Abstract
Ove mutants in the moss Physcomitrella patens can arise from different recessive mutations. These mutants display a much larger number of buds than the wild type (wt) due to a dramatic overproduction of cytokinins (Cks), which are released into the culture medium (T.L. Wang, R. Horgan, D.J. Cove [1981] Plant Physiol 68: 735–738). The amounts of isopentenyladenine (iP) and isopentenyladenosine ([9R]iP) produced by chloronema of different ove mutants were measured. Levels of the major Ck iP in the culture medium of the mutants oveA78, oveA201, oveC200, and oveB300 (cultured at 21°C) were 4-fold (oveA78) to 22-fold (oveB300) higher than for the wt. A new temperature-sensitive ove strain oveST25, which exhibits a strong ove phenotype at 25°C, was also studied. It produced about 260 times more iP than the thiamine auxotrophic wt from which it was derived. To contribute to the physiological understanding of Ck overproduction, in vivo labeling experiments with 3H-[9R]iP were performed. In all ove mutants analyzed, the rate of 3H-[9R]iP conversion to 3H-iP was higher as compared with the wt. In oveST25, the 3-fold increased riboside to base conversion was temperature inducible and correlated with the iP production. Analysis of Ck catabolism revealed no major differences between ove mutants and wt, thus indicating that ove mutants are unlikely to be degradation mutants. The data suggest that in ove mutants the increased riboside to base conversion is part of a generally up-regulated Ck biosynthetic pathway and may play an important role for the enhanced release of iP into the medium.
In the early stages of cytokinin (Ck) research it was found that moss tissue releases Ck-like substances into the culture medium. Bauer (1966) reported the isolation of a kinetin-like substance (called bryokinin) that was found in the culture medium of sporophyte callus cells derived from the moss hybrid Physcomitrium piriforme × Funaria hygrometrica. The substance, which was detected at micromolar concentrations in the culture medium as well as in the tissue, was identified as isopentenyladenine (iP; Beutelmann, 1973).
Cks play a major role in the cellular differentiation of the moss protonema because they induce the formation of buds (Bopp and Atzorn, 1992; Cove, 1992; Reski, 1998). In F. hygrometrica, Ck bases have considerably higher hormonal activities when compared with their ribosides (Whitaker and Kende, 1974). Spiess (1976) tested eight moss species for their ability to form buds after treatment with zeatin and zeatin riboside. All species showed increased budding or callus formation after application of zeatin when compared with the control. After zeatin riboside treatment, three species reacted also with significantly increased budding but five species, including F. hygrometrica, showed either no or only little bud formation.
The base iP, which is considered the biologically active form, was identified as the major Ck in the culture medium of the bud-overproducing ove mutants of Physcomitrella patens (Wang et al., 1980, 1981b). The extracellular iP concentration in ove mutants can exceed that of the wild type (wt) up to 100-fold (Wang et al., 1984). When ove mutants are grown under constant replacement of the culture medium, bud overproduction can be prevented (Wang et al., 1984).
When ove mutants are incubated with radiolabeled adenine, incorporation of radioactivity into the iP fraction is enhanced when compared with the wt (Wang et al., 1981a).
Featherstone et al. (1990) carried out a genetic study with 15 independent ove mutants. This study revealed that the ove mutants belong to three complementation groups: oveA, oveB, and oveC. Furthermore, 14 of the ove mutations are recessive, indicating that their phenotype probably arises from a loss of gene function.
Here, we present a new temperature-sensitive ove mutant, oveST25 (A.H. Hofmann and V.E.A. Russo, unpublished data). OveST25, obtained after UV mutagnesis of wt(thiA1), displays a wt phenotype at 15°C. However, at 25°C it exhibits an ove phenotype with an excess of buds developing to callus-like structures and no functional gametophores. At 21°C oveST25 exhibits an intermediate phenotype with callus-like structures and developed gametophores on the same culture. A similar mutant (oveA409) has been described for its phenotype and Ck production by Futers et al. (1986). However, in this mutant Ck metabolism was not studied.
In this work, we present a comparison of Ck production for all ove mutants available including oveST25. Because the molecular basis for Ck overproduction in ove mutants is completely unknown, we also investigated the possibility that changes in Ck production could be related to alterations in Ck interconversion and catabolism. We focused on the conversion reaction of the Ck riboside isopentenyladenosine ([9R]iP) to the base iP, the last step in the biosynthesis of biologically active Ck bases. Furthermore, we measured Ck breakdown and the formation of Ck nucleotides in ove mutants and wt.
RESULTS
Cytokinin Release by the Temperature-Sensitive Strain oveST25
A new temperature-sensitive ove strain (oveST25) was isolated after UV mutagenesis of wt(thiA1) protonema. Grown at 15°C, its phenotype is comparable to that of wt, whereas at 25°C it shows a clear ove phenotype with a great number of abnormal buds (not shown).
