withdrawal, multiple baseline, and alternating treatments designs. Arch. Phys. Med. Rehabil. 1997;78(10):1145–1153. doi: 10.1016/s0003-9993(97)90142-8. Oct. [DOI] [PubMed] [Google Scholar]
- 65.Graham J.E., Karmarkar A.M., Ottenbacher K.J. Small sample research designs for evidence-based rehabilitation: issues and methods. Arch. Phys. Med. Rehabil. 2012;93(8):S111–S116. doi: 10.1016/j.apmr.2011.12.017. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.