Abstract
Restorative treatment is the most common approach to treating dental caries. However, after cavity preparation, some microorganisms may still persist in the substrate, suggesting the use of cavity disinfectants. Nevertheless, their effect on adhesion to composite resins is not yet fully understood, especially in primary teeth. The study aimed to assess the impact of five different cavity disinfectants on dentin adhesion in primary teeth. A total of 60 primary molars were uniformly flattened at their occlusal thirds and randomly allocated into six groups (n = 10 each): Control; Glutaraldehyde; Chlorhexidine; EDTA; Ethanol; Aloe vera. All disinfectants were actively applied, rinsed, and air-dried. The adhesion procedure was carried out according to the manufacturer's instructions, and the restoration was positioned using a mold. Shear bond strength was evaluated. Data were statistically analyzed (One-way ANOVA with Post-hoc Tukey test), with the level of significance set at 5 %. Glutaraldehyde (14.59 ± 3.89 MPa), Chlorhexidine (11.24 ± 2.25 MPa), and EDTA (11.04 ± 2.95 MPa) did not impair the shear bond strength when compared to the Control group (14.95 ± 2.75 MPa). Ethanol and Aloe vera application significantly lowered SBS. The results suggest that Glutaraldehyde, Chlorhexidine, and EDTA can be used as cavity disinfectants. Nevertheless, further in vitro and clinical research is required.
Keywords: Adhesion, Cavity disinfectants, In vitro technique, Primary teeth, Shear bond strength
Highlights
-
•
Glutaraldehyde, Chlorhexidine, and EDTA did not impair adhesion in primary teeth.
-
•
Ethanol and Aloe vera reduced shear bond strength in primary teeth.
-
•
More research is needed to develop effective clinical protocols.
1. Introduction
According to the most recent estimates concerning the burden of oral conditions from the Global Burden of Disease 2017 Study [1], dental caries is among the most widespread oral health issues, with its prevalence in primary teeth ranging from 443 million to 622 million people worldwide. According to Pitts et al. [2], dental caries is characterized as a biofilm-driven condition influenced by sugars, which causes cyclical demineralization and remineralization of dental hard tissues [2,3]. Its occurrence is due to the interaction between various agents, including genetic, physiological, biological, and environmental factors, and the accurate diagnosis of this disease is of extreme importance to attain health [4].
Microorganisms present in the oral microbiome, the second-largest microbiome, namely Streptococcus spp. and Actinomyces spp., combined with the supply of carbohydrates from food ingestion and other components, such as glycoproteins from saliva, form plaque on the tooth surface through the adhesion of adhesins (bacterial surface components) to its receptors from the salivary coating on tooth surfaces [3,5,6]. This adhesion causes the acidification of the environment and a decrease in oxygen concentration, facilitating the colonization of the plaque by bacteria that are oxygen-labile, such as Streptococcus mutans, thereby increasing the cariogenic potential [5].
Hence, the adequate treatment of dental caries lesions is of primal importance, and restorative treatment is the most commonly used procedure [2,3,7]. Restorative treatment of dental caries aims to control the disease and re-establish form and function [3]. Among the restorations made on primary teeth to treat dental caries lesions, 12.5 % fail, causing microleakage, recurrent caries, dental hypersensitivity, discoloration, and pulpitis [8]. Even after cavity preparation, some microorganisms might still persist, leading to only a minor portion of the cavity being sterile post-preparation. These residual microorganisms can compromise treatment success by leading to secondary caries, tooth sensitivity, and pulpal inflammation [8,9]. Therefore, effective cavity disinfection is crucial for eliminating these microorganisms from the prepared cavity, smear layer, enamel-dentin junction, or dentinal tubules, thereby enhancing the longevity and success of dental restorations [7,10,11].
The aim of the restorative procedure is to establish an effective bond between the teeth and the restorative material, obtained by the infiltration of hydrophilic resin monomers into the exposed and demineralized collagen matrix, forming the hybrid layer [9,11,12]. Not only will the existence of microorganisms affect the adhesion of dentin to the restorative material, but also the activation of matrix metalloproteinases (MMP), a group of 23 zinc and calcium-dependent endopeptidases that contribute to the faster degeneration of the collagen present in the hybrid layer [11,13,14]. When the surrounding inorganic structure of the collagen fibers is eliminated due to dental caries or acid etching, the denuded collagen fibers become susceptible to MMP activity, causing faster degradation of the collagen matrix from the adhesive interface and a possible decrease in bond strength over time [11,[13], [14], [15]].
Studies using different cavity disinfectants have stated that they can reduce bacterial activity and inhibit MMP. However, more studies are needed, given that these disinfectants might negatively impact the bond strength of the restorations, especially concerning primary teeth, which possess micromorphological, compositional, tubular density, intrinsic moisture, and dentinal permeability differences when compared to permanent teeth [9,16].
The aim of this research was to evaluate the impact of 0.20 % Chlorhexidine digluconate, Gluma® (5 % glutaraldehyde), Aloe vera, 17 % Ethylenediaminetetraacetic acid (EDTA), and 100 % Ethanol on the adhesion of composite restorations to dentin of primary teeth. The null hypothesis proposed that there would be no significant differences between disinfectants and control.
2. Materials and methods
All data used in this study were obtained in accordance with the Declaration of Helsinki and its updates, and the patients' information was kept confidential and anonymized. An informed consent form was read, understood, and signed, and the project was approved by the Ethics Committee of the Faculty of Medicine of the University of Coimbra, Portugal (CE-161/2022, December 2022).
Sixty caries-free primary molars extracted from patients from the Dentistry Area of the Faculty of Medicine of the University of Coimbra and from the Paediatric Dentistry Department of the Faculty of Health Sciences of the University Fernando Pessoa (Oporto) were selected and included. The specimens were selected through visual examination and assessment of surface hardness, and teeth with an intact crown enamel and absence of cracks, fractures, and defects were included. The exclusion criteria included the presence of caries, large restorations in the adhesion test area, structural defects, erosion, or abfraction, in addition to teeth presenting endodontic treatment or teeth that fractured during the extraction procedure.
