Abstract
Although goji berry with many main biological activity components is a nutritional medicinal and edible plan and widespread in the northeast of China, its current development also faces a new challenge of overcapacity. So this study was largely focused to investigate the optimal extraction process conditions (extraction water proportion, acidic pH regulating reagent, adjusted pH, extraction temperature, extraction time) for goji berry with increased yield. Box Behnken experimental design (BBD) was used to model the mathematical model of the relationship between the three main influence of pivotal extraction parameters (extraction pH, extraction time and extraction temperature) selected by the single factor experiments on the responses of goji berry pectin yield (%). Morover the predicted models were adequately fitted to the real experimental data (18.507 % ± 0.275 %, P < 0.001) for all the response variables within a short time. The maximum yield of pectin was sufficiently obtained with optimal conditions of the best water-goji ratio of 20:1, extraction pH of 2.2 adjusted by hydrochloric acid, extraction time of 80 min at 76 °C. Interesting, as thickener, goji berry pectin does not only improve the health benefits of yogurt in some studies have reported, but it also improves its sensory properties and viscosity of the yogurt in this paper. It will effectively improve the efficiency of obtaining goji berry pectin, which will provide strong experimental evidence for the widespread application of goji berry pectin. Although goji berry pectin does not have significant advantages in sensory evaluation and yogurt viscosity, it has been proven in some studies to have good functionality in terms of nutrition. This provides a good idea for the development of functional foods urgently needed in the current aging society. Goji berry will be an effective alternative source of pectin on health food industry on large scale. In addition, this paper also provides a feasible approach to effectively solve the problem of goji berry overcapacity.
Keywords: Goji berry, Pectin, Extraction, Yogurt
1. Introduction
In recent years, with the increasing awareness of health care among people, Lycium barbarum (known as goji berry [1] or wolfberry [2]), which have many important functions, are becoming increasingly important in traditional Chinese medicine [3], so their market demand as a traditional Chinese medicine [4] and nutritional supplement [5] has been constantly expanding. Currently, it has been discovered that goji berry contain various nutrients and functional active ingredient, such as carbohydrates, alkaloids, flavonoids, carotenoids, polypeptides, polyphenols and others [4,6,7]. Modern medical research has proven that goji berry have many biological activity functions, including immune regulation, anti-inflammation, anti-tumor, nerve protection, anti-aging, antioxidant, anti-fatigue, hypoglycemic and hypolipidemic, liver protection, protecting myocardial cells, and so on [8]. However, nowadays approximately 75 %–85 % of goji berry fruits widespread in China are usually stored or consumed directly in dry form [9]. Besides dried fruits, the main product varieties are goji juice and goji fermented products [10] (including goji fruit wine, goji fruit vinegar, goji fermented beverages, etc.) [11]. There are not many other types of new goji berry foods or goji berry related products. Therefore, developing new goji related products or widely using goji ingredients will become more meaningful for expanding the goji health huge market quickly.
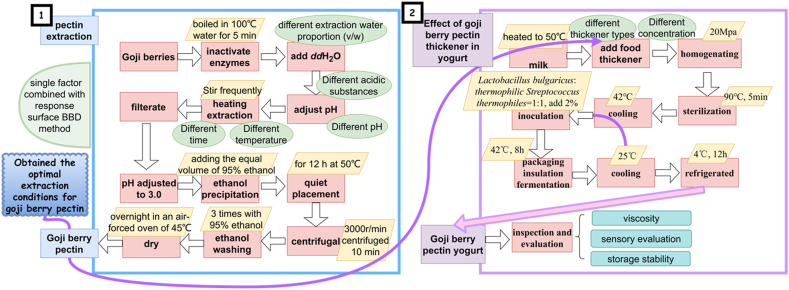
Although Many studies have shown that polysaccharide (e.g. pectin) was the main biological activity component of Chinese herb [12], the current development of goji berries in China like Shanxi and Liaoning Province also faces a new challenge of overcapacity. Therefore, this project aims to further develop goji berries and fully utilize them to solve the current problem of surplus goji berries. Pectin can often be extracted at high temperature by hydrolyzing proto-pectin using acid and isolated by precipitation with alcohol [13]. Some researches [14] showed that the yield of pectin extracted from Lycium ruthenicum by high-temperature acid method are significantly higher than other methods, but this method has not been well optimized. Therefore, we hypothesize that some key procedures (extraction water proportion (v/w), acidic pH regulating reagent, adjusted pH, extraction temperature, extraction time) in the method of extracting goji berry pectin by high temperature acid hydrolysis with ethanol precipitation should be optimized by Box–Behnken experimental designs (BBD). It has been widely known that pectin is a polysaccharide contained in the cell walls of plants and largely used in food industries. So in this paper, the extracted pectin was compared as a yogurt thickener with two known ones to expand the deep use of goji berry pectin in health food industry (Fig. 1).