OveST25, like other ove mutants, induces increased budding in wt plants cultured in its vicinity on the same petri dish; thus, it displays “cross-feeding” characteristics (not shown). At 21°C an intermediate phenotype is observed with less buds and less malformation than is observed with growth at 25°C (not shown). OveST25 was compared with wt(thiA1), a thiamine auxotrophic wt (from which oveST25 was derived).
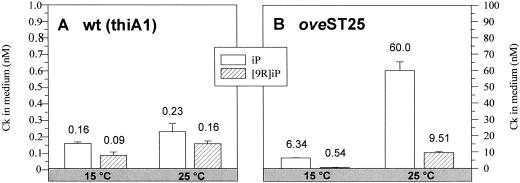
As shown in Figure 1, the Ck production of oveST25 grown at 25°C differs very strongly from that of oveST25 grown at 15°C. At 25°C, 9.5 times more iP and 18 times more [9R]iP are released into the culture medium than at 15°C. These data clearly demonstrate the temperature-dependent induction of the ove phenotype in oveST25. Thus, oveST25 is a conditional ove mutant with a strong temperature-inducible Ck overproduction.
Figure 1.
Thermal induction of Ck overproduction in the mutant oveST25. Comparison of Ck concentrations measured by HPLC-ELISA in the culture medium of oveST25 and wt (thiA1), a thiamine auxotrophic strain. iP and [9R]iP were measured in the medium of liquid cultures cultivated continuously at 15°C or 25°C. The age of the culture was 3 weeks; 2.1 to 3.5 mg chloronema tissue per ml medium was used. Note the different scales in A and B. Error bars indicate sd from four to eight ELISA measurements.
For wt(thiA1), the higher growth temperature leads only to a moderate increase of iP and [9R]iP release (1.4- to 1.7-fold, respectively; see Fig. 1.). This could be explained by a general stimulation of metabolic activities at a higher temperature. Wt(thiA1) was found to be comparable to the nonauxotrophic wt in terms of Ck production and metabolism (not shown).
Cytokinin Released into Culture Medium by wt, oveA78, oveA201, oveB300, and oveC200
Although several papers on iP production in ove mutants have been published by Wang and coworkers, a comprehensive study including measurements of the riboside [9R]iP in all ove mutants is not yet available.
Table I displays the Ck concentration in the culture medium of wt and ove mutants that were cultured, unlike oveST25, at the standard temperature of 21°C. Cks in the culture medium were measured by ELISA. For all genotypes, the base iP is the predominant Ck in the culture medium, and the iP to [9R]iP ratio lies between 1.3 and 4.9.
Table I.
Concentrations and concentration ratios of iP and [9R]iP in the culture medium of P. patens wt and ove mutants after 6 d of culturing at 21°C
| Genotype | iP | [9R]iP | iP + [9R]iP | iP/[9R]iP |
|---|---|---|---|---|
| pM | ||||
| wt | 30 | 23 | 53 | 1.3 |
| oveA78 | 132 | 27 | 159 | 4.9 |
| oveA201 | 170 | 117 | 287 | 1.45 |
| oveB300 | 687 | 445 | 1,132 | 1.6 |
| oveC200 | 149 | 30 | 179 | 4.9 |
For the wt iP and [9R]iP, concentrations were 30 and 23 picomolar, respectively. All ove mutants showed increased iP and [9R]iP concentrations in comparison with wt. The highest iP and [9R]iP release was observed for oveB300 at 22- and 19-fold increased concentrations, respectively.
The relative order of the genotypes for the degree of iP (over) production was confirmed by performing a time course analysis with two cultures for each genotype measured during 8 d (data not shown).
Uptake and in Vivo Metabolism of [9R]iP by ove Mutants and wt
Although the first steps of the Ck biosynthethic route are still unclear, the conversion of the Ck ribosides can be regarded as the last step leading to the formation of the biologically active Ck bases (see Zazimalova et al., 1999). Especially in P. patens, the riboside-base conversion is an essential step for the release of iP into the culture medium.
To monitor the riboside-base conversion, we carried out in vivo experiments with 3H-[9R]iP, which was added to the culture medium. The products of 3H- [9R]iP in vivo metabolism were measured in the culture medium and in tissue extracts.
After addition of 3H-[9R]iP to the culture medium, little apparent uptake of radioactivity (<6%) was measured within 6 h for all genotypes (see Table II).
Table II.
Distribution of radioactive metabolites in tissue and culture medium after 6 h in vivo labeling with 3H-[9R]iP (225 pmol per incubation, 4-mL volume)
| Genotype | Tissue in Incubation | Apparent Uptake of Radioactivity | iP
|
[9R]iP (Substrate)
|
[9R]iP Nucleotides
|
Degradation Products
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cells | Medium | Cells | Medium | Cells | Medium | Cells | Medium | |||
| mg | % | pmol | ||||||||
| wt | 111 | 2.0 | 0.66 | 12 | 0.40 | 168.2 | 1.76 | n.d.a | 1.38 | n.d. |
| oveA78 | 140 | 3.1 | 4.5 | 73.5 | 0.54 | 79.0 | 0.95 | n.d. | 0.61 | n.d. |
| oveA201 | 160 | 5.2 | 6.86 | 99.3 | 0.49 | 50.4 | 0.99 | n.d. | 1.61 | n.d. |
| oveB300 | 120 | 3.2 | 2.21 | 158 | 0.06 | 6.73 | 1.00 | n.d. | 2.4 | n.d. |
| oveST25 | 104 | 4.5 | 5.29 | 96.7 | 0.22 | 47.0 | 1.74 | n.d. | 2.75 | n.d. |
Values are not corrected for recovery losses (about 25%). Other unidentified metabolites ranged below 0.45 pmol and are not listed. Apparent uptake of radioactivity was calculated from the total radioactivity found in extracts. Values important for discussion are in bold face.
n.d., Not detected.