Following extraction, the teeth were rinsed thoroughly under running water to eliminate any blood and adherent tissue, then kept in distilled water at 4 °C for no longer than 6 months, with the storage medium being replaced once every two months, according to ISO/TS 11405:2015 (Dentistry - testing of adhesion to tooth structure) [17].
The primary molars' occlusal thirds were ground to a standard, reproducible, flat surface with a diamond disk (918BF/220, D + Z, Germany) while rinsing with running water. This process aimed to expose a superficial layer of coronal dentin at a right angle to the teeth's long axis. Following this, the teeth were embedded in auto-polymerizing acrylic resin (Probase, Ivoclar Vivadent, Spain) up to the cemento-enamel junction, using a 2.5 cm diameter and 5 cm height polyvinyl chloride (PVC) cylinder.
The specimens were then randomly assigned to 6 groups:
-
-
Group 1: Control - no cavity disinfectants applied (n = 10);
-
-
Group 2: Application of 5 % Glutaraldehyde (5 % w/v) (Gluma®, Heraeus, Germany) (n = 10);
-
-
Group 3: Application of 0.20 % Chlorhexidine (0.20 % w/v) (Parodontax Extra 0.2 %, GSK, United Kingdom (UK)) (n = 10);
-
-
Group 4: Application of 17 % EDTA (17 % w/v) (CanalPro™ EDTA 17 %, Coltène, Germany) (n = 10);
-
-
Group 5: Application of 100 % Ethanol (100 % v/v) (Sigma-Aldrich Corporation, United States of America (USA)) (n = 10);
-
-
Group 6: Application of Aloe vera (Just Javik®, Herbs and Crops Overseas, India) (n = 10).
The components and manufacturers of the disinfectants used are specified in Table 1.
Table 1.
Composition, lot and manufacturer of the cavity disinfectants.
| Material | Composition | Lot | Manufacturer |
|---|---|---|---|
| Paradontax Extra | Aqua, Sorbitol, Glycerin, PEG-40, Hydrogenated Castor Oil, Aroma, Chlorhexidine, Digluconate | 2440350 | GSK, UK |
| CanalPro™ EDTA | Sodium EDTA | 171540 | Coltène, Germany |
| Aloe vera | Aloe barbadensis powder 200:1 | OABP101 | Herbs and Crops Overseas, India |
| Gluma® | 35 % 2-hydroxyethyl methacrylate (HEMA); 5 % Glutaraldehyde | K010536 | Heraeus, Germany |
| Ethanol | 100 % Ethanol | 493511 | Sigma-Aldrich Corporation, USA |
The Aloe vera solution was formulated with Aloe barbadensis powder (Just Jaivik®, Herbs and Crops Overseas, India) at a ratio of 200:1, by dissolving 0.5 g of the powder in 99.5 g of distilled water, resulting in a concentration of 0.5 % w/w.
The dentin surface was treated with the assigned cavity disinfectant for 30 s, followed by a 30-s wash with water and 15 s of air drying. Teeth assigned to the control group underwent no pre-treatment.
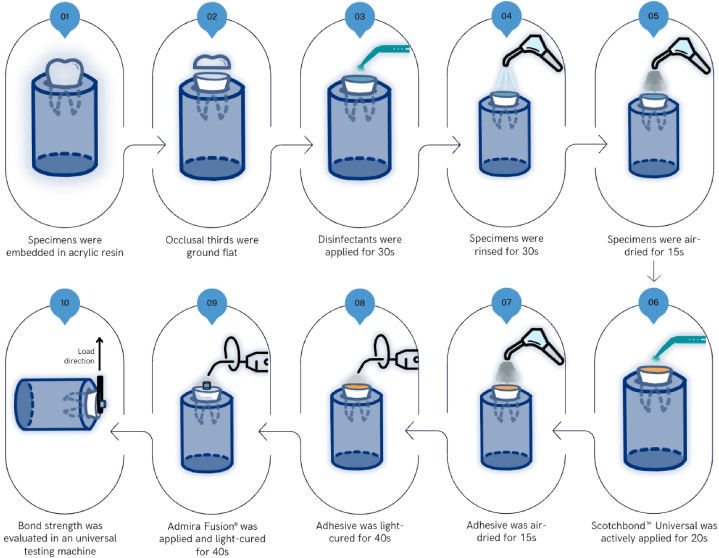
Afterward, Scotchbond™ Universal (3M, USA) was actively brushed on the dentin surface for 20 s. The surface was then dried using a gentle airflow for 10 s, followed by light curing with a LED curing light (1000 mW/cm2, SmartLite Focus, Dentsply Sirona, USA) for 40 s, in accordance with the manufacturer's guidelines. Resin composite (Admira Fusion®, VOCO, Germany) was placed with 1 mm increments using polyethylene tubes measuring 3 mm in diameter and 2 mm in height, and each layer was light-cured for 40 s. All adhesive and restorative procedures were conducted by the same operator. The protocol used is presented in Fig. 1.
Fig. 1.
Schematic diagram of specimen preparation.
After the specimens were prepared, shear bond strength (SBS) was evaluated according to ISO 29022:2013 (Dentistry - Adhesion - Notched-edge shear bond strength test) [18] and DIN 13990 (2017) standards [19] using a Shimadzu universal testing machine (Shimadzu Autograph AG-X-5kN, Japan).
The technician who recorded the SBS measurements was blinded to the group allocations to reduce bias.