Fig. 1.
Overall planning of the paper.
2. Materials and methods
2.1. Plant samples and reagents
Whole, freshly cut, the fruits of Lycium barbarum were harvested in September from the Jinzhou Medical University, Jinzhou of China. The plant material was oven-dried at 50 °C and subsequently crushed into powder (the particle size about 0.150 mm and the moisture for ≤6 %). Milk (purchased from China Huishan Dairy Holdings Company Limited). All solvents used were the analytical grade solvents, purchased from Hongda Co. (Jinzhou, China) were used throughout the study. Distilled and deionized water (ddH2O) were prepared by Ultrapure ™ water purification system (DAI Co., Ltd., Shenyang, China).
2.2. Experimental design
The entire experiment was divided into two parts (Fig. 1). The first part was the optimization of the extraction process of goji berry pectin (the detail procedures described in the blue box), and the second part was the application of goji berry pectin (obtained based on the optimization in the first part) as a thickener in yogurt (the detail procedures described in the purple box).
2.3. Pectin extraction and factor design
The extraction program is based on the previous method of extracting pectin in our laboratory [15] and has been improved (the first part in Fig. 1). By using the index of pectin yield, several main factors (Table 1 I) in the initial screening of pectin extraction process are identified, which limits the scope of further optimization. When one factor was changed to examine, the other examined factors were water-goji ratio of 20:1, adjustment pH = 2.0 using hydrochloric acid at 80 °C for 70 min.
Table 1.
The main factor extraction experimental design.
| I. Single factor experimental design | |||||
|---|---|---|---|---|---|
| Level Factors |
S1 Reagent |
S2 water-goji ratio (v/w) | S3 pH | S4 time (min) | S5 temperature (°C) |
| 1 | Hydrochloric acid | 10:1 | 1.5 | 50 | 40 |
| 2 | Nitric acid | 15:1 | 2.0 | 60 | 50 |
| 3 | Sulphuric acid | 20:1 | 2.5 | 70 | 60 |
| 4 | Citric acid | 25:1 | 3.0 | 80 | 70 |
| 5 | – | 30:1 | 3.5 | 90 | 80 |
| 6 | – | 35:1 | 4.0 | 100 | 90 |
| 7 | – | 40:1 | 4.5 | 110 | 100 |
| II. Experimental Design of BBD Response Surface | |||||
|
Level Factors |
reagent | water-goji ratio (v/w) | A pH | B time (min) | C temperature (°C) |
| −1 | – | – | 1.5 | 70 | 70 |
| 0 | – | – | 2.0 | 80 | 80 |
| 1 | – | – | 2.5 | 90 | 90 |
Optimizing the Acid extraction - alcohol precipitation conditions of pectin from the fruit of goji berry has not been studied yet. In this study, BBD was constructed to evaluate the effects of the following main factors: pH (factor A, the level is set to1.5 to 2.5), extraction time (B, 70–90 min) and extraction temperature (C, 70–90 °C) (Table 1 II). Quadratic regression models from the 3-factorial design were set up according to the experimental data with Design Expert (Version 8.0.6).
2.4. Yield of pectin
Goji berry pectin obtained from the first part of the experiment 2.2 was dried overnight in an air-forced oven at 45 °C. The yield of pectin was calculated using equation (1).
| equation 1 |
Where W is the weight of extracted pectin from the fruit of goji berry and weight paper in g, W0 is the amount of blank paper in g, and W1 is the weight of goji berry in g.
2.5. Thickener application of goji berry pectin in yogurt and factor design
The main process of producing goji berry pectin yogurt was shown in Part 2 of Fig. 1. Different food thickeners were respectively goji berry pectin (obtained under optimal conditions by our laboratory), gelatin (Food-grade, Henan Wan Bang industrial Co., Ltd) and agar (Food-grade, Henan Wan Bang industrial Co., Ltd). Successfully prepared different yogurt samples were subjected to viscosity, sensory evaluation and storage stability determination separately.