Analysis of Culture Medium
The culturing and the labeling experiments for oveST25 and for wt(thiA1) were carried out at 15°C and 25°C (Fig. 2).
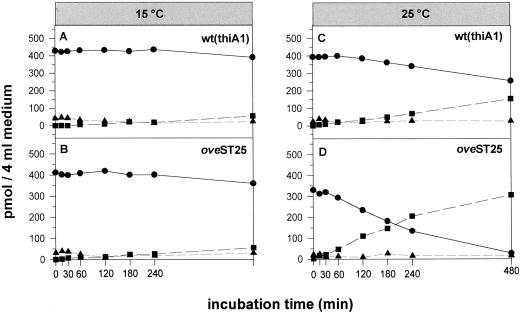
Figure 2.
Temperature-induced increase in the conversion of 3H-[9R]iP (●) to 3H-iP (▪) and unidentified metabolites (▴) measured by HPLC-LS in the culture medium during in vivo labeling of P. patens wt (thiA1) and its temperature-sensitive mutant oveST25. The two strains were grown and incubated at 15°C (A and B) and 25°C (C and D). Each incubation was carried out with 100 to 135 mg of washed chloronema in 4 mL of aerated medium.
Comparing wt(thiA1) and oveST25 grown at 15°C, the kinetics of [9R]iP to iP conversion show no major differences. After 8 h of feeding, both strains have only converted about 13% of the substrate 3H-[9R]iP to 3H-iP (Fig. 2, A and B). However, at 25°C, a temperature leading to Ck overproduction, oveST25 clearly shows a higher conversion rate than wt(thiA1), (Fig. 2, C and D). After 4 h of incubation oveST25 had converted about 62% of the substrate to 3H-iP, whereas wt(thiA1) had only metabolized about 18% of the initial 3H-[9R]iP. After 8 h, almost all 3H-[9R]iP had been converted in the medium of oveST25, whereas the substrate 3H-[9R]iP was still the predominant radiolabeled compound in wt(thiA1). No nucleotides were detected in the culture medium.
When calculating the initial rates for the 3H-[9R]iP to 3H- iP conversion, we found a 3.3-fold higher value for wt(thiA1) when this strain was cultured and incubated at 25°C versus 15°C (Table III). OveST25, however, had a 10-fold higher initial conversion rate at 25°C than at 15°C. At 25°C, oveST25 had a 3-fold higher initial conversion velocity than wt(thiA1).
Table III.
Relative rates for the in vivo conversion of radiolabeled [9R]iP to iP in the temperature-sensitive mutant oveST25 and the control strain wt(thiA1); both strains were cultured and incubated at 15°C and 25°C
| Strain | Temperature | Relative Conversion Rate |
|---|---|---|
| wt(thiA1) | 15°C | 1 |
| wt(thiA1) | 25°C | 3.3 |
| oveST25 | 15°C | 1.1 |
| oveST25 | 25°C | 11 |
The relative rates for the in vivo conversion of radiolabeled [9R]iP to iP for wt and the other ove mutants (cultured and incubated at the standard temperature of 21°C) were: wt, 1; oveA78, 3.4; oveA201, 2.1; oveB300, 2.3; and oveC200, 2.0. These data reveal that ove strains from all three complementation groups have increased initial velocities for the 3H-[9R]iP to 3H-iP conversion relative to the wt.
The increased [9R]iP to iP conversion is consistent with the finding that it is mainly the base iP which is released into the culture medium in Ck-overproducing ove mutants (Table I). These data further suggest that, in all analyzed ove mutants, Ck overproduction is coupled to an enhanced conversion of the riboside [9R]iP to its base iP.
Medium Tissue Distribution of 3H-[9R]iP Metabolites after in Vivo Labeling
Although some Ck metabolites migrate across the plasma membrane, others like Ck nucleotides or adenylic degradation products stay within the cell. To complete the picture of Ck metabolism, protonema of wt and the strains oveA78, oveA201, oveB300, and oveST25 were extracted after 6 h of labeling with 3H-[9R]iP. In this experiment the nonauxotrophic wt was used as a control. Culture and incubation were carried out at 25°C to obtain the ove phenotype for oveST25. Protonema extracts were analyzed for 3H-[9R]iP, 3H-iP, and 3H-[9R]iP nucleotides and the adenylic degradation products (adenine, adenosine, AMP, ADP, and ATP).