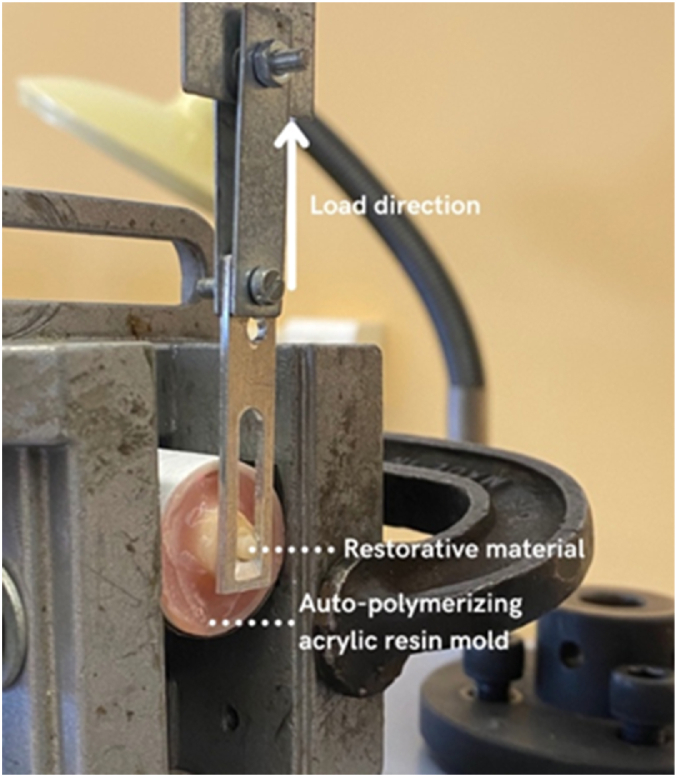
Fig. 2 illustrates the setup used to evaluate the adhesive strength. A stress relief device was constructed, comprising two parallel blades connected by two screws. One side is attached to the clamps, while the other side is connected to a blade with a guillotine window that applies a load on the adhesive joint. The force exerted in an upward direction and parallel to the adhesive joint.
Fig. 2.
Experimental setup for measuring shear bond strength (SBS).
The adhesive test involves applying a progressive uniaxial load until the adhesive joint ruptures, while recording the longitudinal deformation of the material. This process generates a force vs. displacement curve, revealing the behaviour of the adhesive joint and allowing determination of properties such as maximum shear force, shear modulus, or maximum displacement.
The failure mode (cohesive, adhesive, and mixed failures) was analyzed through observation using microscopy (3D Hirox digital microscope) and Scanning Electron Microscope (Hitachi SU3800). Adhesive failure occurs exclusively at the interface between dentin and composite resin. Cohesive failure is considered when the failure occurs inside the dentin or inside the composite resin cylinder. Mixed failure is identified when parts of both are detectable.
The data distribution was assessed for normality using the Shapiro-Wilk normality test. After confirmation of the variables’ normality, One-way ANOVA with Post-hoc Tukey test was conducted. The level of significance was set at 5 %. The software used in data analysis was IBM® SPSS® v.29.0 (IBM Corporation, USA).
3. Results
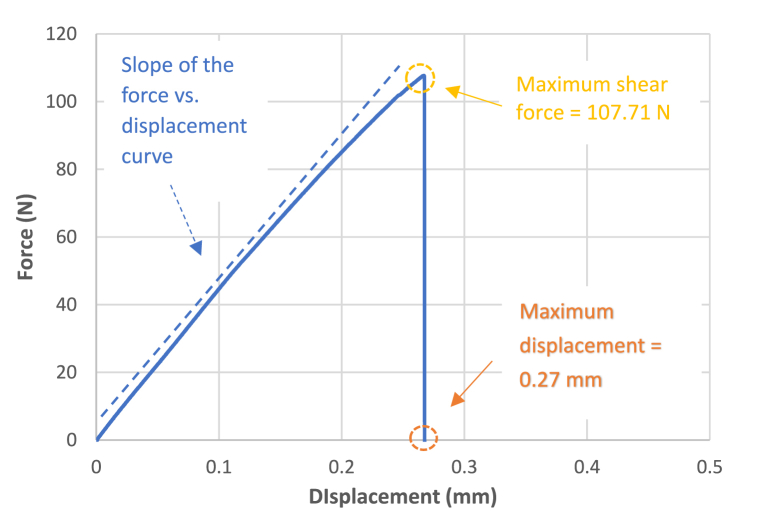
In Fig. 3, the evolution of force (N) with displacement (mm) for a specimen of Group 2 - Disinfection with 5 % Glutaraldehyde can be observed. In general, the curves for the various cavity disinfectants and the Control group exhibited a fragile behaviour, as the rupture of the adhesive system was abrupt, registering a low amplitude displacement. This is contrary to what occurs with ductile adhesive systems, which show displacements of greater amplitudes. Upon analysis of Fig. 3, it was determined that the maximum shear force reached by the adhesive system before rupture was 107.71 N, while the displacement was 0.27 mm. Considering that the diameter of the composite resin cylinder was 3 mm, and normalizing the maximum shear force by the area of the glued joint, a value of 15.24 MPa was obtained for the shear bond strength.
Fig. 3.
Force vs. displacement curve for the specimen 7 from the Group 2 – Disinfection with 5 % Glutaraldehyde.
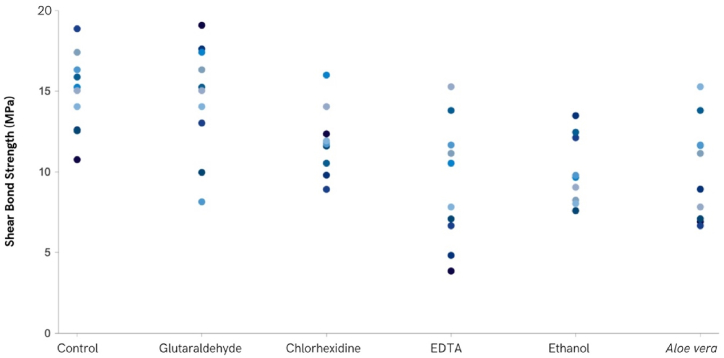
The same procedure was conducted, as described in the materials and methods, for the specimens where the remaining cavity disinfectants were used, and the final results are illustrated in Fig. 4. All force-displacement curves showed a constant slope, indicating the rigidity of the adhesive system, which is defined as the shear modulus.
Fig. 4.
Shear bond strength results for the different cavity disinfectants.
The disinfection method that presented the lowest SBS was the one where Aloe vera was applied (10.09 ± 2.60 MPa), with the highest values observed in the Control group (14.95 ± 2.75 MPa). The SBS mean and standard deviation values obtained, in descending order, were 14.59 ± 3.89 MPa when Glutaraldehyde was applied, 11.24 ± 2.25 MPa for the Chlorhexidine application, 11.04 ± 2.95 MPa when the surface was treated with EDTA and 10.37 ± 2.09 MPa when Ethanol was applied prior to the restorative procedure.