2.6. Viscosity measurement
Viscosity measurement was according to Osamu Kurita et al. [16]. Viscosity of yoghurt was measured with a constant 30 Hz by a sine-wave viscometer (SV-10, A&D, Tokyo, Japan) with a water-jacket assembly (AX-SV-37, A&D) at 25 °C.
2.7. Sensory evaluation
The sensory evaluation of yogurt product contained the texture, smoothness, stability, viscosity and uniformity. 0–4 scores were set followed by the indexes of sensory evaluation, respectively as poor (0 score), medium (1 score), good (2 score), very good (3 score), and excellent (4 score). Each tested product was tested by 20 sensory professionals, and the average score of each product was the last sensory score. The sensory evaluation was approved by the Ethics Com mittee of Jinzhou Medical University and all panelists provided informed written consent before the experiments.
2.8. Storage stability
The storage stability of finished yogurt product was evaluated at 7 days after storage at 4 °C. According to how many were whey precipitations, several grades were set. No whey separation was 0 score. 1–4 scores were set followed by the amount of whey precipitation, respectively as very little, little, much and very much.
2.9. Statistical analysis
Data are expressed as mean ± SD (n = 3). Statistical analysis was performed using a one-way ANOVA and was analyzed further by Tukey HSD test for statistical difference. Different lowercase alphabets in the same column represent significant differences at P ≤ 0.05, and different capital alphabets represent significant differences at P ≤ 0.01, respectively. All data analyses were conducted using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and Design Expert (Version 8.0.6).
3. Results and discussion
3.1. Preliminary screening results of main pectin extraction factors
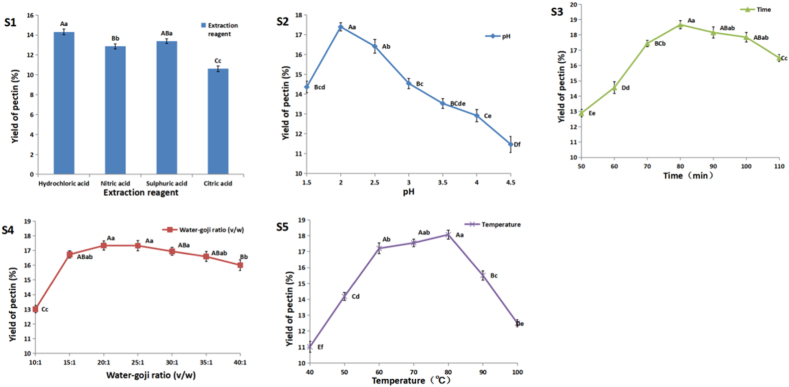
In order to obtain the main compositional parameters of the goji berry pectin extraction, several parameters were investigated. The results about the single factor experimental results were showed in Fig. 2 S1 to S5. The optimal key extraction conditions for goji berries pectin are water-goji ratio of 20:1, adjusting to pH 2.0 with hydrochloric acid (HCl), and extracting at 80 °C for 80 min.
Fig. 2.
Effect of extraction reagent, pH, extraction time, water-goji ratio and extraction temperature on the yield of pectin (%) from goji berry.
In this study, the results showed that in four commonly used in commercial pectin production acid, the best goji berry pectin extraction reagent was HCl and sulphuric acid (H2SO4) (P < 0.05). Compared with the other three inorganic acids, the extraction rate of citric acid (an organic acid) is the lowest (P < 0.01). It is similar to the research about the pectin from hot pepper residues or Kiwi peel obtained with HCl was higher than that extracted with H2SO4 [17] or nitric acid (HNO3) [18]. Then there are also some research results on pectin extraction that are inconsistent with the results of this paper. The pectin from Fuji apple was more easily obtainable using citric acid than two inorganic acids (HCl and HNO3) [19]. In the experiment, the effect of pH (Fig. 2 S2), extraction time (Fig. 2 S3) and extraction temperature (Fig. 2 S5) on the pectin of goji berry changed more obviously than other factors. So these three factors were the major ones to further optimize using response surface methodology.
3.2. Response surface model fitting
In this study, utilized Box-Behnken design (BBD) was applied to arrange and optimize experiment conditions. Combined with the experimental results in section 3.1, the major factors of pH (A), Time (B) and Temperature (C) were selected to optimize by response surface experiments. The experimental conditions were a range of pH (1.5–2.5), time (70–90 min) and temperature (70–90 °C) to be tested. The other conditions were extraction reagent for HCl and water-goji ratio of 20:1. The observed responses of 17 experimental points in the entire design were listed in Table 2 I. The experiments were performed randomly in triplicate.