The analysis of protonema extracts revealed three distinct metabolic pathways for 3H-[9R]iP, thus giving a more complex pattern of metabolites than the analysis of the culture medium (Tables II and IV):
Table IV.
Relative distribution of radioactivity in the fractions of iP, [9R]iP, [9R]iP nucleotides, and adenylic degradation products
| Genotype | iP %
|
[9R]iP (Substrate) %
|
[9R]iP Nucleotides %
|
Degradation Products %
|
||||
|---|---|---|---|---|---|---|---|---|
| Cells | Medium | Cells | Medium | Cells | Medium | Cells | Medium | |
| nM | ||||||||
| wt | 0.35 | 6.51 | 0.21 | 91.20 | 0.95 | n.d.a | 0.74 | n.d. |
| (5.9) | (3.0) | (3.6) | (42.0) | (15.9) | (12.4) | |||
| oveA78 | 2.83 | 46.20 | 0.34 | 49.65 | 0.60 | n.d. | 0.38 | n.d. |
| (32.1) | (18.4) | (3.7) | (19.8) | (6.8) | (4.4) | |||
| oveA201 | 4.30 | 62.20 | 0.31 | 31.57 | 0.62 | n.d. | 1.01 | n.d. |
| (42.9) | (24.8) | (3.1) | (12.6) | (6.2) | (10.1) | |||
| oveB300 | 1.30 | 92.94 | 0.04 | 3.96 | 0.59 | n.d. | 1.41 | n.d. |
| (18.4) | (39.5) | (0.5) | (1.7) | (8.3) | (20.0) | |||
| oveST25 | 3.46 | 63.20 | 0.14 | 30.72 | 1.14 | n.d. | 1.80 | n.d. |
| (50.9) | (24.2) | (2.1) | (11.8) | (16.7) | (26.4) | |||
Values are related to the total amount of these radiolabeled substances detected for each genotype (medium plus tissue extract). Intracellular and extracellular concentrations (nM) of radiolabeled metabolites determined in medium and tissue are in parentheses. Values are derived from Table II and show localization of radiolabeled metabolites in tissue and the medium as well as their concentrations.
n.d., Not detected.
First, 3H-iP was detected as metabolite in all five genotypes analyzed, but was a major metabolite only in ove mutants, with 46% to 92% of the radioactivity in the extracellular iP (Table IV). Thus, tissue analysis confirms the increase in riboside-base conversion for ove mutants.
Second, 3H-[9R]iP was phosphorylated to its nucleotides (mono-, di-, and tri-phosphates). The relative amount of 3H-[9R]iP nucleotides per incubation did not exceed 1.14% of the total intra- and extracellular radioactivity. Nevertheless, 3H-[9R]iP-monophosphate was the dominating radiolabeled internal metabolite in the wt tissue. In the ove mutants, however, the 3H-[9R]iP-monophosphate is a minor metabolite because the 3H-[9R]iP metabolism is directed mainly toward the formation of 3H-iP.
Third, wt and all ove strains analyzed showed degradation of 3H-[9R]iP, leading to a loss of the isopentenyl side chain, i.e. to the formation of adenylic compounds. With the exception of oveA78 the intracellular amounts of degradation compounds exceeded even those found for wt. Thus, the accumulation of Cks due to a defect in Ck catabolism seems very unlikely for ove mutants.
Summarizing the Ck metabolism data, it can be stated that the ove mutants are characterized by an increased in vivo activity for the conversion of the riboside [9R]iP to its base iP, which is released into the culture medium.
DISCUSSION
For a comparison of Ck production in ove mutants and wt, we tested all available genotypes at the same developmental stage. This was achieved by the addition of di-ammonium tartrate to the culture medium which is known to block the sequential cellular differentiation at the level of the chloronema stage (D.J. Cove, personal communication). Thus, all measurements can be related to one single cell type (chloronema) for all genotypes.
Cytokinin Production by ove Mutants
For the strains oveA78, oveA201, oveC200, and oveB300, we measured extracellular iP concentrations ranging from 132 (oveA78) to 687 pM (oveB300) after 1 week of culturing at 21°C (Table I). When arranging the genotypes in order of increasing Ck release, the same order is obtained for iP as well as for [9R]iP: wt < oveA78 < oveC200 < oveA201 < oveB300. This suggests that increased Ck biosynthesis in ove mutants affects both riboside and base production in the same way.
A strong release of Cks into the culture medium was also described for P. patens transformed with the agrobacterial isopentenyltransferase gene (ipt), where 97% to 99% of the iP amount was found in the culture medium (Reutter et al., 1998; Schulz et al., 2000).
Wang et al. (1984) previously measured iP for various ove mutants and reported extracellular concentrations up to 270-fold higher (oveA78) than the values presented in this work. The occurrence of the extracellular [9R]iP was not reported by Wang and coworkers. It is possible that the discrepancies are due to differences in culture conditions. For example, no di-ammonium tartrate was added to the culture medium by Wang and coworkers; thus, their cultures probably also contained caulonema and buds. We also do not exclude that Ck production capacity of some ove mutants might have decreased due to the long period of vegetative growth.