A significant statistical difference in shear bond strength was found among the various groups (F(5.44) = 4.625, p = 0.002), namely between Control and Aloe vera (p = 0.011), Control and Ethanol (p = 0.031) and Aloe vera and Glutaraldehyde (p = 0.023). The results regarding SBS are presented in Fig. 4.
Analysis of the graph in Fig. 4 reveals that the dispersion of values was lower in the case of samples that used ethanol as a cavity disinfectant and greater in the case of samples that used EDTA as a cavity disinfectant. This type of dispersion is characteristic of biological systems that exhibit some heterogeneity.
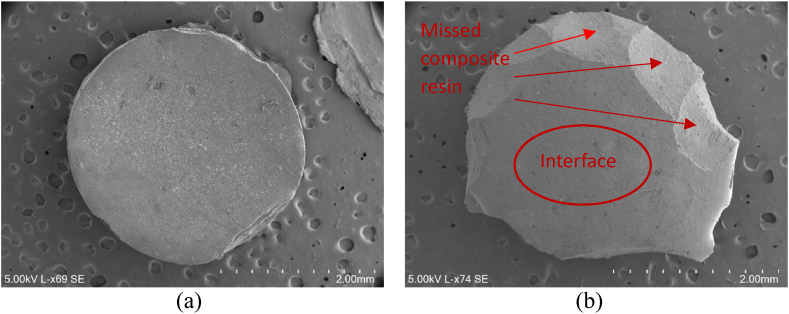
Fig. 5 shows micrographs obtained by Scanning Electron Microscope (SEM) where it is possible to observe the composite resin cylinder from both sides, as it was after the adhesive test. It can be seen that the composite resin cylinder was intact from one side (the side opposite to the joint (Fig. 5a)), and on the side of the adhesive joint (Fig. 5b), faceted planes can be seen where there is evidence of a lack of composite, which was adhered to the dentin. This type of mixed failure, in which part of the interface (flat zone) and part of the torn-off material can be seen, was observed for most of the specimens.
Fig. 5.
Micrographs obtained by SEM showing the mixed failure mode: (a) side opposite to the joint; (b) side of the adhesive joint.
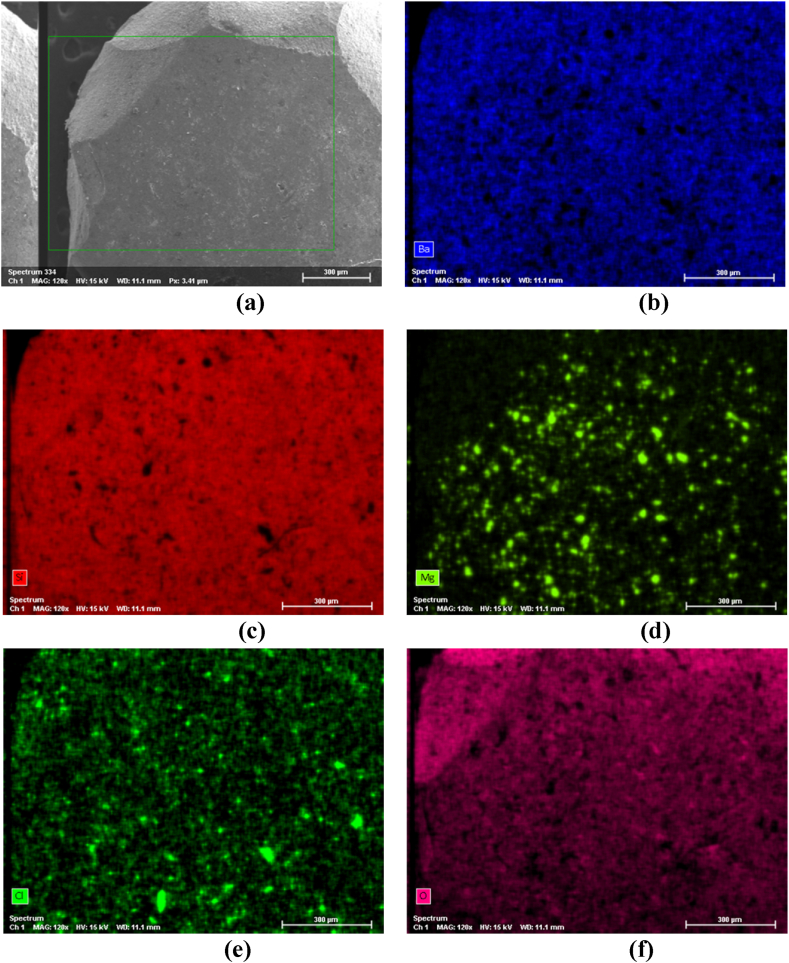
Fig. 6(a–f) shows SEM/EDS (Energy Dispersive X-ray Spectroscopy) mapping, where it is possible to observe the chemical composition of the cylinder part shown in Fig. 5b.
Fig. 6.
SEM/EDS mapping showing the chemical composition of the composite cylinder shown in Fig. 5b: (a) SEM micrograph showing the mapping region; (b) spectrum of Ba; (c) spectrum of Si; (d) spectrum of Mg; (e) spectrum of Cl; (f) spectrum of O.
4. Discussion
In order to achieve the objective of this study, extracted primary teeth were used to conduct the tests. According to the literature, some chemical and micromorphological differences over permanent teeth were stated, namely a lower inorganic and higher organic content and a reduced area of intertubular dentin caused by the higher tubular dentin density and diameter, with the peritubular dentin being 2 to 5 times thicker [10,[20], [21], [22], [23]]. The buffering capacity of these teeth is reduced, given that the dentin is less mineralized and the reactivity to acidic solutions is enhanced [20]. Furthermore, considering that primary teeth dentin owns less inorganic content, the hybrid layer will be more susceptible to deterioration [10,13,21,24].