Table 2.
Response surface experimental design, result (I), summary of ANOVA (II), R2 analysis of multiple model (III) and optimum conditions(IV).
| I. Response surface experimental design and Result | ||||
|---|---|---|---|---|
| Independent variables |
Dependent variables |
|||
| Run | pH A |
Time (min) B |
Temperature (°C) C |
Yield of pectin (%) Y |
| 1 | 2 | 90 | 70 | 16.54 |
| 2 | 2 | 80 | 80 | 18.75 |
| 3 | 2 | 90 | 90 | 17.53 |
| 4 | 2 | 80 | 80 | 18.31 |
| 5 | 2 | 80 | 80 | 18.75 |
| 6 | 2.5 | 80 | 90 | 16.71 |
| 7 | 2 | 80 | 80 | 18.07 |
| 8 | 1.5 | 90 | 80 | 15.92 |
| 9 | 2.5 | 80 | 70 | 18.32 |
| 10 | 2 | 70 | 70 | 17.56 |
| 11 | 2.5 | 90 | 80 | 17.22 |
| 12 | 1.5 | 80 | 70 | 15.74 |
| 13 | 1.5 | 70 | 80 | 14.41 |
| 14 | 1.5 | 80 | 90 | 15.53 |
| 15 | 2.5 | 70 | 80 | 16.49 |
| 16 | 2 | 70 | 90 | 15.76 |
| 17 | 2 | 80 | 80 | 18.64 |
|
II. Summary of ANOVA | ||||||
|---|---|---|---|---|---|---|
| Sourcea | SUMb | Dfc | MSd | F-Value | p-value | Significant |
| Model | 26.897 | 9 | 2.989 | 26.528 | 0.0001 | ∗∗ |
| A-A | 6.372 | 1 | 6.372 | 56.565 | 0.0001 | ∗∗ |
| B-B | 1.118 | 1 | 1.118 | 9.920 | 0.0162 | ∗ |
| C-C | 0.865 | 1 | 0.865 | 7.675 | 0.0277 | ∗ |
| AB | 0.152 | 1 | 0.152 | 1.350 | 0.2833 | |
| AC | 0.490 | 1 | 0.490 | 4.350 | 0.0755 | |
| BC | 1.946 | 1 | 1.946 | 17.274 | 0.0043 | ∗∗ |
| A2 | 8.056 | 1 | 8.056 | 71.512 | <0.0001 | ∗∗ |
| B2 | 5.195 | 1 | 5.195 | 46.112 | 0.0003 | ∗∗ |
| C2 | 1.254 | 1 | 1.254 | 11.132 | 0.0125 | ∗ |
| Residual | 0.789 | 7 | 0.113 | |||
| Lack of Fit | 0.423 | 3 | 0.141 | 1.543 | 0.3338 | |
| Pure Error | 0.366 | 4 | 0.091 | |||
| Cor Total | 27.685 | 16 | ||||
| III. R2analysis of multiple model | ||||||
| Model | Standard deviation | R2 | Adjusted R2 | Predicted R2 | ||
| Linear | 1.22 | 0.3018 | 0.1406 | −0.0925 | ||
| 2FI | 1.29 | 0.3953 | 0.0324 | −0.5726 | ||
| Quadratic | 0.34 | 0.9715 | 0.9349 | 0.7349 | Suggested | |
| Cubic | 0.3 | 0.9868 | 0.9472 | Aliased | ||
| Regression equations of the fitted quadratic model: YYield of pectin(%) = −86.37400 + 32.63700 × A (pH)+1.33458 × B(Time)+0.42233 × C (Temperature) −0.039000 × AB–0.070000 × AC+6.97500E-003 × BC–5.53300 × A2–0.011108 × B2–5.45750E-003 × C2 | ||||||
| IV. Optimum conditions and the predicted and experimental values of response under optimum conditions | ||||||
|
pH A |
Time (min) B |
Temperature (°C) C |
Yield of pectin (%) Y |
|||
| Optimum conditions | 2.19 | 80.04 | 75.80 | 18.7411 | ||
| Modified conditions | 2.20 | 80.00 | 76.00 | 18.7399 (predicted) 18.507 ± 0.275 (experiments) |
||
The results of the analysis of variance (ANOVA) for formulation variables from BBD were showed in Table 2 II. The F-test suggested that this model was highly significant (model F-value = 26.528) and no significant lack of fit (P > 0.05). Quadratic Model was obviously significant (P < 0.01). One quadratic parameter (A2 and B2) and two interaction parameters of BC were obviously significant (P < 0.01). These results meant that this model was fitted for this research. The yield of pectin was significantly affected by pH (A) (P < 0.01), followed by time (B) and temperature (C) (P < 0.05).