However, Ck concentration in oveST25 can reach levels 260-fold higher than those of wt(thiA1) when cultured at 25°C (Fig. 1). Also, at 15°C oveSt25 exhibits higher Ck concentrations than wt (40-fold higher iP and 6-fold higher [9R]iP), but at 15°C they do not visibly affect the phenotype. For the reason of its strong temperature-sensitive Ck production oveST25 is an interesting conditional mutant for the study of Ck metabolism.
Metabolism of 3H-[9R]iP
Because it is completely unknown which factors govern Ck overproduction in ove mutants, we decided to compare the uptake and metabolism of 3H-[9R]iP by in vivo labeling studies. The substrate concentration of about 100 nm is similar to that measured in the medium of certain ove mutants (see Table I, Fig. 1). Our in vivo labeling studies with ove mutants confirmed that with respect to the whole labeling assay most of the 3H-iP amount, deriving from the substrate 3H-[9R]iP, is located extracellularly (Table IV). This is mainly due to the high ratio of medium volume versus tissue volume (16:1–40:1) and to the fact that the 3H-iP concentrations are in the same order of magnitude in cells and medium.
We found that the substrate 3H-[9R]iP moves into the cells where it does not accumulate to concentrations higher than in the medium. However, the mechanism of 3H-[9R]iP uptake is unclear. Because no extracellular activities for the deribolisation of 3H-[9R]iP could be detected (not shown), we assume that it is metabolized intracellularly to 3H-iP, which then leaves the cells following a concentration gradient. The concentration of 3H-iP is about 1.7 to 2.1 times higher in the tissue compared with the medium. The only exception to this is the oveB300 strain, where 3H-iP is 2.1 times higher concentrated in the medium (Table IV). In oveB300, in contrast to the other strains, the extracellular substrate has been metabolized almost completely (92%) to the base 3H-iP. Because no new 3H-iP can be formed in oveB300, it can be suggested that there is a net influx of 3H-iP, which is subject to intracellular Ck breakdown resulting in a lower 3H-iP concentration. In this context, we assume that the bidirectional transport of iP is based on passive diffusion. For tobacco (Nicotiana tabacum) cell cultures it has been shown that Ck bases migrate easily across the plasma membrane (Laloue and Pethe, 1982).
The conversion of Ck ribosides to their bases seems to be important when comparing the relative hormonal activity of these Ck forms in mosses. It seems possible that under experimental conditions with a low density of tissue, Ck ribosides added to the medium are only poorly converted to the corresponding base and therefore show little or no effect, as it has been shown for zeatin riboside in certain mosses (Whitaker and Kende, 1974; Spiess, 1976). The relative activities of Ck ribosides and bases in mosses should be redetermined taking into account that Ck ribosides can be efficiently converted to the base.
The role of Ck oxidase for Ck breakdown has been described for F. hygrometrica by Gerhäuser and Bopp (1990). The fact that P. patens releases large amounts of iP seems to be consistent with the finding that in this plant there is no efficient metabolism of iP except from degradation via Ck oxidase (K. von Schwartzenberg and M. Laloue, unpublished data): (a) In previous studies with Physcomitrella wt, we have shown that there is only little phosphoribosylation of iP via adenine phosphoribosyltransferase (K. von Schwartzenberg and M. Laloue, unpublished data), which is known to form Ck nucleotides in higher plants (Moffatt et al., 1991; Schnorr et al., 1996). Instead, Ck interconversion in Physcomitrella uses the adenosine kinase pathway to form iP-nucleotides from the riboside [9R]iP (Schwartzenberg et al., 1998); and (b) The analysis of tissue extracts after labeling with 3H-iP did not reveal a significant formation of glucoside conjugates or hydroxylated Ck forms such as zeatin (K.v. Schwartzenberg and M. Laloue, unpublished data) but showed degradation to adenylic compounds. Thus, degradation via Ck oxidase and the cellular efflux seem to be the only two major processes determining the metabolic fate of iP.
In this work, we show that oveA78, oveA201, oveB300, and oveST25 are capable to degrade Cks. In oveA201, oveB300, and oveST25, Ck degradation is even found to be higher than in the wt (see Table IV). First, in vitro experiments on Ck breakdown revealed similar Ck oxidase activities in protein extracts of ove mutants and wt (data not shown). Therefore, we conclude that ove mutants are very unlikely to be mutated with respect to Ck degradation.
At this time, we are not sure about the enzyme(s) responsible for the deribolization of [9R]iP to iP (Fig. 2; Tables II and III). In higher plants, [9R]iP has been shown to be deribosylated to iP via adenosine nucleosidase (Chen and Kristopeit, 1981). But when performing in vitro enzyme assays for purine nucleosidase with desalted enzyme extracts of P. patens wt, we were not able to identify any nucleosidase activity converting [9R]iP to iP. Further biochemical work must be done to purify the [9R]iP-converting enzyme(s).