Although adhesion to enamel is nowadays considered predictable, adhesion to dentin is more complex, particularly due to its lesser inorganic content and higher intrinsic moisture [25]. To standardize and obtain optimal conditions when testing dentin, superficial caries-free dentin was tested, which has more organic content, a higher percentage of intertubular dentin, fewer dentinal tubules, and is in accordance with the ISO/TS 11405:2015 (Dental materials - testing of adhesion to tooth structure) [17], which states the tooth substrate and storage to be used, and how the surface should be prepared, the specimen production, handling, and storage. Every selected specimen was a molar, and, after its extraction, the teeth were kept in distilled water until the experimental procedures to prevent the any alteration of the chemical and physical properties of the dentin substrates by the storage medium. As stated in the ISO mentioned above, the storage period of the samples did not exceed six months. Once the adhesive and restorative procedures were achieved, the specimens were maintained in water at 23 °C.
The degradation of the dentin-resin interface is primarily attributed to the deterioration of the hybrid layer, made not only by extrinsic factors, such as water, oral fluids, and polymeric expansion but also intrinsic host factors, namely MMP, and mechanical factors, particularly mastication forces, temperature, and pH variations [22,26,27]. Therefore, optimizing the longevity of the adhesive interface is crucial.
The development of newer bonding agents, such as universal adhesives, made possible a variety of clinical applications as well as the simplification of the restorative protocol and the reduction of chair time, which is of particular importance when treating children, where the treatment effectiveness is directly related to patient collaboration [14,28,29]. The adhesive used was Scotchbond™ Universal (3M, USA), in a self-etch mode, where the acidic primer does not eliminate the smear layer but instead incorporates it into the hybrid layer [23,30]. Its ultra-mild pH (pH = 2.7) grants this bonding agent the capacity to prevent excessive dentin demineralization, when compared to phosphoric acid [23,31]. Its presence gives this bonding agent its chemical and micromechanical adhesion. 10-MDP, an acidic monomer, adheres to calcium and causes minimal dentin dissolution, enabling slight calcium release and MDP-Ca salt formation in a nano-layer, preventing calcium loss from its dentinal matrix [23,30,32].
Regardless of the advantages of using this bonding agent, the demineralized dentin can leave the collagen matrix susceptible to the activity of MMP. Once the pH drops, caused by dental caries or acid etching, the MMP trapped in the extracellular matrix is activated, and the circumjacent inorganic structure is removed, leaving the denuded collagen fibers exposed to MMP activity, so the application of exogenous MMP inhibitors could be beneficial to preserve the collagen matrix structural integrity and hinder the hybrid layer collagenolytic and gelatinolytic degradation [11,13,14,25].
Besides the impact of MMP in the degradation of the adhesive interface, it was observed that some fermentative organisms were still viable after 139 days in non-antiseptic restorations, thus, cavity disinfection might be useful to reduce the bacterial load after mechanical removal of the carious dentin [33].
Commonly used for post-operative hypersensitivity, glutaraldehyde also possesses antibacterial properties and is an MMP inhibitor [34,35]. It works as a cross-linking agent that improves the collagen matrix's resistance to degradation, and its mechanism of action is due to the reaction between the aldehyde group from glutaraldehyde and the amine groups found in lysine and hydroxylysine within collagen. Glutaraldehyde application prior to the restorative procedure did not impair the SBS, possibly because of the surface energy increase due to the expansion of the demineralized collagen, which facilitated the penetration of resin monomers [34]. In studies conducted in permanent teeth [34,36], the SBS was also not impaired when glutaraldehyde was used as a cavity disinfectant. However, studies using primary teeth [10] stated that there was a significant reduction of SBS, contrary to the findings from this in vitro study.
Chlorhexidine is regarded as the oral antiseptic gold standard, with a wide range of efficacy against both gram-positive and negative bacteria, namely Streptococcus mutans, as well as fungi, and some viruses. It interacts with the negative charge of the bacterial cell wall, changing the osmotic balance of the microorganism and ultimately leading to their death [25,37]. It is a bioactive biguanide, water-soluble, with a physiological pH, and it acts by chelating calcium and zinc ions. Its interaction with the MMP-2, 8, and 9 also prevents collagen degradation and the hybrid layer disintegration over time [9,11,14,22,28]. Given that this compound has high substantivity, it enables a long-term effect, since when all protease binding sites are oversaturated, it remains connected to the collagen fibers to achieve a later release [22]. It can prevent secondary caries and post-operative hypersensitivity, has a rehydrating ability to the etched dentin, leaving the collagen matrix expanded, and lowers the convective and evaporative water flows in the dentinal substrate [11,13,15,25]. However, chlorhexidine is also able to bind to the hydroxyapatite phosphate group present in the smear layer, interfering with the action of the acidic primer, as stated by some authors [9,21,28]. Velayos-Galán et al. [14], Ebrahimi et al. [21], and Oznurhan et al. [38] found that immediate bond strength was not impaired when chlorhexidine was applied, using the same adhesion mode tested in this study. When considering other adhesion protocols, the same results were reported in some studies [16,22,25,39]. However, studies testing delayed SBS [13,22] showed significantly higher SBS when chlorhexidine was applied.
EDTA is more commonly used as an irrigant in endodontic treatments, performing the chelation of calcium and potassium ions and preventing collagen degradation [41]. It also acts as an MMP-2 and 9 inhibitor and dissolves the inorganic compounds of the smear layer; nonetheless, given that EDTA is soluble in water, it can be washed off the treated surface [10,22,35,39,40]. Parsaie et al. [22] and Mohammadi et al. [39] stated that the EDTA application did not result in SBS impairment, similarly to the findings observed in this in vitro study.
Ethanol application expels water from the dentinal subtract, keeping the collagen matrix expanded and supported from the replacement of water with ethanol, allowing the resin to infiltrate deeper into the substrate [35,[41], [42], [43], [44]]. An insufficient infiltration of the hydrophilic monomer present in the adhesive in the demineralized dentin might be caused by the incomplete removal of water and its replacement with ethanol from the substrate [44]. Yu et al. [42] reported that SBS was not impaired after ethanol application. Nonetheless, the findings of this study indicate that its application caused an SBS reduction. Similar results were observed by Simões et al. [45] in permanent teeth.