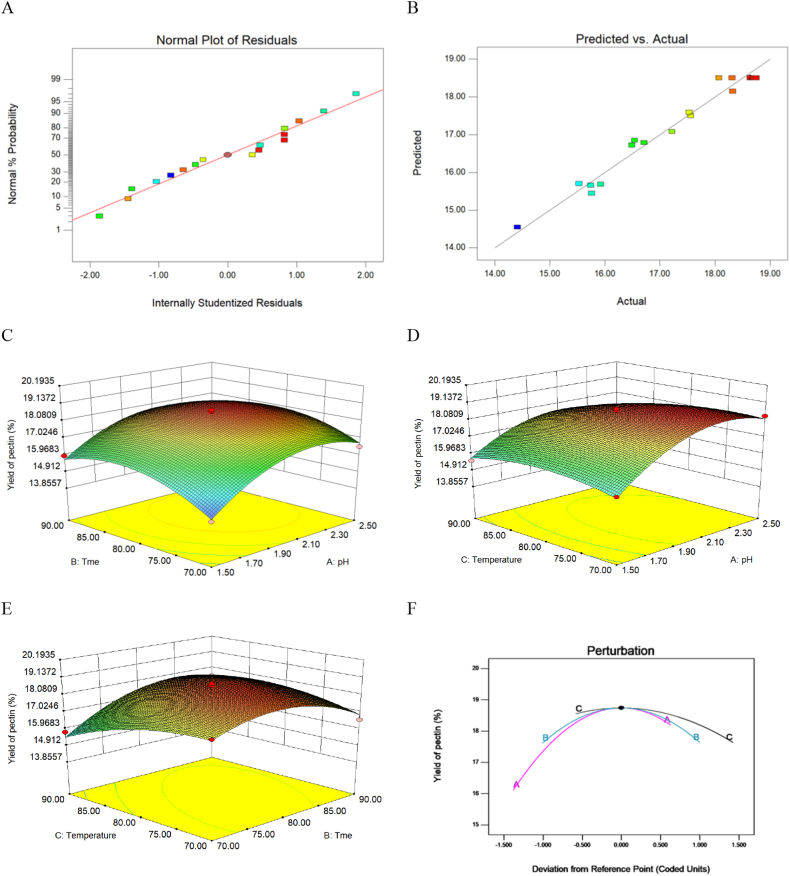
Based on the central composite design principle of Box-Behnken method, linear, 2FI, quadratic equation, and cubic equation prediction models were established. The values of R2, adjusted R2 and predicted R2 are analyzed and shown in Table 2 III. The Design Expert software recommends using a quadratic model (Quadratic), in which determination coefficient R2 is 0.9715>0.8 (meaning a well fitted model, P < 0.01), indicating that the predicted results of the model (adjusted R2 = 0.9349) are more in line with the actual results (predicted R2 = 0.7349). It meant that the calculated model explained 97.2 % of the results and the model was significant to represent the relationship between yield of pectin from goji berry and the independent variables (pH, extraction time and extraction temperature). Therefore, it can be concluded that the quadratic model has the smallest deviation (Fig. 3A) and higher fitting degree (Fig. 3B) than other models.
Fig. 3.
Response surface of any two extraction factors and other model graphs.
Note: (A) Normal Plot of Residuals. (B) Predicted vs. actual of yield of pectin. (C) Response surface of pH and extraction time. (D) Response surface of pH and extraction temperature. (E) Response surface of extraction time and extraction temperature. (F) Perturbation of yield of pectin, when Factor A = 2.19, B = 80.04 and C = 75.80.
3.3. Analysis of response surface
Two factors in the quadratic regression equations are graphically analyzed by three-dimensional response surface (3-DRS), whereas the third factor was at its optimum value [20]. From the 3-DRS curves (Fig. 3C and D E and F), the independent and interaction effects of extraction conditions on the yield of pectin of goji berry can be seen. Fig. 3 displayed the pectin yield obviously increased with the pH from 1.5 to 2.2, extraction time from 70 to 80 min and extraction temperature from 70 to 75 °C, and decreased with pH from 2.2 to 2.5, time from 80 to 90 min and temperature from 75 to 90 °C.