It is astonishing that ove mutants of all three complementation groups as well as the genetically uncharacterized oveST25 showed a similar increase in [9R]iP to iP conversion in vivo. To investigate whether the increase in riboside-base conversion is induced by the overproduced Ck, we compared the riboside-base conversion in a Ck overproducing ipt transformant PC22ipt8 with that of wt. PC22ipt8 produced up to 530 times more iP and 40 times more [9R]iP than the wt (Reutter et al., 1998; Schulz et al., 2000). It is surprising that PC22ipt8 had a [9R]iP to iP conversion rate that was comparable to that of the wt (data not shown). Also, based on the finding that oveST25 cultured at 15°C displays [9R]iP and iP concentrations that are 5 and 40 times higher than in wt(thiA1) but has no significant increase in riboside-base conversion (Figs. 1 and 2), we hypothesized that elevated Ck concentrations themselves do not up-regulate the riboside-base conversion in P. patens.
Our data on the temperature-dependent Ck production (Fig. 1) are in accordance with the work of Futers et al. (1986) who found increased iP concentrations in wt cultures grown at 24°C compared with 15°C. The fact that Ck production is temperature sensitive per se is also reflected in the about 3-fold increased rate of riboside to base conversion found for the wt at 25°C when compared with 15°C (Table III). The molecular basis for the stronger conversion (10-fold) in oveST25 is so far unclear.
We presume that the Ck biosynthetic pathway in ove mutants is affected at several levels and is generally up-regulated. When trying to attribute a function to the uncharacterized genes mutated in the recessive ove mutants, we agree with Cove (1992), who presumes that ove genes code for negative regulator(s), e.g. a repressor of Ck biosynthesis. We suggest that deribolization of [9R]iP, as the last biosynthetic step in the formation of the base iP, is also negatively regulated by the ove gene products. The increased velocity in the riboside to base conversion as part of the up-regulated Ck biosynthesis may play an important role for the enhanced iP formation in ove mutants. Further experiments will reveal the identity of the deregulated genes and enzyme(s).
MATERIALS AND METHODS
Moss Strains
The following strains of Physcomitrella patens (Hedw.) B.S.G. were generously provided by Dr. D.J. Cove (University of Leeds, England): (a) Cambridge wt and wt(thiA1), a thiamin auxotrophic wt. Both strains were used as control; (b) ove mutants oveA78, oveA201, oveB300, and oveC200 (all recessive mutations), which were generated as described by Ashton et al. (1979).
The strain oveST25 was obtained by UV mutagenesis from protonema of the thiamine auxotrophic wt strain wt(thiA1), and was isolated from nonselective medium based on its phenotype (A. Hofmann and E. Russo, unpublished data).
Culture Conditions
P. patens was cultivated in liquid culture using a medium described by Wang et al. (1980): Ca(NO3)2, 0.359 mm; FeSO4, 0.035 mm; MgSO4, 1.01 mm; KH2PO4, 1.84 mm; and KNO3,10 mm; 1 mL of Hoagland trace element solution was added (Ashton and Cove, 1977). Di-ammonium tartrate was added to a final concentration of 5 mm and the pH was adjusted to 6.5 (KOH). Five hundred milliliters of culture medium was inoculated with about 300 mg of protonema filaments that had been freshly cut up with a Ultra-Turrax (IKA, Staufen, Germany) to filaments of 10 to 20 cells. Culture flasks (Pyrex 1000 mL) were aerated with water saturated sterile air (about 600 mL min−1). Maintainance of strains was as described by Ashton and Cove (1977).
Cultures were grown in white light (Philips TLM, Hamburg, Germany ) at 100 μE m−2 s−1 under a light:dark cycle of 16:8 h.
For experiments with wt, oveA78, oveA201, oveB300, and oveC200, a culture temperature of 21°C was used (standard).
The temperature-sensitive mutant oveST25 was cultured at 25°C to express the ove phenotype, or at 15°C to obtain the wt phenotype.
Preparation of Culture Medium for Ck Determination
Concentration of Cks from liquid culture medium was previously described by Schulz et al. (2000).
HPLC: Separation of Cytokinin Metabolites
Samples were concentrated to a volume of 100 μL by rotary film evaporation, then resuspended in 2.2 mL buffer A (10 mm triethylamine/acetic acid, pH 5.4). Fractions containing Ck nucleotides, ribosides, and bases were separated on a reverse phase HPLC column (Lichrospher 60 RP-Select B, 5 μm, Merck, Darmstadt, Germany). The elution was performed using a gradient of buffer A (see above) and methanol with a flow rate of 0.8 mL min−1. Methanol concentration was raised from 10% to 100% (w/v) within 27 min. The optical density of the effluent was determined by UV detection (269 nm). Fractions of 0.8 mL were collected, dried, and redissolved in 3.75% (v/v) methanol prior to liquid scintillation counting for yield calculations and ELISA.
Cytokinin Determination by Enzyme Immunoassay
iP and [9R]iP in HPLC fractions were determined using an indirect competitive ELISA as described by Schwartzenberg et al. (1988). The validity of the ELISA method for Ck determination in P. patens had previously been verified (Schulz et al., 2000).