Aloe vera possesses anthraquinone, which is bactericidal, and aloins, which inhibit MMP-2 and 9 [11,12,46]. Its phytotherapeutic agents can possibly inhibit the proliferation of some oral microorganisms, such as S. mutans, S. Sanguis, and Candida albicans, preventing secondary caries, and they can also extend the lag phase of some microorganisms, inhibiting their enzymatic activity [15,46]. The results obtained in permanent teeth concluded that there was an increase in SBS with statistical significance for Sinha et al. [15], even though three other studies [11,12,35] didn't find any statistically significant difference. However, to the authors' knowledge, there were no prior studies found using Aloe vera in primary teeth, even though the findings of this study found a significant reduction in SBS when Aloe vera was applied.
In summary, the null hypothesis was accepted when Glutaraldehyde, Chlorhexidine, and EDTA were applied. Nonetheless, there were significant differences when the disinfectants used prior to the restorative procedure were Ethanol and Aloe vera.
This study faced some limitations that should be taken into account when interpreting the results. The sample size of 10 specimens per group, while consistent with similar in vitro studies, may restrict the applicability and robustness of the conclusions drawn. A larger sample size could strengthen the statistical power of the results. Additionally, the in vitro design of the study does not completely simulate the intricate conditions of the oral environment and may not account for all clinical variables. The study also focused exclusively on shear bond strength as the outcome measure, without evaluating other critical factors like microleakage, secondary caries, or the long-term durability of restorations. These factors could significantly impact the clinical success of dental treatments and warrant further investigation. Despite efforts to standardize procedures and reduce variability, potential confounding factors such as inherent differences in dentin substrates between primary teeth and slight variations in handling techniques should be considered when interpreting the results.
Furthermore, the limited number of existing studies on this topic underscores the need for additional research to strengthen scientific evidence and develop a robust foundation for clinical applications. Future studies should explore various adhesion and restorative protocols, different disinfectants, application times, and substrates, including caries-affected dentin and varying dentin depths. Long-term bonding durability should also be assessed to offer a more thorough insight into the impact of cavity disinfectants on composite adhesion.
5. Conclusions
The results showed that Aloe vera and Ethanol can significantly reduce shear bond strength, suggesting their potential to impair the bonding process when used prior to adhesive procedures. In contrast, Glutaraldehyde demonstrated the highest shear bond strength, comparable to the control group, suggesting it may be a more suitable option for cavity disinfection without adversely affecting adhesion. Chlorhexidine showed moderate effects on adhesion, consistent with previous findings indicating its potential to maintain bond durability over time. EDTA also did not significantly impair adhesion.
Given the results, it is critical to carefully consider the choice of cavity disinfectant in clinical practice to optimize restorative outcomes in primary teeth.
Nevertheless, further in vitro and clinical studies are warranted to validate and strengthen these results. Future investigations should aim at optimizing cavity disinfectant protocols and exploring innovative materials, while also assessing the balance between antimicrobial efficacy and bonding integrity, ensuring that the benefits of cavity disinfection are fully realized without unintended negative consequences on restoration performance.
All data used in the present study was obtained according to the Declaration of Helsinki and its updates, and the patients' information was kept confidential and anonymized. An informed consent form was read, understood, and signed, and the project was approved by the Ethics Committee of the Faculty of Medicine of the University of Coimbra, Portugal (CE-161/2022, December 2022).
CRediT authorship contribution statement
Ana Coelho: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Luís Vilhena: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Formal analysis, Conceptualization. Mariana Cordeiro: Writing – review & editing, Writing – original draft, Investigation, Formal analysis. Inês Amaro: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Conceptualization. Anabela Paula: Writing – review & editing, Investigation, Formal analysis. Carlos Miguel Marto: Writing – review & editing, Investigation, Formal analysis. Cristina Cardoso Silva: Writing – review & editing, Investigation, Formal analysis. Manuel Marques Ferreira: Writing – review & editing, Methodology, Conceptualization. Eunice Carrilho: Writing – review & editing, Visualization, Validation, Methodology, Conceptualization. Amílcar Ramalho: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Conceptualization.
Data and code availability
Data is referenced in the article.
Funding
This research did not receive any specific funding.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bernabe E., Marcenes W., Hernandez C.R., Bailey J., Abreu L.G., Alipour V., Amini S., Arabloo J., Arefi Z., Arora A., et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the global burden of disease 2017 study. J. Dent. Res. 2020;99:362–373. doi: 10.1177/0022034520908533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitts N.B., Zero D.T., Marsh P.D., Ekstrand K., Weintraub J.A., Ramos-Gomez F., Tagami J., Twetman S., Tsakos G., Ismail A. Dental caries. Nat. Rev. Dis. Prim. 2017;3:1–16. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 3.MacHiulskiene V., Campus G., Carvalho J.C., Dige I., Ekstrand K.R., Jablonski-Momeni A., Maltz M., Manton D.J., Martignon S., Martinez-Mier E.A., et al. Terminology of dental caries and dental caries management: consensus report of a workshop organized by ORCA and cariology research group of IADR. Caries Res. 2020;54:7–14. doi: 10.1159/000503309. [DOI] [PubMed] [Google Scholar]