3.4. Optimization of extraction conditions and construction of the fitted quadratic model
The aim of this study was to find the optimization extraction conditions to give the maximum yield of pectin of goji berry by a convenient and fast experiment (Table 2 IV). Software (Design Expert 8.0.5b) predicted the optimum extraction pH, extraction time and extraction temperature as 2.19, 80.04 min and 75.8 °C, respectively. And the yield of pectin was predicted to 18.7411 % by the software. After experimental verification, the optimal conditions were pH of 2.2, extraction time of 80 min and temperature of 76 °C, and the actual results (18.507 % ± 0.275 %) was very close to the predicted value (18.7399 %), indicating that the optimization model was reliable in this study. Compared with the pectin content of other berries [21] (strawberry 9.0 ± 0.7 %, redcurrant 8.8 ± 1.3 %, blackberry 9.1 ± 1.3 %, raspberry 10.9 ± 0.01 %), goji berries contain more pectin. The extraction rate of goji berry pectin in this paper is higher than that of Ziyang Wu's extraction of Lycium ruthenicum pectin by three different extraction methods [22] (the high-temperature water-extracted pectin 3.83 ± 0.73 %, .high-temperature acid-extracted pectin 6.77 ± 0.54 % and high-temperature alkali-extracted pectin 6.19 ± 0.71 %). All of these indicate that this experimental procedure can effectively improve the efficiency of obtaining goji berry pectin, which will provide strong experimental evidence for the widespread application of goji berry pectin.
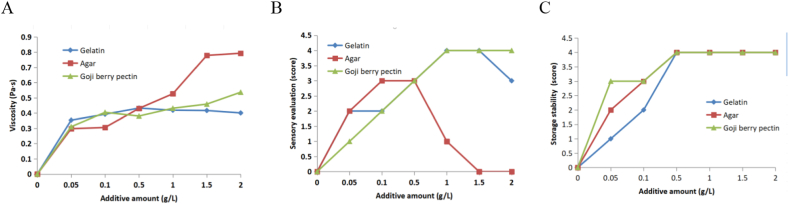
3.5. Compared the effect of goji berry pectin, gelatin and agar on the quality of yogurt
The results of Fig. 4 A, B, and C showed that agar increased the viscosity of yogurt most obviously. Compared with the commonly used thickener, such as gelatin and agar, goji berry pectin had more stable and higher sensory evaluation. The viscosity of yogurt could rise by the augmented concentration of goji berry pectin. It can form a gel under the sugar and acid conditions in yogurt, thus increasing the viscosity. The effects of goji berry pectin on the storage stability score and sensory evaluation score of yogurt were good. Because goji berry pectin has mang strong glycosidic bonds, so its structure remains stable and yogurt adding it is also has a stable stability. As thickener, goji berry pectin with more nutritious and healthy properties was more suitable added in yogurt. These results indicate that the addition of goji berry pectin improves its sensory properties and viscosity of the yogurt. This view is similar to some published researches [23,24].
Fig. 4.
Effect of the different additive concentration of goji berry pectin, gelatin and agar as food thickening agent (g/L) on viscosity, sensory evaluation and storage stability of yogurt.
4. Conclusions
This paper optimizes the key conditions for extracting pectin from goji berry, which are water-goji ratio of 20:1, extraction pH of 2.2 adjusted by hydrochloric acid, extraction time of 80 min and temperature of 76 °C, and the maximum amount of pectin obtained is 18.507 %. It will effectively improve the efficiency of obtaining goji berry pectin, which will provide strong experimental evidence for the widespread application of goji berry pectin. This experiment further proves that the addition of goji berry pectin as thickener improves its sensory properties and viscosity of the yogurt. Although goji berry pectin does not have significant advantages in sensory evaluation and yogurt viscosity, it has been proven in some studies to have good functionality in terms of nutrition. This provides a good idea for the development of functional foods urgently needed in the current aging society. Goji berry will be an effective alternative source of pectin on health food industry on large scale. In addition, this paper also provides a feasible approach to effectively solve the problem of goji berry overcapacity.