In Vivo Labeling with 3H-[9R]iP
For radiolabeling, [9R][2-3H]iP, named 3H-[9R]iP, was either prepared according to Laloue and Fox (1987; specific activity 18 Ci mmol−1) or was obtained from the Institute of Experimental Botany (Prague, Czech Republic; 33.8 Ci mmol−1). Protonema tissue of P. patens was grown in liquid culture as described above. To reduce interference from non-labeled plant derived Cks, the protonema tissue was intensively rinsed with fresh medium prior to the experiment.
One hundred to 160 mg of chloronema (fresh weight) from 9- to 11-d-old cultures was incubated in 4 mL of aerated liquid medium. 3H-[9R]iP was added to a final concentration of 50 to 100 nm. After 0 to 8 h of labeling the culture medium was separated from the protonema by filtration using a glass fiber filter (Whatman No. 6, Whatman, Maidstone, UK).
The culture medium was centrifuged (15,000g, 5 min) and analyzed directly by reverse phase HPLC (for separation conditions see above) in combination with online liquid scintillation counting (Canberra Packard, Dreieich, Germany).
For the tissue analysis, protonema was harvested after incubation by filtration on glass fiber filter (Whatman, No. 6) and rinsed for 2 s with 10 mL of fresh ice-cold culture medium.
Analysis of Cytokinin Metabolites from Protonema Tissue
After radiolabeling, 1 mL of Bieleski's reagent (methanol:chloroform:formic acid:water, 12:5:1:2 [v/v], Bieleski, 1964, altered) was added to 100 to 160 mg of protonema. The tissue was ground in the presence of glass beads (diameter 0.5 mm) by vortex and incubated overnight at −20°C to inactivate phosphatases. Water (0.5 mL) was then added and a phase separation was achieved. After centrifugation (15,000g, 10 min), the upper water layer, containing the Ck metabolites, was collected and dried by rotary film evaporation. The residue was dissolved in buffer A prior to HPLC (see HPLC separation) coupled to online liquid scintillation counting. Radioactivity bound to [9R]iP-monophosphate, [9R]iP, and iP was measured directly at the corresponding retention times (20, 24, and 26 min respectively). For the analysis of di-and triphosphates of [9R]iP and Ck degradation products, the HPLC-effluent eluting between 2 and 10 min was collected and dried by evaporation. After dissolving the residue in digestion buffer (Tris-HCl, 10 mm; MgCl2, 10 mm [pH 7.5]), calf intestine alkaline phosphatase (10 units, MBI Fermentas, St. Leon-Rot, Germany) was added and the mixture was incubated for 1 h at 37°C. The mixture was run over HPLC again for the analysis of 3H-[9R]iP, which corresponds to the amount of [9R]iP-nucleotides in the primary extract. Cytokinin degradation was also determined during this second HPLC by measuring the amount of radioactivity in the fractions of adenine and adenosine.
ACKNOWLEDGMENTS
The authors thank Regis Maldiney (University of Paris VI, France) for help performing parts of the Ck measurements. This article is based in part on a doctoral study by P.S. in the Faculty of Biology, University of Hamburg (Germany).
Footnotes
Initial work was financed by the European Union Framework IV Biotech project “EUROMOSS” as part of the AMICA Project of Technical Priority. This work was further supported by the Deutsche Forschungsgemeinschaft in the program “Molekulare Analyse der Phytohormonwirkung” (to K.v.S.; grant no. Schw 687/2).
LITERATURE CITED
- Ashton NW, Cove DJ. The isolation and preliminary characterization of auxotrophic and analogue resistant mutants of the moss, Physcomitrella patens. Mol Gen Genet. 1977;154:87–95. [Google Scholar]
- Ashton NW, Cove DJ, Featherstone DR. The isolation and physiological analysis of mutants of the moss Physcomitrella patens, which overproduce gametophores. Planta. 1979;144:437–442. doi: 10.1007/BF00380119. [DOI] [PubMed] [Google Scholar]
- Bauer L. Isolierung und Testung einer kinetinartigen Substanz aus Kalluszellen von Laubmoossporophyten. Z Pflanzenphysiol. 1966;54:241–253. [Google Scholar]
- Beutelmann P. Untersuchungen zur Biosynthese eines Cytokinins in Calluszellen von Laubmoossporphyten. Planta. 1973;112:181–190. doi: 10.1007/BF00388588. [DOI] [PubMed] [Google Scholar]
- Bieleski RL. The problem of halting enzyme action when extracting plant tissues. Anal Biochem. 1964;9:431–442. doi: 10.1016/0003-2697(64)90204-0. [DOI] [PubMed] [Google Scholar]
- Bopp M, Atzorn R. Hormonelle Regulation der Moosentwicklung. Naturwissenschaften. 1992;79:337–346. [Google Scholar]