- 4.Pitts N.B., Twetman S., Fisher J., Marsh P.D. Understanding dental caries as a non-communicable disease. Br. Dent. J. 2021;231:749–753. doi: 10.1038/s41415-021-3775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi N. Microbial ecosystem in the oral cavity: metabolic diversity in an ecological niche and its relationship with oral diseases. Int Congr Ser. 2005;1284:103–112. [Google Scholar]
- 6.Deo P.N., Deshmukh R. Oral microbiome: unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019;23:122–128. doi: 10.1016/j.ics.2005.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma V., Rampal P., Kumar S. Shear bond strength of composite resin to dentin after application of cavity disinfectants - SEM study. Contemp. Clin. Dent. 2011;2:155–159. doi: 10.4103/0976-237X.86438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chisini L.A., Collares K., Cademartori M.G., Oliveira L.J.C., Conde M.C.M., Demarco F.F., Corrêa M.B. Restorations in primary teeth: a systematic review on survival and reasons for failures. Int. J. Paediatr. Dent. 2018;28:123–139. doi: 10.1111/ipd.12346. [DOI] [PubMed] [Google Scholar]
- 9.Kimyai S., Mohammadi N., Bahari M., Pesyanian E., Pesyanian F. Effect of cavity disinfection with chlorhexidine on marginal gap of class V composite restorations bonded with a universal adhesive using self-etch and etch-and-rinse bonding strategy. Front Dent. 2020;17:1–7. doi: 10.18502/fid.v17i1.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coelho A., Amaro I., Apolónio A., Paula A., Saraiva J., Ferreira M.M., Carrilho E., Ramalho A. Effect of cavity disinfectants on adhesion to primary teeth—a systematic review. Int. J. Mol. Sci. 2021;22:1–17. doi: 10.3390/ijms22094398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goel S., Sinha D.J., Singh U.P., Ahuja U., Haider N., Sharma N. Comparative evaluation of effect of chlorhexidine, Azadirachta indica (neem), and Aloe barbadensis miller (Aloe vera) on resin-dentin bond stabilization using shear bond testing: an in vitro study. J. Conserv. Dent. 2019;22:300–304. doi: 10.4103/JCD.JCD_11_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha D.J., Jaiswal N., Vasudeva A., Garg P., Tyagi S.P., Chandra P. Comparative evaluation of the effect of chlorhexidine and Aloe barbadensis Miller (Aloe vera) on dentin stabilization using shear bond testing. J. Conserv. Dent. 2016;19:406–409. doi: 10.4103/0972-0707.190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leitune V.C.B., Portella F.F., Bohn P.V., Collares F.M., Werner S.M., Collares S.M. Dental materials influence of chlorhexidine application on longitudinal adhesive bond strength in deciduous teeth. Braz. Oral Res. 2011;25:388–392. doi: 10.1590/s1806-83242011000500003. [DOI] [PubMed] [Google Scholar]
- 14.Velayos-Galán L., Molinero-Mourelle P., Sevilla P., Fonseca M., Mourelle-Martínez M.R., Vera-González V. Influence of 2% chlorhexidine on the bond strength of three adhesive systems on primary molars: an in vitro study. Appl. Sci. 2022;12:1–11. doi: 10.3390/app12062964. [DOI] [Google Scholar]
- 15.Sinha D.J., Jandial U.A., Jaiswal N., Singh U.P., Goel S., Singh O. Comparative evaluation of the effect of different disinfecting agents on bond strength of composite resin to dentin using two-step self-etch and etch and rinse bonding systems: an in-vitro study. J. Conserv. Dent. 2018;21:424–427. doi: 10.4103/JCD.JCD_66_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenzi T.L., Tedesco T.K., Soares F.Z.M., Loguercio A.D., Rocha R.O. Chlorhexidine does not increase immediate bond strength of etch-and-rinse adhesive to caries-affected dentin of primary and permanent teeth. Braz. Dent. J. 2012;23:438–442. doi: 10.1590/s0103-64402012000400022. [DOI] [PubMed] [Google Scholar]
- 17.ISO/TS 11405 . 2015. Dentistry - Testing of Adhesion to Tooth Structure. Geneva, Switzerland. [Google Scholar]
- 18.ISO 29022 Dentistry - Adhesion - Notched-edge shear bond strength test. 2013 Dublin, Ireland. [Google Scholar]
- 19.DIN 13990:2017-04 Dentistry - Test methods for shear bond strength of adhesives for orthodontic attachments. 2017 Berlin, Germany. [Google Scholar]
- 20.Ricci H.A., Sanabe M.E., Costa C.A.S., Hebling J. Bond strength of two-step etch-and-rinse adhesive systems to the dentin of primary and permanent teeth. J. Clin. Pediatr. Dent. 2010;35:163–168. doi: 10.17796/jcpd.35.2.958893530534m503. [DOI] [PubMed] [Google Scholar]
- 21.Ebrahimi M., Naseh A., Abdollahi M., Shirazi A.S. Can chlorhexidine enhance the bond strength of self-etch and etch-and-rinse systems to primary teeth dentin? J. Contemp. Dent. Pract. 2018;19:404–408. [PubMed] [Google Scholar]
- 22.Parsaie Z., Firouzmandi M., Mohammadi N. Evaluating the effect of pretreatment with matrix metalloproteinase inhibitors on the shear bond strength of composite resin to primary teeth dentin: a 6-month in vitro study. Contemp. Clin. Dent. 2021;12:408–413. doi: 10.4103/ccd.ccd_662_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gindri L.D.O., Fröhlich T.T., Rosso C.R., Rocha R.O. Etching time and bonding of adhesive systems to dentin of primary teeth: a systematic review and meta-analysis. Int. J. Paediatr. Dent. 2021;31:122–130. doi: 10.1111/ipd.12711. [DOI] [PubMed] [Google Scholar]
- 24.Lenzi T.L., Tedesco T.K., Soares F.Z.M., Loguercio A.D., Rocha R.O. Chlorhexidine application for bond strength preservation in artificially-created caries-affected primary dentin. Int. J. Adhesion Adhes. 2014;54:51–56. doi: 10.1016/j.ijadhadh.2014.04.007. [DOI] [Google Scholar]
- 25.Manfro A.R.G., Reis A., Loguercio A.D., Imparato J.C.P., Raggio D.P. Effect of chlorhexidine concentration on the bond strength to dentin in primary teeth. Rev odonto ciênc. 2010;25:88–91. [Google Scholar]