CRediT authorship contribution statement
Lijing Geng: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Project administration, Funding acquisition, Data curation. Qianqian Shi: Writing – original draft, Validation, Data curation. Wei Zhou: Writing – review & editing, Funding acquisition, Data curation. Dan Wang: Writing – review & editing, Visualization, Validation, Data curation. Fu Hang: Writing – review & editing, Visualization, Validation. Huang Wei: Writing – review & editing, Visualization, Validation. Mbinga Isequias Elamba Tertuliano: Writing – review & editing, Visualization, Validation. Muhammad Zain Ul Aabideen: Writing – review & editing, Visualization, Validation.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional information files.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Scientific Research and Academic Ethics (Ethics) Committee of Jinzhou Medical University (NO.2022092911. Approved on September 29, 2022).
Data availability statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Consent for publication
Not applicable.
Funding
This work was supported by “Liaoning Province Applied Basic Research Program (20230242)” and “Liaoning Provincial College Student Innovation and Entrepreneurship Project (S202310160003X)”.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Lijing Geng, Wei Zhou reports financial support was provided by Liaoning Province Applied Basic Research Program. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Contributor Information
Lijing Geng, Email: gengli777@126.com.
Wei Zhou, Email: zhoutuntun@163.com.
References
- 1.Zhang A.A., Xie L., Wang Q.H., Xu M.Q., Pan Y., Zheng Z.A., Lv W.Q., Xiao H.W. Effect of the ripening stage on the pulsed vacuum drying behavior of goji berry (Lycium barbarum L.): ultrastructure, drying characteristics, and browning mechanism. Food Chem. 2024 doi: 10.1016/j.foodchem.2024.138489. [DOI] [PubMed] [Google Scholar]
- 2.Kong Q., Han X., Cheng H., Liu J., Zhang H., Dong T., Chen J., So K.F., Mi X., Xu Y., Tang S. Lycium barbarum glycopeptide (wolfberry extract) slows N-methyl-N-nitrosourea-induced degradation of photoreceptors. Neural Regen Res. 2024:2290–2298. doi: 10.4103/1673-5374.390958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J., Guo H., Dong Y., Yuan S., Wei X., Zhang Y., Dong L., Wang F., Bai T., Yang Y. Polysaccharides from Chinese herbal medicine: a review on the hepatoprotective and molecular mechanism. Chin. J. Nat. Med. 2024:4–14. doi: 10.1016/S1875-5364(24)60558-3. [DOI] [PubMed] [Google Scholar]
- 4.Yu Z., Xia M., Lan J., Yang L., Wang Z., Wang R., Tao H., Shi Y. A comprehensive review on the ethnobotany, phytochemistry, pharmacology and quality control of the genus Lycium in China. Food Funct. 2023:2998–3025. doi: 10.1039/d2fo03791b. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q., Fan J., Lin L., Zhao M. Combination of Lycium barbarum L. and Laminaria japonica polysaccharides as a highly efficient prebiotic: optimal screening and complementary regulation of gut probiotics and their metabolites. Int. J. Biol. Macromol. 2023 doi: 10.1016/j.ijbiomac.2023.125534. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira F., Silva A.M., Delerue-Matos C., Rodrigues F. Lycium barbarum berries (Solanaceae) as source of bioactive compounds for healthy purposes: a review. Int. J. Mol. Sci. 2023:4777. doi: 10.3390/ijms24054777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X., Gao H., Radani Y., Yue S., Zhang Z., Tang J., Zhu J., Zheng R. Integrative transcriptome and metabolome analysis reveals the discrepancy in the accumulation of active ingredients between Lycium barbarum cultivars. Planta. 2024:74. doi: 10.1007/s00425-024-04350-0. [DOI] [PubMed] [Google Scholar]
- 8.Lee H.S., Choi C.I. Black goji berry (Lycium ruthenicum murray): a review of its pharmacological activity. Nutrients. 2023:4181. doi: 10.3390/nu15194181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni J., Ding C., Zhang Y., Song Z. Impact of different pretreatment methods on drying characteristics and microstructure of goji berry under electrohydrodynamic (EHD) drying process. Innovat. Food Sci. Emerg. Technol. 2020 doi: 10.1016/j.ifset.2020.102318. [DOI] [Google Scholar]