- Chen C-M, Kristopeit SM. Metabolism of cytokinin: deribolization of cytokinin ribonucleoside by adenosine nucleosidase from wheat germ cells. Plant Physiol. 1981;68:1020–1023. doi: 10.1104/pp.68.5.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove DJ. Regulation of development in the moss, Physcomitrella patens. In: Russo VEA, Brody S, Cove D, Ottolenghi S, editors. Development: The Molecular Genetic Approach. Berlin, Heidelberg, New York: Springer Verlag; 1992. pp. 179–193. [Google Scholar]
- Featherstone DR, Cove DJ, Ashton NW. Genetic analysis by somatic hybridization of cytokinin overproducing developmental mutants of the moss, Physcomitrella patens. Mol Gen Genet. 1990;222:217–224. doi: 10.1007/BF00633821. [DOI] [PubMed] [Google Scholar]
- Futers TS, Wang TL, Cove DJ. Characterization of a temperature-sensitive gametophore over-producing mutant of the moss, Physcomitrella patens. Mol Gen Genet. 1986;203:529–532. [Google Scholar]
- Gerhäuser D, Bopp M. Cytokinin oxidases in mosses: I. Metabolism of kinetin and benzyladenine in vivo. J Plant Physiol. 1990;135:680–685. [Google Scholar]
- Laloue M, Fox JE. The synthesis of tritiated ribosylzeatin with high specific activity. Phytochemistry. 1987;26:987–989. [Google Scholar]
- Laloue M, Pethe C. Dynamics of cytokinin metabolism in tobacco cells. In: Wareing PW, editor. Plant Growth Substances 1982. London: Academic Press; 1982. , 185–195. [Google Scholar]
- Moffat B, Pethe C, Laloue M. Metabolism of benzyladenine is impaired in a mutant of Arabidopsis thaliana lacking adenine phosphoribosyltransferase activity. Plant Physiol. 1991;95:900–908. doi: 10.1104/pp.95.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reski R. Development, genetics and molecular biology of mosses. Bot Acta. 1998;111:1–15. [Google Scholar]
- Reutter K, Atzorn R, Hadeler B, Schmülling T, Reski R. Expression of the bacterial ipt gene in Physcomitrella rescues mutations in budding and in plastid division. Planta. 1998;206:196–203. [Google Scholar]
- Schnorr KM, Gaillard C, Biget E, Nygaard P, Laloue M. A second form of adenine phosphoribosyltransferase in Arabidopsis thaliana with relative specificity towards cytokinins. Plant J. 1996;9:891–898. doi: 10.1046/j.1365-313x.1996.9060891.x. [DOI] [PubMed] [Google Scholar]
- Schulz P, Reski R, Maldiney R, Laloue M, Schwartzenberg vK. Kinetics of cyokinin production and bud formation in Physcomitrella: analysis of wild type, a developmental mutant and two of its ipt transgenics. J Plant Physiol. 2000;156:768–774. [Google Scholar]
- Schwartzenberg vK, Lutze K, Hahn H. Determination of cytokinin content in needles of spruce (Picea abies (L.) Karst.) by an indirect enzyme-linked immunosorbent assay. J Plant Physiol. 1988;156:529–534. [Google Scholar]
- Schwartzenberg vK, Kruse S, Reski R, Moffatt B, Laloue M. Cloning and characterization of an adenosine kinase from Physcomitrella involved in cytokinin metabolism. Plant J. 1998;13:249–257. doi: 10.1046/j.1365-313x.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- Spiess LD. Developmental effects of zeatin, ribosyl-zeatin, and agrobacterium tumefaciens b6 on certain mosses. Plant Physiol. 1976;58:107–109. doi: 10.1104/pp.58.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TL, Beutelmann P, Cove DJ. Cytokinin biosynthesis in mutants of the moss Physcomitrella patens. Plant Physiol. 1981a;68:739–744. doi: 10.1104/pp.68.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TL, Cove DJ, Beutelmann P, Hartmann E. Isopentenyladenine from mutants of the moss, Physcomitrella patens. Phytochemistry. 1980;19:1103–1105. [Google Scholar]
- Wang TL, Futers TS, McGeeary F, Cove DJ. Moss mutants and the analysis of cytokinin metabolism. In: Crozier A, Hillman JR, editors. The Biosynthesis and Metabolism of Plant Hormones. Cambridge, UK: Cambridge University Press; 1984. p. 135. [Google Scholar]
- Wang TL, Horgan R, Cove DJ. Cytokinins from the moss Physcomitrella patens. Plant Physiol. 1981b;68:735–738. doi: 10.1104/pp.68.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker BD, Kende H. Bud formation in Funaria hygrometrica: a comparison of the activities of three cytokinins with their ribosides. Planta. 1974;121:93–96. doi: 10.1007/BF00384010. [DOI] [PubMed] [Google Scholar]
- Zazimalova E, Kaminek M, Brezinova A, Motyka V. Control of cytokinin biosynthesis and metabolism. In: Hooykaas PJJ, Hall MA, Libbenga KR, editors. Biochemistry and Molecular Biology of Plant Hormones. Rotterdam, The Netherlands: Elsevier Science BV; 1999. pp. 141–160. [Google Scholar]