- 26.Krifka S., Petzel C., Bolay C., Hiller K., Spagnuolo G., Schmalz G., Schweikl H. Activation of stress-regulated transcription factors by triethylene glycol dimethacrylate monomer. Biomater. 2011;32:1787–1795. doi: 10.1016/j.biomaterials.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 27.Krifka S., Hiller K., Bolay C., Petzel C., Spagnuolo G., Reichl F., Schmalz G., Schweikl H. Function of MAPK and downstream transcription factors in monomer-induced apoptosis. Biomater. 2012;33:740–750. doi: 10.1016/j.biomaterials.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Nisar S.S., Irfan F., Hammad H., Abdulla A.M., Kamran M.A., Barakat A., Niazi F., Baig E.A., Qureshi A. Disinfection of caries-affected dentin using potassium titanyl phosphate laser, rose bengal and ozonated water on shear bond strength of deciduous teeth. Photodiagnosis Photodyn. Ther. 2022;40:1–6. doi: 10.1016/j.pdpdt.2022.103044. [DOI] [PubMed] [Google Scholar]
- 29.Spagnuolo G., Annunziata M., Rengo S. Cytotoxicity and oxidative stress caused by dental adhesive systems cured with halogen and LED lights. Clin. Oral Invest. 2004;8:81–85. doi: 10.1007/s00784-003-0247-y. [DOI] [PubMed] [Google Scholar]
- 30.Perdigão J., Araujo E., Ramos R.Q., Gomes G., Pizzolotto L. Adhesive dentistry: current concepts and clinical considerations. J. Esthetic Restor. Dent. 2021;33:51–68. doi: 10.1111/jerd.12692. [DOI] [PubMed] [Google Scholar]
- 31.Perdigão J. Current perspectives on dental adhesion: (1) Dentin adhesion – not there yet. Jpn Dent Sci Rev. 2020;56:190–207. doi: 10.1016/j.jdsr.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrilho E., Cardoso M., Ferreira M.M., Marto C.M., Paula A., Coelho A.S. 10-MDP based dental adhesives: adhesive interface characterization and adhesive stability-A systematic review. Materials. 2019;12:1–18. doi: 10.3390/ma12050790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalkilic E.E., Arisu H.D., Kivanc B.H., Uctasli M.B., Omurlu H. Effect of different disinfectant methods on the initial microtensile bond strength of a self-etch adhesive to dentin. Laser Med. Sci. 2012;27:819–825. doi: 10.1007/s10103-011-0987-x. [DOI] [PubMed] [Google Scholar]
- 34.Davalloo R., Seyedeh M.T., Ebrahimi H., Darabi F., Mahmoudi S. In vitro comparative evaluation of newly produced desensitizer, chlorhexidine and gluma on bond strength and bond longevity of composite to dentin. J Dent Shiraz Univ Med Sci. 2020;21:111–118. doi: 10.30476/DENTJODS.2019.77756.0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coelho A., Vilhena L., Amaro I., Antunes M., Paula A., Marto C.M., Saraiva J., Ferreira M.M., Carrilho E., Ramalho A. Effect of different cavity disinfectants on adhesion to dentin of permanent teeth. J. Funct. Biomater. 2022;13:1–13. doi: 10.3390/jfb13040209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiang Y.-S., Chen Y.-L., Chuang S.-F., Wu C.-M., W P.-J., Han C.-F., Lin J.-C., Chang H.-T. Riboflavin-ultraviolet-A-induced collagen cross-linking treatments in improving dentin bonding. Dent. Mater. 2013;29:682–692. doi: 10.1016/j.dental.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Vieira R.S., Silva I.A. Bond strength to primary tooth dentin following disinfection with a chlorhexidine solution: an in vitro study. Pediatr. Dent. 2003;25:49–52. [PubMed] [Google Scholar]
- 38.Oznurhan F., Ozturk C., Ekci E.S. Effects of different cavity-disinfectants and potassium titanyl phosphate laser on microtensile bond strength to primary dentin. Niger. J. Clin. Pract. 2015;18:400–404. doi: 10.4103/1119-3077.151774. [DOI] [PubMed] [Google Scholar]
- 39.Mohammadi N., Parsaie Z., Jafarpour D., Bizolm F. Effect of different matrix metalloproteinase inhibitors on shear bond strength of composite attached to primary teeth dentin. Eur J Gen Dent. 2020;9:147–151. [Google Scholar]
- 40.Gandolfi M.G., Taddei P., Pondrelli A., Zamparini F., Prati C., Spagnuolo G. Demineralization, collagen modification and remineralization degree of human dentin after EDTA and citric acid treatments. Mater. 2018;12:1–20. doi: 10.3390/ma12010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhn E., Farhat P., Teitelbaum A.P., Mena-Serrano A., Loguercio A.D., Reis A., Pashley D.H. Ethanol-wet bonding technique: clinical versus laboratory findings. Dent. Mater. 2015;3:1030–1037. doi: 10.1016/j.dental.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Yu J., Zhao Y., Shen Y., Yao C., Guo J., Yang H., Huang C. Enhancing adhesive–dentin interface stability of primary teeth: from ethanol wet-bonding to plant-derived polyphenol application. J. Dent. 2022;126:1–10. doi: 10.1016/j.jdent.2022.104285. [DOI] [PubMed] [Google Scholar]
- 43.Hussein A.A.A., Al-Shamma A.M.W. Effect of chlorhexidine and/or ethanol pre-bonding treatment on the shear bond strength of resin composite to dentin. Int. J. Med. Res. Health Sci. 2019;8:150–159. [Google Scholar]
- 44.Ozsoy A., Erdemir U., Yucel T., Yildiz E. Effects of cavity disinfectants on bond strength of an etch-and-rinse adhesive to water- or ethanol-saturated sound and caries-affected dentin. J. Adhes. Sci. Technol. 2015;29:2551–2564. doi: 10.1080/01694243.2015.1073825. [DOI] [Google Scholar]
- 45.Simões D.M.S., Basting R.T., Amaral F.L.B., Turssi C.P., França F.M.G. Influence of chlorhexidine and/or ethanol treatment on bond strength of an etch-and-rinse adhesive to dentin: an in vitro and in situ study. Operat. Dent. 2014;39:64–71. doi: 10.2341/12-486-L. [DOI] [PubMed] [Google Scholar]
- 46.Goel S., Sinha D.J., Singh U.P., Ahuja U., Haider N., Sharma N. Comparative evaluation of effect of chlorhexidine, Azadirachta indica (neem), and Aloe barbadensis miller (Aloe vera) on resin-dentin bond stabilization using shear bond testing - an in vitro study. J. Conserv. Dent. 2019;22:300–304. doi: 10.4103/JCD.JCD_11_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is referenced in the article.