- 10.Wang M., Ouyang X., Liu Y., Liu Y., Cheng L., Wang C., Zhang B. Comparison of nutrients and microbial density in goji berry juice during lactic acid fermentation using four lactic acid bacteria strains. J. Food Process. Preserv. 2021 doi: 10.1111/jfpp.15059. [DOI] [Google Scholar]
- 11.Yu J., Yan Y., Zhang L., Mi J., Yu L., Zhang F., Lu L., Luo Q., Li X., Zhou X., Cao Y. A comprehensive review of goji berry processing and utilization. Food Sci. Nutr. 2023:7445–7457. doi: 10.1002/fsn3.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D.Q., Li J., Dong H.L., Li X., Zhang J.Q., Ramaswamy S., Xu F. Pectin in biomedical and drug delivery applications: a review. Int. J. Biol. Macromol. 2021:49–65. doi: 10.1016/j.ijbiomac.2021.06.088. [DOI] [PubMed] [Google Scholar]
- 13.Qin C., Yang G., Zhu C., Wei M. Characterization of edible film fabricated with HG-type hawthorn pectin gained using different extraction methods. Carbohydr. Polym. 2022 doi: 10.1016/j.carbpol.2022.119270. [DOI] [PubMed] [Google Scholar]
- 14.Wu Z., Qin D., Li H., Guo D., Cheng H., Sun J., Huang M., Ye X., Sun B. Physicochemical and functional properties of Lycium ruthenicum pectin by different extraction methods. Front. Nutr. 2022 doi: 10.3389/fnut.2022.946606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng L., Zhou W., Qu X., Chen W., Li Y., Liu C., Sun J., Yu X., Wang H., Zhang Z., Li J., Wang L. Optimization of the preparation of pectin from Aloe using a Box-Behnken design. Carbohydr. Polym. 2014:193–199. doi: 10.1016/j.carbpol.2014.01.069. [DOI] [PubMed] [Google Scholar]
- 16.Kurita O , Fujiwara T , Yamazaki E .Characterization of the pectin extracted from citrus peel in the presence of citric acid, Carbohydrate Polym. (200) 725-730. doi:10.1016/j.carbpol.2008.04.033.
- 17.Xu H., Tai K., Wei T., Yuan F., Gao Y. Physicochemical and in vitro antioxidant properties of pectin extracted from hot pepper (Capsicum annuum L. var. acuminatum (Fingerh.)) residues with hydrochloric and sulfuric acids. J. Sci. Food Agric. 2017:4953–4960. doi: 10.1002/jsfa.8372. [DOI] [PubMed] [Google Scholar]
- 18.Karbuz P., Tugrul N. Microwave and ultrasound assisted extraction of pectin from various fruits peel. J. Food Sci. Technol. 2021:641–650. doi: 10.1007/s13197-020-04578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J., Liu D., Xia W., Guo Y., Luo Y., Xue J. Physicochemical and functional properties of RG-I enriched pectin extracted from thinned-young apples. Int. J. Biol. Macromol. 2023 doi: 10.1016/j.ijbiomac.2023.123953. [DOI] [PubMed] [Google Scholar]
- 20.Piasecka I., Brzezińska R., Kalisz S., Wiktor A., Górska A. Response surface methodology for optimization of ultrasound-assisted antioxidants extraction from blackberry, chokeberry and raspberry pomaces. Plants. 2024 Apr 17;13(8):1120. doi: 10.3390/plants13081120. PMID: 38674528; PMCID: PMC11053409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muñoz-Almagro N., Ruiz-Torralba A., Méndez-Albiñana P., Guerra-Hernández E., García-Villanova B., Moreno R., Villamiel M., Montilla A. Berry fruits as source of pectin: conventional and non-conventional extraction techniques. Int. J. Biol. Macromol. 2021:962–974. doi: 10.1016/j.ijbiomac.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Wu Z., Qin D., Li H., Guo D., Cheng H., Sun J., Huang M., Ye X., Sun B. Physicochemical and functional properties of Lycium ruthenicum pectin by different extraction methods. Front. Nutr. 2022 doi: 10.3389/fnut.2022.946606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H., Goff H.D., Liu C., Luo S., Hu X. Preparation of liquid yogurt in the presence of pectin and its formation mechanism. Food Chem. 2024 doi: 10.1016/j.foodchem.2024.139473. [DOI] [PubMed] [Google Scholar]
- 24.Bankole A.O., Irondi E.A., Awoyale W., Ajani E.O. Application of natural and modified additives in yogurt formulation: types, production, and rheological and nutraceutical benefits. Front. Nutr. 2023 doi: 10.3389/fnut.2023.1257439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Additional information files.
The data used to support the findings of this study can be made available by the corresponding author upon request.